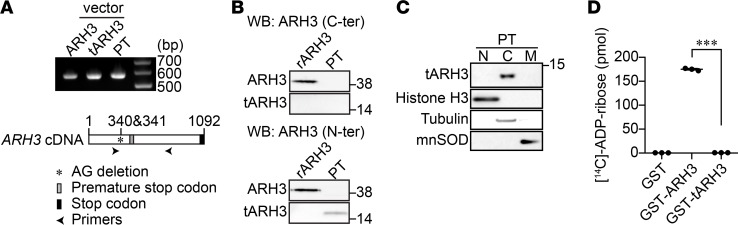
Figure 1. Truncated ARH3 expressed in patient fibroblasts lacks PAR-degrading activity.
(A) RT-PCR was performed to detect ARH3 mRNA transcript expression in patient fibroblasts (PTs) using ARH3-specific primers, as described in Supplemental Table 1. Plasmid vectors encoding ARH3 WT and truncated ARH3 were used as expression controls. (B) Expression of truncated ARH3 (tARH3), but not full-length ARH3, in patient fibroblasts. Cells were subjected to Western blotting using anti-ARH3 antibodies recognizing the C- (C-ter) and N-terminal regions of ARH3. Recombinant human ARH3 protein (rARH3) was used as a positive control. (C) Expression of truncated ARH3 in the cytoplasm. Purity of nuclear (N), cytoplasmic (C), and mitochondrial (M) fractions was confirmed using protein markers: histone H3 (nucleus), tubulin (cytoplasm), and manganese superoxide dismutase (MnSOD, mitochondria). (D) PAR-degrading activity of ARH3. [14C]-labeled PAR (52,012 cpm, 245 pmol) was incubated with GST-tagged proteins (200 nM) for 60 minutes. As described in Methods, [14C]-ADP-ribose was separated by HPLC using an LC-18T column; radioactivity was measured with a liquid scintillation counter. Data are mean ± SEM of values obtained from 3 samples. ***P < 0.001 by one-way ANOVA with Tukey’s post-hoc test.