Abstract
The aim of this study was to determine whether skin wounding induces monocyte expansion in bone marrow and whether interleukin-1 receptor 1 (IL-1R1) signaling regulates this process. Our data show that skin wounding increases myeloid lineage committed multipotent progenitors (MPP3 subset) and monocytes in bone marrow but this expansion is not impaired in Il1r1−/− mice. We also demonstrate that macrophage colony stimulating factor-induced differentiation of myeloid progenitors into monocytes is not impaired by the loss of IL-1R1 ex vivo, indicating that IL-R1 deficiency does not abrogate myeloid progenitor differentiation potential. In addition, we observed modestly delayed wound closure in Il1r1−/− mice associated with higher frequency of Ly6Clo monocytes in the circulation at baseline and in wounds early after injury. Thus, in contrast to other models of inflammation that involve IL-1R1-dependent monopoiesis, our results demonstrate that skin wounding induces monocyte progenitor and monocyte expansion independently of IL-1R1 signaling
Introduction
Monocyte/macrophages (Mo/Mp) are required for repair of a variety of tissues via their roles in the inflammatory, proliferative and remodeling phases of wound healing (1–4). The importance of Mo/Mp in skin wound repair is evident from studies demonstrating impaired wound healing in mice following selective Mp ablation (3, 5). Mo/Mp accumulate in wounds either by egressing from circulation or migrating and proliferating from their local pool (6–9). However, it is unclear whether skin wounding induces signals that increase Mo production (monopoiesis) in bone marrow (BM) and whether such damage-induced monopoiesis contributes to Mo/Mp accumulation that is required for normal healing of skin wounds.
Published reports have provided evidence that interleukin-1 receptor 1 (IL-1R1) signaling plays an important role in inflammatory responses to wounding. For example, one study showed reduced infiltration of inflammatory cells in Il1r1−/− mice leading to decreased scar formation in deep wounds but that skin wound closure was not affected (10). In addition, sustained activation of IL-1R1 signaling in IL-1 receptor antagonist (IL-1Ra) deficient mice exhibited aberrant wound healing in association with chronic inflammation indicating that timely regulation of IL-1R1 signaling is required for normal wound healing (11). Furthermore, our finding of delayed early skin wound healing in mice deficient for NLRP3 (NLR family, pyrin domain-containing 3), which is required for activation and release of IL-1β, indicates a necessity of IL-1β-induced inflammation in the early stages of normal skin wound healing (12). Finally, we found that blocking IL-1R1 signaling in diabetic db/db mice via local administration of neutralizing antibody to wounds or using Il1r1−/− BM transplantation induces a switch from pro-inflammatory to healing associated Mp phenotypes and improves diabetic wound healing (13). However, much remains to be learned about the role of IL-1R1 in regulating the inflammatory response in wound healing.
A recent study demonstrated that chronic IL-1β exposure induces myelopoiesis and myeloid lineage biased progenitor expansion implicating IL-1R1 signaling in myelopoiesis (14). Another study demonstrated that alum adjuvant induces hematopoietic stem and progenitor cell (HSPC) expansion and subsequent emergency granulopoiesis in wild-type mice but that this response was blunted in Il1r1−/− mice (15). Similarly, myocardial infarction-induced hematopoietic stem cell (HSC) expansion and myelopoiesis was blunted in Il1r1−/− mice, supporting the importance of IL-1R1 signaling in tissue damaged-induced myelopoiesis (16). However, whether damage to other tissues, such as skin wounding, induces HSPC and monocyte expansion and whether such a response may be regulated by IL-1R1 signaling has not been reported. The present study aims to fill this gap in the literature.
Material and methods
Animals
Interleukin-1 receptor 1 knock out mice (Il1r1−/−) and C57Bl/6 WT controls were obtained from Jackson laboratories. Experiments were performed on 8- to 12-week-old mice. All experimental procedures involving animals were approved by Animal Care Committee at the University of Illinois at Chicago.
Excisional skin wounding
Mice were anesthetized with isoflurane and their dorsum was shaved and cleaned with alcohol swab. Two 8-mm excisional wounds were made on the back of each mouse with a dermal biopsy punch and wounds were covered with Tegaderm (3M).
In vivo BrdU incorporation assay
Following 2 days of skin wounding BrdU (Sigma, B9285) was injected i.v. through retro-orbital route at 2 mg per mouse. After 4 hours of BrdU injection mice were sacrificed and BM cells were fixed, permeabilized, treated with DNase-1 and stained with FITC conjugated anti-BrdU antibody (Biolegend, 364103) following surface antibody staining.
Cell isolation
Cells from mouse excisional wounds were dissociated using enzymatic digest as described previously (17). For bone marrow (BM) cells femurs were isolated and marrow was flushed with cold fluorescence activated cell sorting (FACS) buffer.
Flow cytometry
For stem cell populations, BM single cells were incubated with biotin conjugated lineage antibodies against CD45R, CD3e, Gr1, TER-119, CD11b, CD4, CD8a, CD19, NK1.1 and CD127 (BioLegend) followed by either streptavidin-APC-Cy7 (BioLegend, 405208), Sca-1-PerCP-Cy5.5 (BioLegend, 108124), cKit-Alexa Fluor 647 (BioLegend, 105818), Flk2-BV421 (BioLegend, 135315), CD150-PE-Dazzle594 (BioLegend, 115935) and CD48-PE (BioLegend, 103405) or streptavidin-APC-Cy7, Sca-1-PerCP-Cy5.5, cKit-Alexa Fluor 647, FcRγ-V450 (BD Horizon, 560539) and CD34-PE (BioLegend, 128609). For myeloid cells in BM and blood, cells were stained with antibodies against Ly6G-BV421 (BioLegend, 127628), CD11b-APC-Fire750 (BioLegend, 101262), CD115-PE (BioLegend, 135505) and Ly6C-PerCPCy5.5 (BD Pharmingen, 560525). For wound myeloid cells, enzymatically dissociated single cells were stained for Zombie-BV421 (BioLegend, 423113), CD45-FITC (BioLegend, 103108), Ly6G-PE-CF594 (BD Horizon, 562700), CD11b-APC-eFluor780 (eBiosciences, 47–0112), F4/80-PE (BioLegend, 123110) and Ly6C-PerCPCy5.5. For BM cells sorting magnetically enriched progenitors, Lineage- BM cells were stained for streptavidin-eFluor450 (eBiosciences, 48–4317), Sca-1-PE-Cy7 (BioLegend, 108114) and cKit-Alexa Fluor 647. For assessing Mo differentiation in culture, differentiated cells were stained for CD11b-APC-Fire750 and Ly6C-PerCPCy5.5. For wound cell sorting wound cells isolated by enzymatic digestion were stained for Zombie-BV421 (BioLegend, 423113), CD45-FITC (BioLegend, 103108), Ly6G-PE-CF594 (BD Horizon, 562700) and CD11b-APC-eFluor780 (eBiosciences, 47–0112). Cell sorting was performed on MoFlo Astrios (Beckman Coulter) and cell analyses were done using LSR II Fortessa (Becton Dickenson).
IL-1β measurement in BM plasma
BM plasma was collected as mentioned previously (18). IL-1β protein was measured in BM plasma using ELISA kit (R&D Systems, MLB00C) following manufacturer’s instructions.
Cell culture
Sorted myeloid progenitor (MyP) cells were grown in StemPro34 medium (Invitrogen) supplemented with penicillin (50 U ml−1)/streptomycin (50 U ml−1) and L-glutamine (2 mM), SCF (25 ng ml−1) and Flt3L (25 ng ml−1) (Peprotech). IL-1β (Peprotech, 211–11B) was added at 5 or 25 ng ml−1 and M-CSF (R&D Systems, 416-ML) at 25 ng ml−1.
Statistics
Results are expressed as mean±SD. Statistical analyses were performed using Prism 7.0 software (GraphPad). Data between two groups were compared using 2 way ANOVA and different time points in the same group using 1 way ANOVA (Kruskal-Wallis test) in kinetic assays. Two time points or two groups were compared using t-test (Mann-Whitney). P values less than 0.05 were considered as significant (at 95% confidence intervals).
Results
Skin wounding induces expansion of myeloid biased hematopoietic progenitor compartment through an IL-1R1-independent mechanism
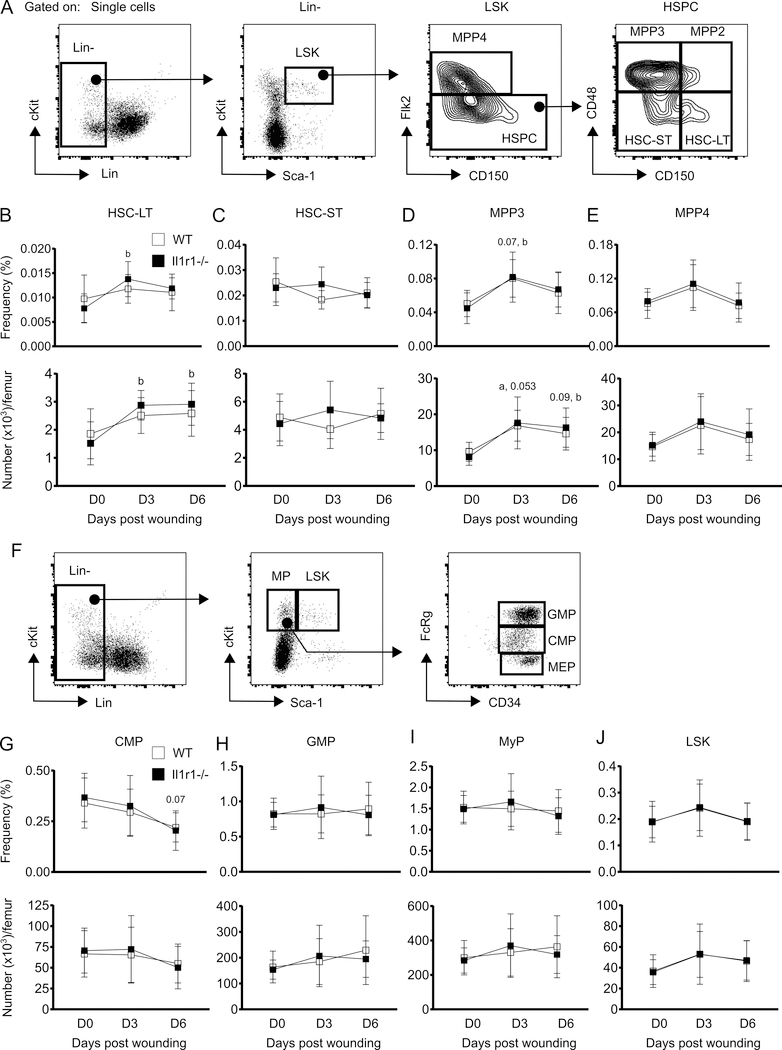
We examined the kinetics of hematopoietic stem cells-long term (HSC-LT) and short term (HSC-ST), multipotent progenitors 2 and 3 (MPP2 and MPP3), common myeloid progenitors (CMP) and granulocyte macrophage progenitors (GMP) (19) in the BM of WT and Il1r1−/− mice following excisional skin wounding. There was no change in the frequency or number of either HSC-LT (Lin-Sca-1+cKit+Flk2-CD48-CD150+) or HSC-ST (Lin-Sca-1+cKit+Flk2-CD48-CD150-) in WT mice in response to wounding (Figure 1B and 1C). On the other hand, Il1r1−/− mice showed an increase in the number and frequency of HSC-LT at both 3 days and 6 days post-injury (Figure 1B). Similar to WT controls, Il1r1−/− mice also did not show any change in HSC-ST subsets following wounding.
Figure 1:
Skin wounding-induced expansion of MPP3 compartment is not affected by IL-1R1 signaling deficiency. (A) Representative flow-cytograms showing gating strategy for flow-cytometry analysis of HSPC subsets in BM. (B-E) Percentage (of total BM cells (BMC); upper panels) and number (lower panels) of LT-HSC (Lin-Sca-1+cKit+(LSK)Flk2-CD48-CD150+), ST-HSC (LSK Flk2-CD48-CD150-), MPP3 (LSK Flk2-CD48+CD150-) and MPP4 (LSK Flk2+CD150-) in BM. (F) Gating strategy for MyP subsets and LSK in BM. (G-J) Percentage (of total BMC; upper panels) and number (lower panels) of CMP (Lin-Sca-1-cKit+FcRγloCD34+), GMP (Lin-Sca-1-cKit+FcRγhiCD34+), MyP (Lin-Sca-1-cKit+) and LSK in BM. Results are represented as mean ± SD, n=7 mice for each strain and each time point. a, mean value significantly different from day 0 in WT; b, mean value significantly different from day 0 in Il-1r1−/−. p≤0.05 is significant.
MPP3 (Lin-Sca-1+cKit+Flk2-CD48+CD150-) are specific progenitors for myeloid lineage cells (19) and were increased both in WT and Il1r1−/− mice at 3 days after wounding (Figure 1D). MPP4 (Lin-Sca-1+cKit+Flk2+CD150-), which are progenitors for lymphoid lineage cells at homeostatic condition but may switch to producing myeloid cells during inflammatory episodes (19), appeared to show a similar trend as MPP3, but this did not reach statistical significance (Figure 1E). Further analysis of myeloid progenitors revealed that the frequency of CMP (Lin-Sca-1-cKit+FcRγloCD34+) trended lower in both WT and Il1r1−/− mice at 6 days after wounding although their number appeared to be unaltered. In addition, GMP (Lin-Sca-1-cKit+FcRγhiCD34+) and myeloid progenitor (MyP, Lin-Sca-1-cKit+) subsets were not affected by skin injury in either of the strains (Figure 1G-I). Similarly, LSK (Lin-Sca-1+cKit+) cells were also found not to be altered by injury in either of the strains (Figure 1J). Altogether, the data indicate that, among all myeloid progenitors, only MPP3 is expanded in BM in response to skin wounding. Expansion of HSPC and MPP populations was observed following alum administration in mice (15); our MPP3 population would be found in the HSPC population of this previous study based on the markers used. However, whereas the response to alum was abrogated in Il1r1−/− mice, loss of IL-1R1 signaling did not influence the MPP3 response following skin wounding.
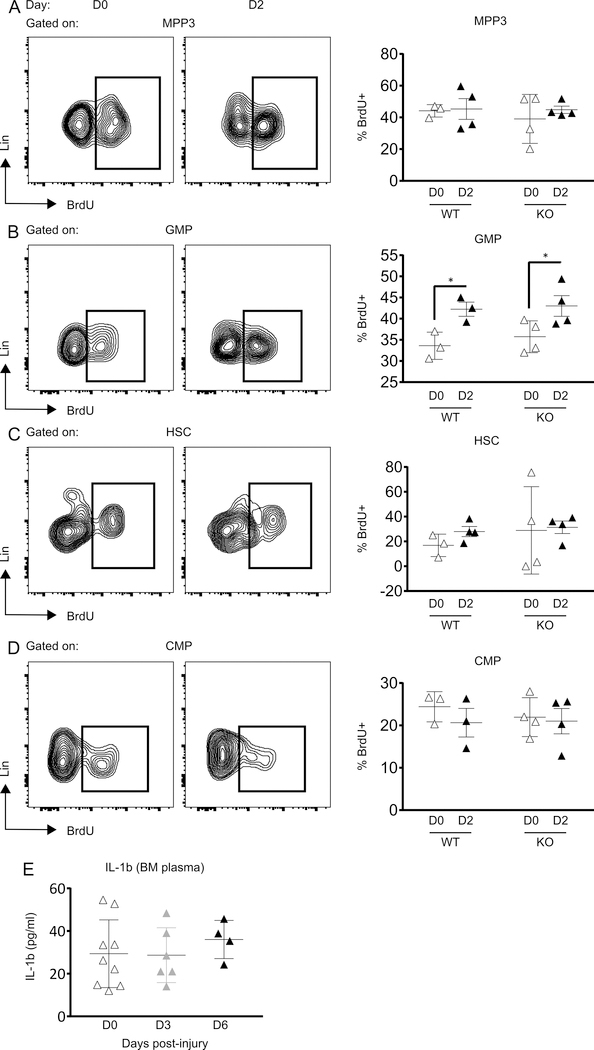
To determine whether skin wounding-induced expansion of MPP3 cells on day 3 post-wounding was due to increased proliferation, we assessed 5-Bromo-2′-deoxyuridine (BrdU) incorporation 2 days post-wounding (Figure 2). Unexpectedly, the wounding-induced increase in MPP3 cells was not associated with increased frequencies of BrdU+ MPP3 compared to unwounded controls in either wild-type or Il1r1−/− mice (Figure 2A). This was also the case when BrdU incorporation was assessed on day 3 post-wounding in wild-type mice (not shown). On the other hand, BrdU incorporation into GMP cells was increased in both WT and Il1r1−/− mice at 2 days following wounding, whereas their numbers were not increased at any time point after wounding (Figure 2B). Similar to MPP3, BrdU incorporation into HSC and CMP cells was unaltered in response to injury (Figure 2C and 2D).
Figure 2:
Skin wounding increases GMP proliferation IL-1R1 independently. Representative flow-cytograms showing BrdU+ cells gated on MPP3 (A), GMP (B), HSC (C) and CMP (D) (left panels) and percentage of BrdU+ cells in the corresponding cell populations (right panels). Results are represented as mean ± SD, n=3 to 4 mice for each strain and each time point. *p≤0.05. (E) IL-1β concentration in BM plasma. Results are represented as mean ± SD, n=4 to 9 mice for each time point.
IL-1β has been reported to serve as a soluble inflammatory mediator which is transported to BM via the circulation from damaged tissue leading to increased myelopoiesis following myocardial infarction (16). We hypothesized that similar to myocardial infarction, skin wounding may also elevate IL-1β in BM and induce increased monopoiesis. Hence, we measured IL-1β protein in BM plasma of WT mice following skin wounding and our results showed no difference in IL-1β level in BM of wounded mice as compared to unwounded controls (Figure 2E).
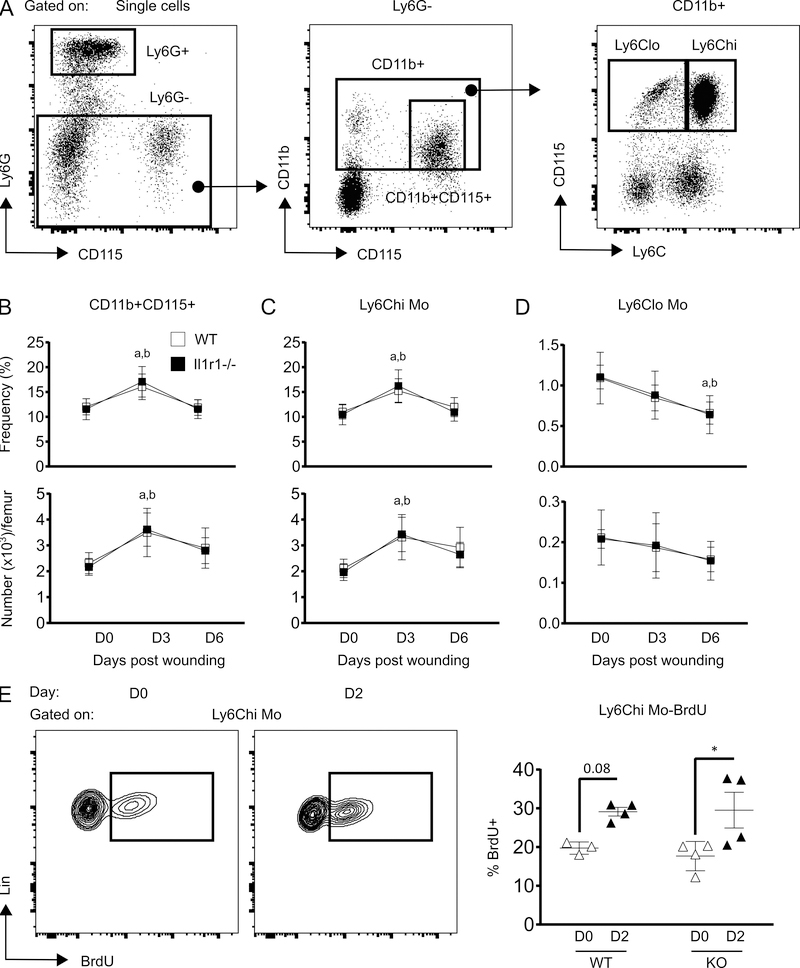
Skin wounding-induced expansion of Ly6Chi Mo in BM is not affected by IL-1R1 deficiency
The majority of wound Mo/Mp are thought to be derived from circulating cells that are in turn produced in the BM (8, 20). Hence, we assessed Mo kinetics in BM post-wounding and tested whether IL-1R1 deficiency influenced Mo kinetics. Results showed significant increase in total Mo (Ly6G-CD11b+CD115+) population in WT BM at day 3 post-injury as compared to non-injured, which reverted back almost to non-injured levels at day 6 post-injury (Figure 3B). This expansion of BM Mo correlates with the kinetics of BM MPP3 following skin injury (Figure 1D). Skin wounding also increased the number of Ly6Chi Mo (Ly6G-CD11b+CD115+Ly6Chi) and this was associated with increased BrdU incorporation; the frequency of BrdU+ Ly6Chi Mo trended higher in the BM of wounded mice after 2 days of injury compared with unwounded controls (Figure 3E). In contrast, Ly6Clo Mo (Ly6G-CD11b+CD115+Ly6Clo) were found to be decreased in WT BM as the healing progressed and reached their lowest level at 6 days after injury (Figure 3C and 3D). However, the kinetics of all Mo populations as well as BrdU+ Ly6Chi Mo in Il1r1−/− BM showed similar pattern when compared to their WT counterparts (Figure 3B-E). Macrophage colony stimulating factor (M-CSF) has been reported to induce myelopoiesis in WT HSCs by upregulating myeloid associated transcription factor PU.1, thus indicating an important role of M-CSF receptor (M-CSFR) signaling in myelopoiesis (21). Hence, we measured surface M-CSFR on HSPC subsets post wounding to examine whether M-CSFR signaling is involved in skin wounding associated monopoiesis. Results, showed a trend in increased surface M-CSFR protein on LSK and GMP in response to wounding whereas CMPs did not show wound induced alteration in surface M-CSFR (Supplementary figure 1). Interestingly, surface M-CSFR on GMP and CMP coincided with BrdU incorporation in these subsets (Supplementary Figure 1, Figure 2B and 2D). Furthermore, our unpublished data revealed that GMPs from type 2 diabetic mice (db/db) display higher M-CSFR in correlation with increased myeloid expansion in db/db mice (not shown). Together, these data show that skin wounding-induced monopoiesis in BM is not altered by the loss of IL-1R1 signaling but that M-CSFR signaling may be involved.
Figure 3:
IL-1R1 deficiency does not alter skin wounding-induced monocyte expansion in BM. (A) Representative flow-cytograms showing gating strategy for flow-cytometry analysis of Mo subsets in BM. (B-D) Percentage (of total BMC; upper panels) and number (lower panels) of total Mo (Ly6G-CD11b+CD115+), Ly6Chi Mo (Ly6G-CD11b+CD115+Ly6Chi) and Ly6Clo Mo (Ly6G-CD11b+CD115+Ly6Clo) in BM. Results are represented as mean ± SD, n=7 mice for each strain and each time point. a, mean value significantly different from day 0 in WT; b, mean value significantly different from day 0 in Il-1r1−/−. (E) Representative flow-cytograms showing BrdU+ cells gated on Ly6Chi (left panel) and percentage of Ly6Chi BrdU+ cells (right panel). Results are represented as mean ± SD, n=3 to 4 mice for each strain and each time point. *p≤0.05.
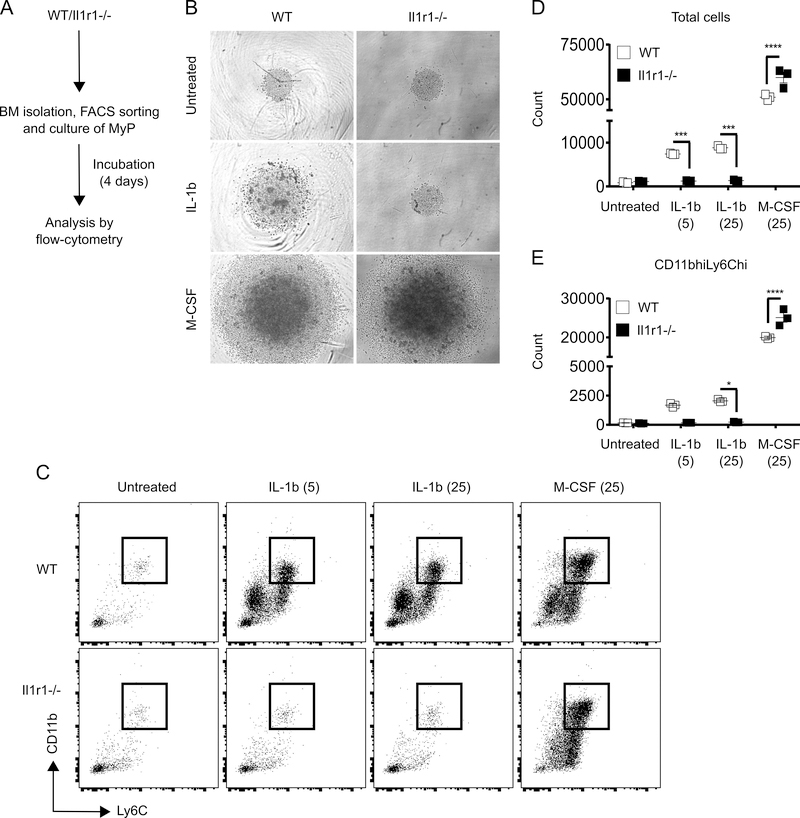
IL-1R1 signaling deficiency does not impair monopoiesis ex vivo
To investigate whether monopoiesis is affected by deficiency of IL-1R1 signaling we isolated MyP from non-injured WT and Il1r1−/− mice and monitored their differentiation into Ly6Chi Mo in the presence or absence of IL-1β or M-CSF in liquid culture. As anticipated, MyP cultured from WT mice differentiated and expanded significantly after 4 days in the presence of either IL-1β or M-CSF as compared to untreated controls; both the total cell and Ly6Chi Mo numbers were increased remarkably (Figure 4). Also as expected, IL-1β-induced differentiation and expansion was abrogated in MyP cultures derived from Il1r1−/− mice, confirming that IL-1R1 signaling is indeed non-functional in those progenitors (Figure 4). However, M-CSF-induced expansion of MyP cultures was not inhibited by the deficiency in IL-1R1 signaling. In fact, compared to WT controls the expansion of Il1r1−/− MyP was significantly higher in response to M-CSF (Figure 4). Together, our data indicates that although IL-1β-induced monopoiesis is dependent on IL-1R1 signaling, M-CSF-induced monopoiesis is not impaired in the absence of IL-R1 signaling.
Figure 4:
IL-1R1 signaling deficiency does not abrogate expansion potential of MyP. (A) Experimental scheme. (B) Representative microscopic images of MyP cultures after 4 days of incubation. Images taken at 10x magnification. (C) Representative flow-cytograms for flow-cytometry analysis of CD11bhiLy6Chi Mo after 4 days of culture. Number of total cells (D) and CD11bhiLy6Chi Mo (E) after 4 days of culture. Results are represented as mean ± SD. BM cells were collected from 2 mice for each strain and experiment was done once in triplicates. *p≤0.05, ***p≤0.001, ****p≤0.0001.
Frequency of Ly6Clo Mo is higher in the circulation of Il1r1−/− mice at steady state
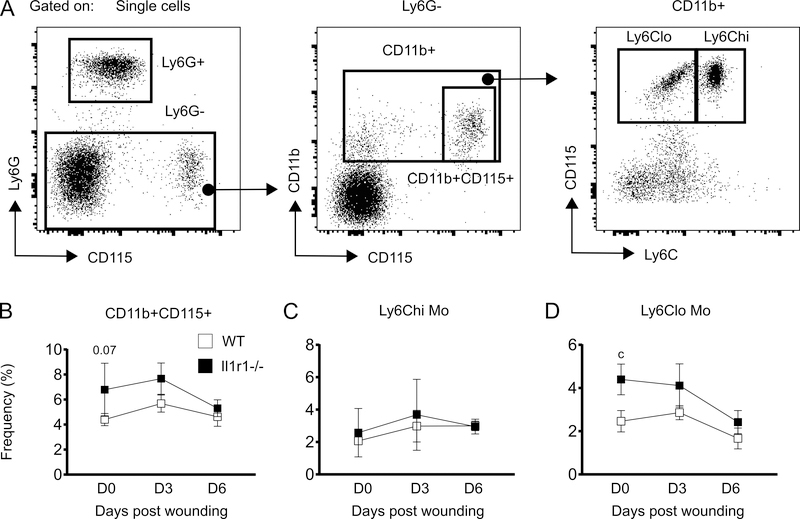
Unlike BM, skin wounding did not induce an increase in the frequencies of total, Ly6Chi or Ly6Clo Mo in peripheral blood of either WT or Il1r1−/− mice (Figure 5B-D). However, there was a trend and significantly higher percentage of total and Ly6Clo Mo respectively, in the circulation of Il1r1−/− compared to WT mice prior to wounding, in the steady state (Figure 5B and 5D); Ly6Chi Mo did not differ between strains (Figure 5C). In contrast to peripheral blood, Ly6Clo Mo did not differ in BM of WT versus Il1r1−/− mice at any time point (Figure 3D). Thus, it is possible that the absence of IL-1R1 signaling may increase conversion of Ly6hi into Ly6Clo Mo in the circulation through an as yet undefined mechanism. However, we did not observe a complementary decrease in Ly6Chi Mo in the circulation that might be expected with such an increase in conversion.
Figure 5:
Frequency of Ly6Clo Mo is higher in the circulation of Il1r1−/− mice at steady state. (A) Representative flow-cytograms showing gating strategy for flow-cytometry analysis of Mo subsets in peripheral blood. (B-D) Percentage (of total BMC; upper panels) and number (lower panels) of total Mo (Ly6G-CD11b+CD115+), Ly6Chi Mo (Ly6G-CD11b+CD115+Ly6Chi) and Ly6Clo Mo (Ly6G-CD11b+CD115+Ly6Clo) in peripheral blood. Results are represented as mean ± SD, n=3 mice for each strain and each time point. c, mean value significantly different between two strains at same time point. p≤0.05 is significant.
Frequency of Ly6Clo Mo is higher in wounds of Il1r1−/− mice during early healing response
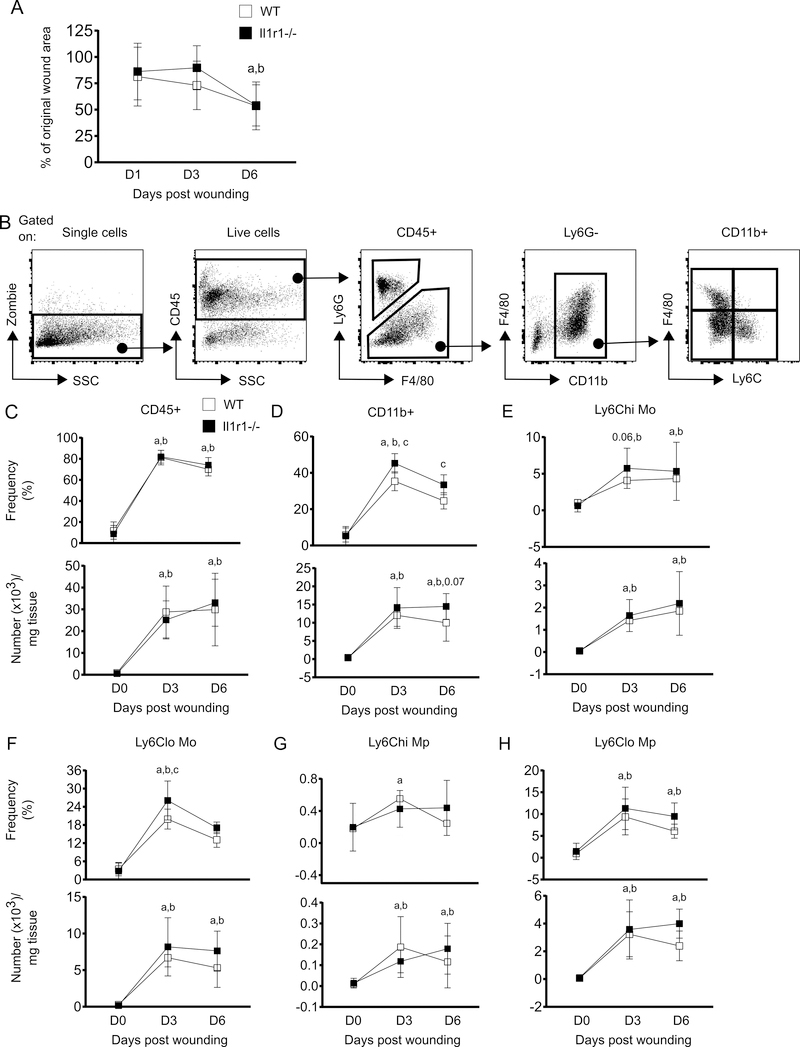
To examine the effect of IL-1R1 deficiency on the early stages of wound healing, we first measured wound closure in WT and Il1r1−/− mice in digital photographs of excisional skin wounds. Wound area was reduced in both strains of mice over time, but remained somewhat larger in Il1r1−/− compared to WT mice at 3 days after injury (Figure 6A). At 6 days post-injury wound area was found to be similar in both strains (Figure 6A). Thus, wound closer is modestly slower in Il1r1−/− mice which catches up with WT as healing progresses.
Figure 6:
IL-1R1 signaling deficiency increases Ly6Clo Mo during early wound repair. (A) Percentage of wound area as compared with day 0 wounds. ImageJ software was used to measure wound area. (B) Representative flow-cytograms showing gating strategy for flow-cytometry analysis of innate immune cells at wounds. (C-H) Percentage (of live cells; upper panels) and number (lower panels) of total leukocytes (CD45+), Mo/Mp (CD45+Ly6G-CD11b+), Ly6Chi Mo (CD45+Ly6G-CD11b+F4/80-Ly6Chi), Ly6Clo Mo (CD45+Ly6G-CD11b+F4/80-Ly6Clo), Ly6Chi Mp (CD45+Ly6G-CD11b+F4/80+Ly6Chi) and Ly6Clo Mp, (CD45+Ly6G-CD11b+F4/80+Ly6Clo) in the wounds. Results are represented as mean ± SD, n=7 mice for each strain and each time point. a, mean value significantly different from day 0 in WT; b, mean value significantly different from day 0 in Il1r1−/−; c, mean value significantly different between two strains at same time point. p≤0.05 is significant.
As leukocytes play critical roles in wound repair, we examined whether the deficiency in IL-1R1 signaling influenced leukocyte infiltration into the wounds. As anticipated, both the frequency and number of total leukocytes (live CD45+) increased remarkably in WT wounds at 3 days after injury and remained high through day 6. Il1r1−/− mice showed a nearly identical increase in CD45+ cells indicating that wound infiltration of total leukocytes is not affected by IL-1R1 deficiency (Figure 6C). Further, Mo/Mp (live CD45+Ly6G-CD11b+) were also elevated in WT wounds at 3 and 6 days post-injury as compared to non-injured skin. This increment in Mo/Mp populations followed a similar pattern in Il1r1−/− mice; however, Il1r1−/− wounds showed a higher frequency of Mo/Mp than WT controls at both time points (Figure 6D).
Wound repair is regulated by both the number and phenotype of recruited Mo/Mp, and impaired wound healing has been linked with dysregulation of Mo/Mp subsets (22–24). We used CD11b, Ly6C and F4/80 to identify different Mo and Mp subsets in the wounds by flow cytometry (1, 4, 17) (Figure 6B). Our data indicate that wound Mo and Mp subsets (both Ly6Chi and Ly6Clo, defined in the figure legend) were increased in both strains in a similar fashion (Figure 6E-H). But notably, among all Mo/Mp populations only the frequency of Ly6Clo Mo was found to be significantly higher in Il1r1−/− wounds at 3 days post-injury (Figure 6F). Thus, the higher frequency of total wound Mo/Mp found in Il1r1−/− mice could be attributed to higher Ly6Clo Mo (Figure 6D and 6F). In addition, the higher frequency of Ly6Clo Mo in day 3 wounds was associated with higher baseline circulating Ly6Lo Mo in Il1r1−/− mice (Figure 5D). Altogether, these data may indicate that loss of IL-1R1 signaling leads to higher proportion of Ly6Clo Mo early in wound healing.
Discussion
Mo/Mp accumulation in skin wounds is thought to be the result of rapid infiltration of circulating Mo which, in turn, are supplied by BM (8, 20, 25–27). However, the possibility that skin wounding may produce signals that can communicate with BM to increase monopoiesis has not been addressed. The present study reveals that skin wounding induces Mo expansion in BM during early wound healing. Further, we demonstrate that in parallel with Mo, the myeloid committed multipotent progenitor MPP3 compartment is also expanded in the bone marrow following skin wounding. Importantly, the loss of IL-1R1 did not affect skin wounding-induced expansion of bone marrow Mo and MPP3 or Mo production by MyPs ex vivo.
Our findings of Mo expansion in combination with trends of increased Mo proliferation indicates that skin wounding accelerates monopoiesis in BM during early wound healing. In addition, the higher rate of GMP proliferation in response to wounding suggests their role in this increased monopoiesis. Furthermore, although myeloid lineage committed MPP3 pool size was increased their proliferation was not affected by skin wounding. One possible explanation for these seemingly disparate findings could be that the typical sequential differentiation of HSPCs to GMPs then to Mo is disturbed during periods of urgent Mo need such as in the case of wound healing (28). During such periods, the high demand of Mo early after wounding may be supplied directly by GMP pool instead of the usual full monopoietic pathway. As a result, the MPP3 pool may increase due to reduced differentiation although their proliferation is unaltered. In addition, a recent study from our group has shown that although LSK cell number is increased in WT mouse BM at day 3 post-hind limb ischemia the rate of LSK proliferation is maximum at day 1 and comes back to normal at day 3 post-ischemia (18). A similar MPP3 response might occur in the response to skin wound healing - MPP3 proliferation might increase at an earlier time point than we assessed, for example day 1 post-wounding, leading to increased MPP3 numbers on day 3. Further studies are needed to test these speculative explanations for our findings. Nevertheless, it is evident from the current study that skin wounding causes increased monopoiesis in BM which likely contributes to wound Mo accumulation during the healing process.
Skin wounding releases a variety of damage associated molecular patters (DAMPs) with ability to activate inflammatory signaling pathways such as NLRP3 inflammasome and toll-like receptors (TLRs) causing increased inflammatory cytokine production (29–32). Activated NLRP3 inflammasome triggers cleavage of pro-IL-1β and pro-IL-18 into their active forms which function through IL-1R1 signaling (33). Thus the findings of elevated IL-1β and IL-18 at normal skin wounds suggest that IL-1R1 signaling may play important roles in skin wound healing (13, 34–36). In addition, it is known that IL-1β is transported to BM from infarcted tissue and increases myelopoiesis following myocardial infarction (16). Induction of myelopoiesis by IL-1β exposure and inhibition of alum-induced emergency granulopoiesis in mice deficient for IL-1R1 further consolidates critical roles of IL-1R1 signaling in increased myelopoiesis following injury and inflammatory stimuli (14, 15). We hypothesized that IL-1β, being a soluble factor may induce monopoiesis by traveling to BM during skin wound healing. But, unlike myocardial infarction we did not observe increased level of IL-1β in BM at any time points post-skin wounding. Besides, identical expansion of BM Mo in both WT and Il1r1−/− mice during early skin wound healing suggests that IL-1β does not communicate with BM for inducing monopoiesis post skin wounding. In addition, increased differentiation potential of Il1r1−/− MyPs ex vivo in response to M-CSF indicates that monopoiesis is still functional in Il1r1−/− BM, and in fact M-CSF mediated monopoiesis of Il1r1−/− MyPs is even higher than their WT counterparts. Thus, it is likely that another signaling pathway is involved in skin wounding-induced monopoiesis. Our results showing trends of an increase in surface M-CSFR on LSK and GMP in BM following skin wounding, indicating that M-CSFR signaling could be associated with skin wound-induced monopoiesis and we plan to further investigate this link in future studies.
The current study showed no significant difference in wound closure between WT and Il1r1−/− mice which is correlated with similar monopoiesis in the BM of these strains. In contrast, our previous studies have shown sustained IL-1β impairs healing of wounds in diabetic mice and that neutralization of this response by blocking IL-1R1 and NLRP3 inflammasome signaling pathways improves diabetic wound healing (13, 37). Together, these suggest that although IL-1R1 signaling does not affect skin wound healing during normal inflammatory conditions, its dysregulation can promote chronic inflammation and impair wound healing. Whether diabetes- associated chronic inflammation impacts monopoiesis and plays important roles in impaired diabetic wound healing awaits further investigation.
This study focused on the role of IL-1R1 in regulating monopoiesis during skin wound healing based on previous studies that identified such regulation following alum administration or myocardial infarction (15, 16). One limitation of our data is that, although they show definitively that IL-1R1 is not required for MPP3 and monocyte expansion in BM induced by skin wounding, the pathways that regulate monocyte expansion during skin wound healing will be investigated in future studies. Another limitation of our study is that we have not examined a wider range of kinetics to fully elucidate the dynamics of HSPC proliferation and differentiation, as well as monocyte mobilization and death; these processes also await further study.
In short, our data show that skin wounding induces expansion of the myeloid committed MPP3 compartment in association with increased monopoiesis in the BM during the early stages of wound healing. Further, contrary to alum adjuvant and myocardial infarction-induced myelopoiesis (15, 16), skin wound-induced monocyte expansion is not affected by IL-1R1 deficiency.
Supplementary Material
Key points.
Skin wounding promotes monocyte expansion in mouse bone marrow
IL-1R1 signaling is not involved in skin wounding induced monocyte expansion
Acknowledgments
The authors thank Dr. Giamila Fantuzzi for her input on experimental design and on aspects of presentation of this manuscript.
This work was supported by grants from the National Institutes of Health R01GM092850 to T.J.K.
Abbreviations used in this article
- BM
bone marrow
- IL-1R1
interleukin-1 receptor 1
- HSPC
hematopoietic stem and progenitor cell
- KO
knockout
- WT
wild-type
- LSK
lineage2Sca-1+c-Kit+
- MyP
myeloid progenitor
- Mo/Mp
monocyte/macrophages
- DAMP
damage-associated molecular pattern
- NLRP3
NLR family, pyrin domain-containing 3
- HSC
hematopoietic stem cells
- MPP
multipotent progenitor
- CMP
common myeloid progenitor
- GMP
granulocyte macrophage progenitor.
References
- 1.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, and Chazaud B 2007. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, and Iredale JP 2005. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, and Eming SA 2010. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977. [DOI] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, and Pittet MJ 2007. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza R, DiPietro LA, and Koh TJ 2009. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 175: 2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida Y, Gao JL, and Murphy PM 2008. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 180: 569–579. [DOI] [PubMed] [Google Scholar]
- 7.Koh TJ, and DiPietro LA 2011. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodero MP, Licata F, Poupel L, Hamon P, Khosrotehrani K, Combadiere C, and Boissonnas A 2014. In vivo imaging reveals a pioneer wave of monocyte recruitment into mouse skin wounds. PLoS One 9: e108212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, and Eming SA 2012. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120: 613–625. [DOI] [PubMed] [Google Scholar]
- 10.Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, and Albina JE 2009. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol 174: 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y, Kondo T, Kimura A, Matsushima K, and Mukaida N 2006. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol 176: 5598–5606. [DOI] [PubMed] [Google Scholar]
- 12.Weinheimer-Haus EM, Mirza RE, and Koh TJ 2015. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One 10: e0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza RE, Fang MM, Ennis WJ, and Koh TJ 2013. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 62: 2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, Nerlov C, Steidl U, Manz MG, Schroeder T, and Passegue E 2016. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol 18: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda Y, Cain DW, Kuraoka M, Kondo M, and Kelsoe G 2009. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol 182: 6477–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, and Nahrendorf M 2015. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 132: 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirza R, and Koh TJ 2011. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 56: 256–264. [DOI] [PubMed] [Google Scholar]
- 18.Fang MM, Barman PK, Thiruppathi M, Mirza RE, McKinney RD, Deng J, Christman JW, Du X, Fukai T, Ennis WJ, Koh TJ, Ushio-Fukai M, and Urao N 2018. Oxidant Signaling Mediated by Nox2 in Neutrophils Promotes Regenerative Myelopoiesis and Tissue Recovery following Ischemic Damage. J Immunol 201: 2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietras EM, Reynaud D, Kang YA, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Gottgens B, and Passegue E 2015. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 17: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, and Kubota Y 2011. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 117: 5264–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, and Sieweke MH 2013. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature 497: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, and Mace KA 2013. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech 6: 1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daley JM, Brancato SK, Thomay AA, Reichner JS, and Albina JE 2010. The phenotype of murine wound macrophages. J Leukoc Biol 87: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak ML, and Koh TJ 2013. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol 183: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiPietro LA, Burdick M, Low QE, Kunkel SL, and Strieter RM 1998. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 101: 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiPietro LA, Polverini PJ, Rahbe SM, and Kovacs EJ 1995. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol 146: 868–875. [PMC free article] [PubMed] [Google Scholar]
- 27.Gillitzer R, and Goebeler M 2001. Chemokines in cutaneous wound healing. J Leukoc Biol 69: 513–521. [PubMed] [Google Scholar]
- 28.Ema H, Morita Y, and Suda T 2014. Heterogeneity and hierarchy of hematopoietic stem cells. Exp Hematol 42: 74–82 e72. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Guo S, Ranzer MJ, and DiPietro LA 2013. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 133: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito H, Kanbe A, Sakai H, and Seishima M 2018. Activation of NLRP3 signalling accelerates skin wound healing. Exp Dermatol 27: 80–86. [DOI] [PubMed] [Google Scholar]
- 31.Jo EK, Kim JK, Shin DM, and Sasakawa C 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilgus TA 2018. Alerting the body to tissue injury: The role of alarmins and DAMPs in cutaneous wound healing. Curr Pathobiol Rep 6: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschopp J, and Schroder K 2010. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215. [DOI] [PubMed] [Google Scholar]
- 34.Kampfer H, Kalina U, Muhl H, Pfeilschifter J, and Frank S 1999. Counterregulation of interleukin-18 mRNA and protein expression during cutaneous wound repair in mice. J Invest Dermatol 113: 369–374. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, and Ohshima T 1996. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med 108: 231–236. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y, and Ohshima T 2000. The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: a preliminary study for forensic wound age estimation (II). Int J Legal Med 113: 140–145. [DOI] [PubMed] [Google Scholar]
- 37.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, and Koh TJ 2014. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 63: 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.