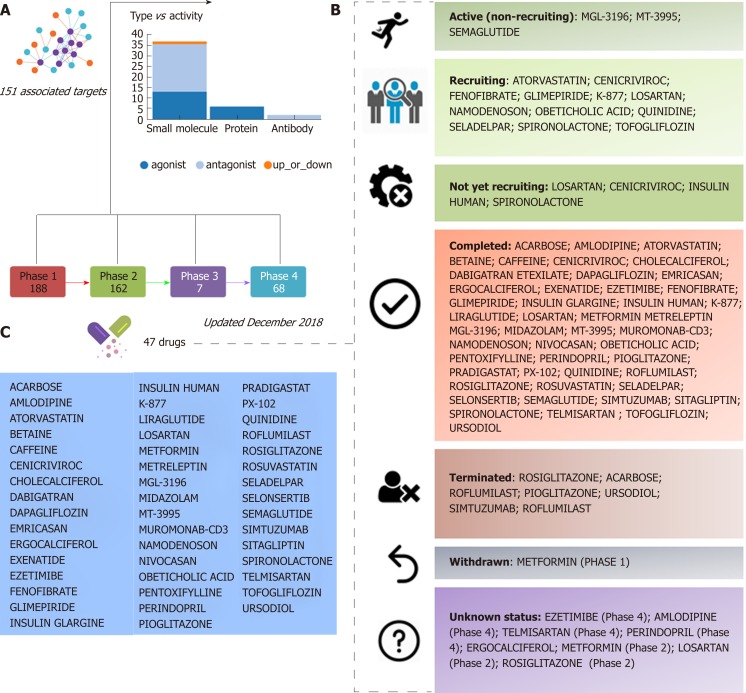
Figure 1.
Clinical trials for the treatment of nonalcoholic steatohepatitis. A and B: Figure highlights 47 drugs that are currently under investigation for the treatment of nonalcoholic steatohepatitis in different pharmacological phases (from phase 1 to phase 4): Information on clinical trial status (recruitment status) as well as prediction of potential associated targets were retrieved from the Target Validation Platform available at https://www.targetvalidation.org; C: Drugs listed in the most advanced pharmacological phase updated December 2018 concerning to privately and publicly funded clinical studies. Not yet recruiting: The study has not started recruiting participants; Recruiting: The study is currently recruiting participants; Active, not recruiting: The study is ongoing, and participants are receiving an intervention or being examined, but potential participants are not currently being recruited or enrolled; Terminated: The study has stopped early and will not start again; participants are no longer being examined or treated; Completed: The study has ended normally, and participants are no longer being examined or treated (that is, the last participant's last visit has occurred); Withdrawn: The study stopped early, before enrolling its first participant; Unknown: A study on ClinicalTrials.gov whose last known status was recruiting; not yet recruiting; or active, not recruiting but that has passed its completion date, and the status has not been last verified within the past 2 years).