FIG 3.
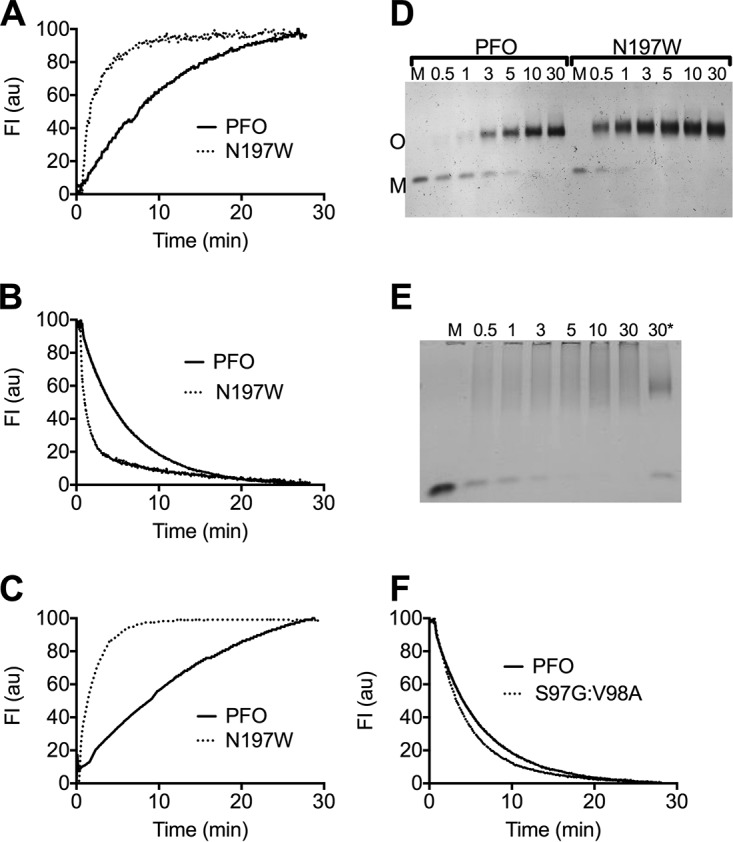
Structural transitions required for oligomerization and pore formation are accelerated in PFON197W. (A) The rate of membrane binding was determined by following the increase in the emission over time of the UDP tryptophan residues in D4 (19), as monomers of PFO (solid line) and PFON197W (dashed line) bind to the liposomes. (B) The rate of monomer oligomerization into the prepore complex was determined by following the disengagement of β5 from β4. This disengagement was detected by monitoring the decrease in the emission over time of NBD-modified cysteine-substituted V322 in PFOV322C (solid line) or PFOV322C-N197W (dashed line). V322 is buried beneath the α1β5 loop (Fig. 4A, left panel), and upon the disengagement of β5 from β4 during oligomerization, NBD is exposed to water, which quenches its emission (20). (C) The membrane insertion of the β-barrel was determined by the increase in the fluorescence emission of NBD positioned on cysteine-substituted A215 (18), a membrane-facing residue in transmembrane β-hairpin 1, in PFOA215C (solid line) and PFOA215C-N197W (dashed line). All data were normalized to 100 and are representative of 3 independent experiments. (D and E) PFO, PFON197W, or DLY was injected into buffer containing cholesterol-rich liposomes, and samples were withdrawn at the times indicated and immediately placed into SDS-PAGE sample buffer to quench the assembly of the pore. The samples were then separated by SDS-AGE to separate the monomer (M) from the oligomer (O). Note that the disappearance of the DLY monomer is rapid, as it assembles into the pore complex, which tends to form a smear in the absence of heat. *, same as the DLY 30-min sample that was additionally heated to 95°C for 3 min to show that the smeared oligomers migrate as a single band when heat is applied. (F) The rate of the disengagement of β5 from β4 in PFOS97G-V98A compared to PFO was measured as described in the legend to panel B. All fluorescence assays were carried out at 37°C. M, soluble toxin monomer in the absence of liposomes.
