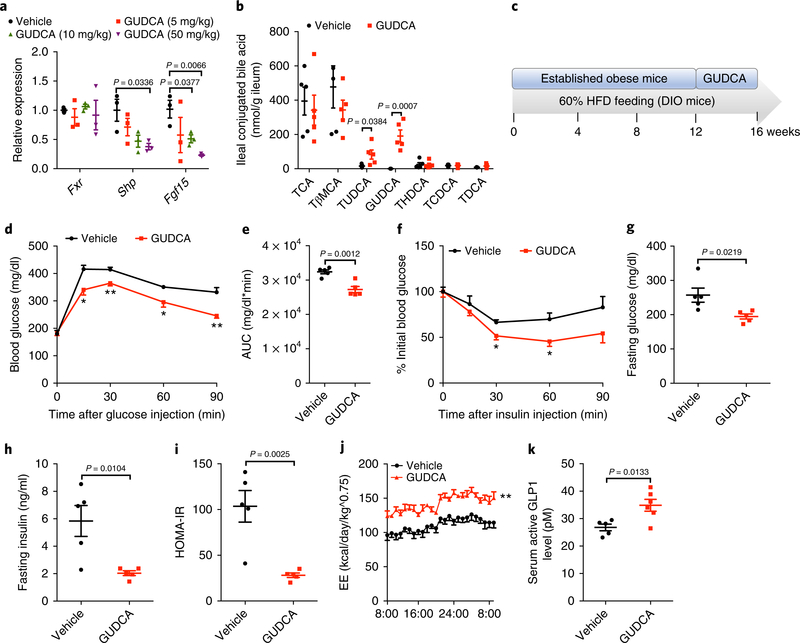
Fig. 6 |. GuDCA supplementation had therapeutic effects in improving glucose tolerance dependent on intestinal FXR.
a, Intestinal Fxr and its target gene mRNAs relative abundance after 1-week GUDCA treatment by gavage (5 mg/kg/d, 10 mg/kg/d and 50 mg/kg/d) on a HFD. n = 3 mice/group. Shp: t4 = 3.178, Fgf15: t4 (10 mg/kg) = 3.06, t4 (50 mg/kg) = 5.171. b, Bile acid profiles in the ileum after 1-week GUDCA treatment by gavage (50 mg/kg/d). n = 5 mice/group. TUDCA: t8 = 2.475, GUDCA: t8 = 5.369. c, The schematic diagram of animal model applied in GUDCA therapeutic experiments; after a 12-week HFD treatment, mice were given vehicle or GUDCA (50 mg/kg/d) for 4 weeks. DIO, diet-induced obesity. d,e GTT (d) and AUC (e). n = 5 mice/ group. e: t8 = 4.891. (f) ITT. n = 5 mice/group. g,h Fasting glucose (g) and insulin (h) levels. n = 5 mice/group. (g): t8 = 2.839, (h): t8 = 3.332. i, HOMA-IR. n = 5 mice/group. t8 = 4.321. j, Energy expenditure. n = 5 mice/group. k, Serum active GLP1 levels. Vehicle or GUDCA-treated (50 mg/kg/d) mice on a HFD for 1 week. n = 5 or 6 mice/group. t9 = 3.072. All P values were determined by two-tailed Student’s t-test, *P < 0.05, **P < 0.01 versus vehicle. All data are presented as the mean ± s.e.m.