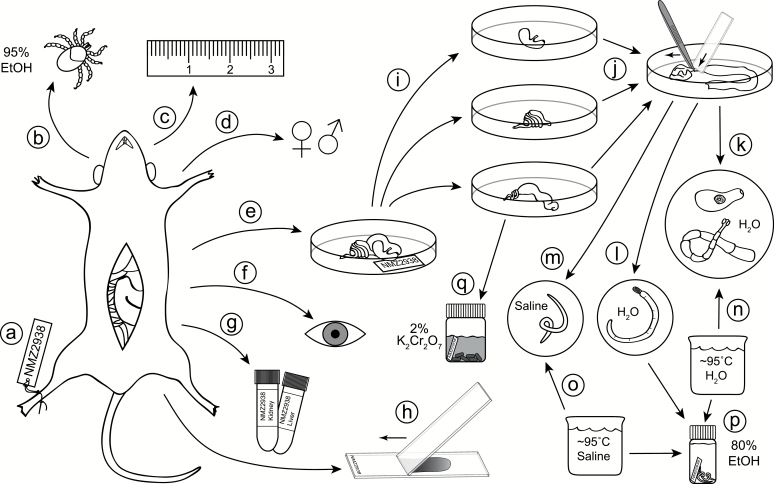
Fig. 1.
Flowchart for small mammal necropsy: a unique identifier is assigned to the mammal immediately upon capture (a); the mammal is swept for ectoparasites (b); standard mammal measurements are recorded (c); reproductive data are recorded (d); the gastrointestinal (GI) tract is transferred to a Petri dish and labeled (e); the body cavity and organs (e.g., liver) are visually inspected for parasites (e.g., encysted metacestodes, nematodes, sarcocysts) (f); host tissues are collected and preserved (g); thin blood smears can be prepared from freshly euthanized hosts to sample blood-borne pathogens (h); major sections of the GI tract are separated (stomach, small intestine, large intestine), straightened, and individually opened lengthwise (i); the lining is scraped by pulling it beneath the end of a microscope slide using forceps, and washed with saline to reveal helminths (j); after transfer of helminths to a new dish, trematodes and cestodes are washed in saline or water (k); acanthocephalans are soaked in water until the proboscis extends and the worm dies (l); and nematodes are washed in saline (m); trematodes and cestodes are simultaneously relaxed and killed by swirling in hot water (n); nematodes are killed using hot saline (o); helminths are preserved in ethanol (p); fecal pellets are collected from the colon and stored in potassium dichromate solution to sample coccidians (q).