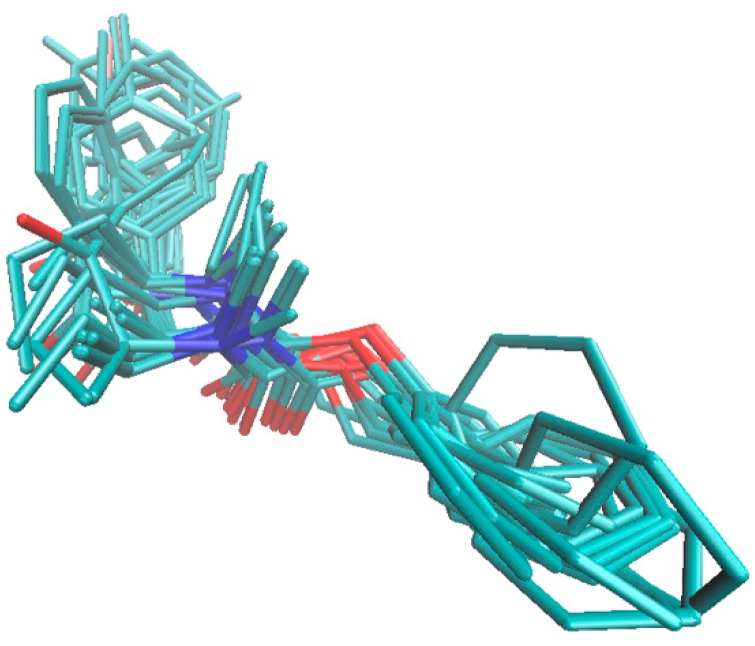
Figure 6.
Comparison of the poses for the most active BChE inhibitors in the enzyme active site AC2. As an additional experiment galanthamine (GLT), which is a very active AChE/BChE inhibitor (IC50 < 3 µM) was docked in the enzyme active site AC2. Although the structural variations between GLT and RIV and the most active molecules are easily noticeable, similar spatial patterns of the host–BChE complex within the catalytic core was observed for the negatively charged atoms (nitrogen and oxygen), as presented in Figure 7.