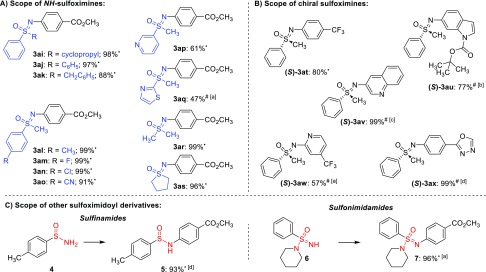
Figure 3.
(A) Scope of NH-sulfoximines. (B) Scope of enantiopure substrates. (C) Scope of other sulfoximidoyl derivatives. Reaction conditions: NH-sulfoximine (1) (0.25 mmol, 1.0 equiv), methyl 4-bromobenzoate (2j) (0.275 mmol, 1.1 equiv), [Ir]-Cat (0.15 mol %), [Ni-2]-Cat (0.20 mol %), TMG (1.2 equiv), dry and degassed MeCN (•) or DMA (#) (0.25 M), irradiation at 455 nm for 17 h; (a) 0.5 mol % of [Ir]-Cat, 1.0 mol % of [Ni-2]-Cat; (b) 0.5 mol % of [Ir]-Cat, 5.0 mol % of [Ni-2]-Cat; (c) 1.0 mol % of [Ir]-Cat, 5.0 mol % of [Ni-2]-Cat; (d) 0.5 mol % of [Ir]-Cat, 2.0 mol % of [Ni-2]-Cat.