Table 1. Optimization of the Reaction Conditionsa,b.
| entry | 1a/2a (equiv) | [Ir]-Cat(mol %) | [Ni]-Cat (mol %) | TMG (equiv) | yieldc (%) |
|---|---|---|---|---|---|
| 1 | 1.5:1.0 | 1.0 | [Ni-1]-Cat (5.0) | 1.5 | 94 |
| 2 | 1.5:1.0 | 0.15 | [Ni-1]-Cat (5.0) | 1.5 | 96 |
| 3 | 1.5:1.0 | 0.15 | [Ni-1]-Cat (1.0) | 1.5 | 95 |
| 4 | 1.5:1.0 | 0.15 | [Ni-2]-Cat (0.20) | 1.5 | 76 |
| 5 | 1.0:1.1 | 0.15 | [Ni-2]-Cat (0.20) | 1.5 | 99 |
| 6 | 1.0:1.1 | 0.15 | [Ni-2]-Cat (0.20) | 1.2 | 99 |
| 7 | 1.0:1.1 | 0.15 | [Ni-2]-Cat (0.20) | 1.2 | 99d |
[Ir]-Cat = [Ir(ppy)2(dtbbpy)]PF6, [Ni-1]-Cat = NiBr2 + dtbbpy (1.0:0.20 equiv) added separately, [Ni-2]-Cat = preformed [Ni(dtbbpy]Br2, TMG = 1,1,3,3-tetramethylguanidine,
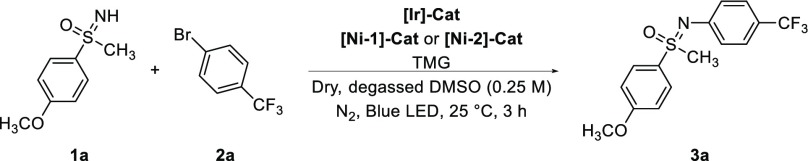
Reaction conditions: 1a (0.25 mmol, 1.0 equiv), 2a (0.28 mmol, 1.1 equiv), [Ir]-Cat (0.15 mol %), [Ni-2]-Cat (0.20 mol %), TMG (0.30 mmol, 1.2 equiv), dry and degassed DMSO (0.25 M, 1.0 mL), irradiation at 455 nm for 3 h.
Yields were determined by GC analysis with naphthalene as internal standard.
Reaction was up-concentrated to 0.75 M and run for 17 h, and the yield is reported after purification via automated flash-column chromatography.