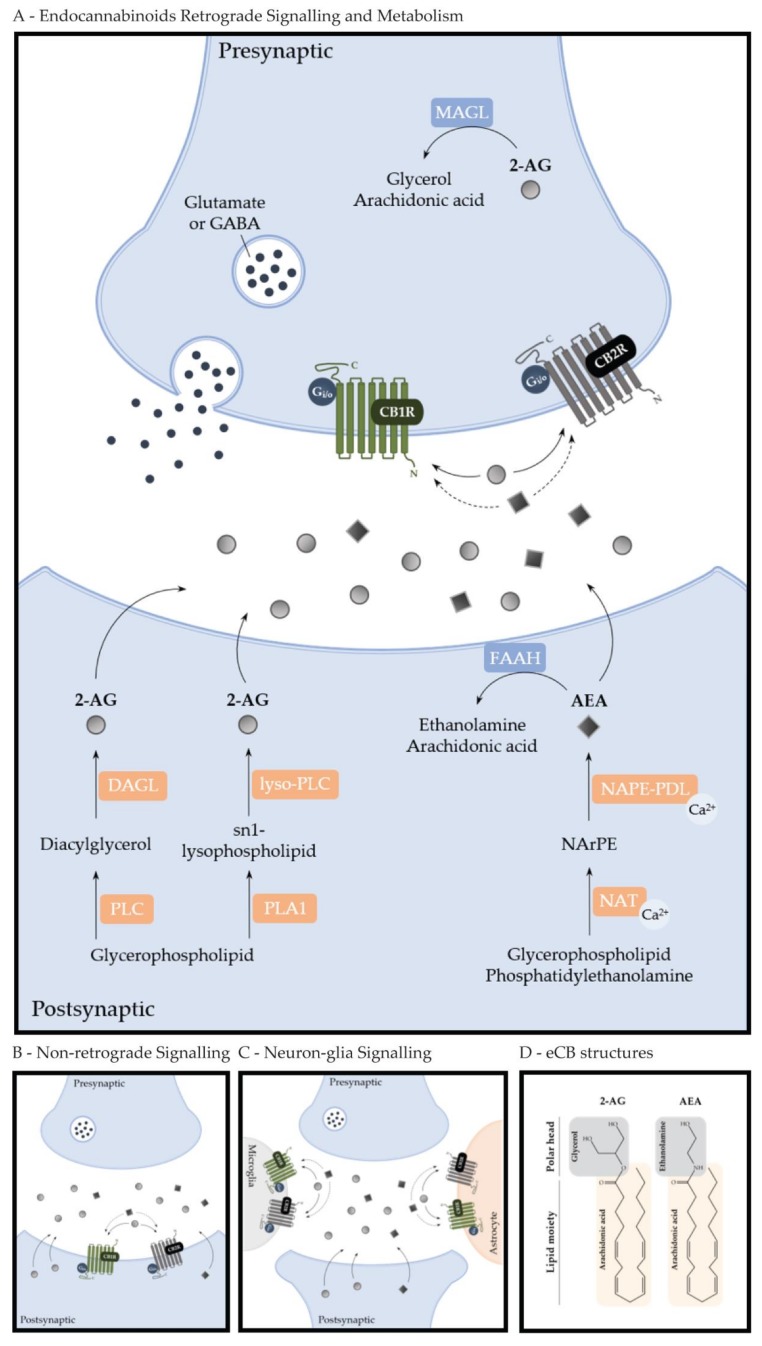
Figure 1.
Endocannabinoid signaling. (A) Endocannabinoid Retrograde Signaling and Metabolism. 2-arachidonoglycerol (2-AG) is synthesized by two different metabolic pathways: via the cleavage of diacylglycerol by diacylglycerol lipase (DAGL), where diacylglycerol is released from membrane phospholipids by phospholipase C (PLC) or via the action of phospholipase A1 (PLA1), releasing an sn-1 lysophospholipid from membrane phospholipids, which is cleaved by lyso-PLC in order to generate 2-AG. On the other hand, a calcium-dependent trans-acylase (NAT) acts on glycerophospholipids and phosphatidylethanolamine, resulting in N-arachidonoyl-phosphatidyl ethanolamine (NArPE), which is then cleaved by a calcium-dependent NAPE (N-acyl-phosphatidylethanolamine)-specific phospholipase D (NAPE-PLD), releasing N-arachidonoylethanolamine (anandamide, AEA) from membrane lipids. While hydrolysis of AEA occurs postsynaptically, via fatty acid amide hydrolase (FAAH) into arachidonic acid and ethanolamine, 2-AG is hydrolyzed by monoacylglycerol lipase (MAGL) into arachidonic acid and glycerol presynaptically. AEA and 2-AG are usually synthesized postsynaptically and are released “on demand” to the synaptic cleft, where they modulate presynaptic glutamatergic or GABAergic signaling by binding to CB1R or CB2R. (B) Non-retrograde Signaling. AEA and 2-AG signal autocrinally and non-retrogradely, the postsynaptic neuron, modulating synaptic transmission. (C) Neuron-glia Signaling. Endocannabinoids produced by neurons can bind to the cannabinoid receptors expressed in astrocytes and microglia. This neuron-glia signaling is able to modulate several responses. (D) eCB structures. 2-AG and AEA have similar molecular structures. They are both polar ester lipids formed by the bond of the omega-6 fatty acid arachidonic acid with either glycerol (to form 2-AG) or ethanolamine (to form AEA).