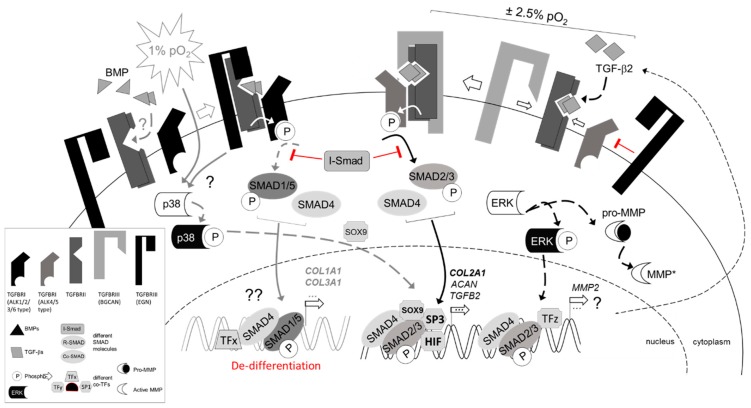
Figure 8.
Simplified schematic of proposed TGF-β signal transduction under physoxic conditions. TBSF signaling is mediated via specific heteromeric complexes of type I (activin A receptor type II-like kinases, ALKs) and type II (TGFBRII) serine/threonine kinase receptors. Canonically, TGF-β interacts with TGFBRII and ALK5, but in some cells (e.g., chondrocytes) signaling through ALK1 may also occur [29]. Physoxia prominently upregulates TGF-β2, while severe hypoxia was also reported to result in nuclear SOX9 accumulation upon BMP-2 stimulation in a p38 MAPK-dependent, but Smad-independent manner [31]. This is shown on the left in gray, as it was not studied here. BMPs commonly signal through BMPRII, ActRIIA, or ActRIIB as type II receptors and type I receptors ALK1, 2, 3, and 6. Co-receptors (TGFBRIII) betaglycan and endoglin can modulate TGFBRII/ALK5 and TGFBRII/ALK1 signaling in a cell type-dependent manner. Intracellular signaling can be divided into two main Smad-mediated signaling pathways. ALK1, 2, 3 and 6 induce phosphorylation of Smad1, 5 and 8, while ALK5 induces phosphorylation of Smad2 and Smad3, respectively. Activated R-Smads form heteromeric complexes with common Smad4 to translocate into the nucleus, where they can act as transcription factors (TF) in complexes together with gene specific co-TFs, like ID or SP molecules, to regulate expression of specific target genes. The ratio of these co-TFs may fine-tune gene expression of, for example, collagen molecules. Physoxia stimulates TGF-β2 and its co-receptors BGCAN, while suppressing EGN. To this end, physoxia seems to stimulate ALK5-mediated signaling pathways, shown on the right. Putative involvement of ERK1/2 and MMP-2 is indicated. Arrows (black) indicate stimulation (gray, putative), while blocks (red) indicate inhibitory effects. Please note: To simply the cartoon, only ALK1 and ALK5 were used to represent both pathways, type II and type III receptors are not illustrated as dimers, and soluble co-receptors, reported in other cells, were excluded. [31]