Fig. 2.
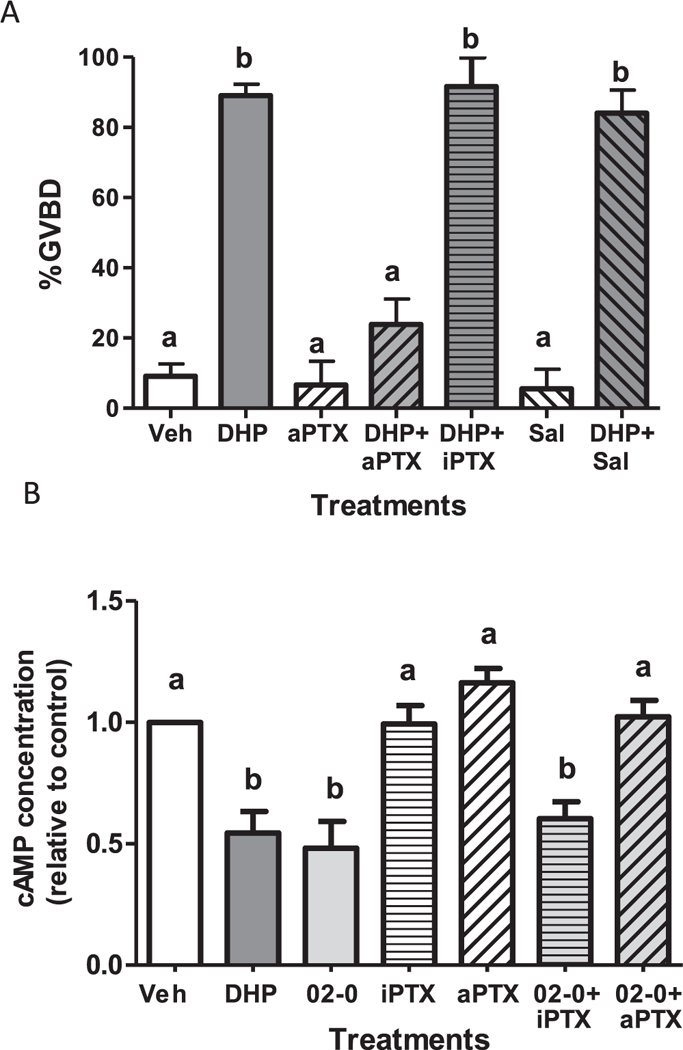
Effects of pertussis toxin treatments on DHP induction of OM (A) and DHP downregulation of cAMP production (B). (A) Effects of microinjection with activated pertussis toxin (aPTX 0.5 μg/μl) or heat-inactivated PTX (iPTX) on the maturation response of zebrafish follicle-enclosed oocytes to 5 nM DHP in the in vitro OM bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). Mature vitellogenic oocytes of diameter ~ 550 μm were injected with 1 nl PTX or saline (Sal) control (< 1% of oocyte volume). An entire experiment was conducted on oocytes from a single donor. The experiment was repeated seven times and the results pooled. The total number of oocytes in the vehicle-, DHP- and aPTX-treated groups was over 100, whereas the number of oocytes in the other treatment groups ranged from 31 to 39. (B). Effects of pre-treatment of oocyte membranes with aPTX or iPTX for 3 h on cAMP production in response to treatment with 10 nM Org OD 02–0. Production of cAMP in response to 5 nM DHP alone was included as a positive control. All data represent means ± S.E.M. Results were analyzed by two-way ANOVA followed by the Bonferroni test. Significant interactive effects of aPTX in the presence of DHP on GVBD and cAMP production were detected in two-way ANOVAs, which was predicted because aPTX blocked OM and the decrease in cAMP levels in response to DHP. Different letters denote significant differences between the treatment groups (p < 0.05) in the post hoc test.
