Figure 3. AcrIIA2 Is a Much Less Effective Inhibitor Compared to AcrIIA4.
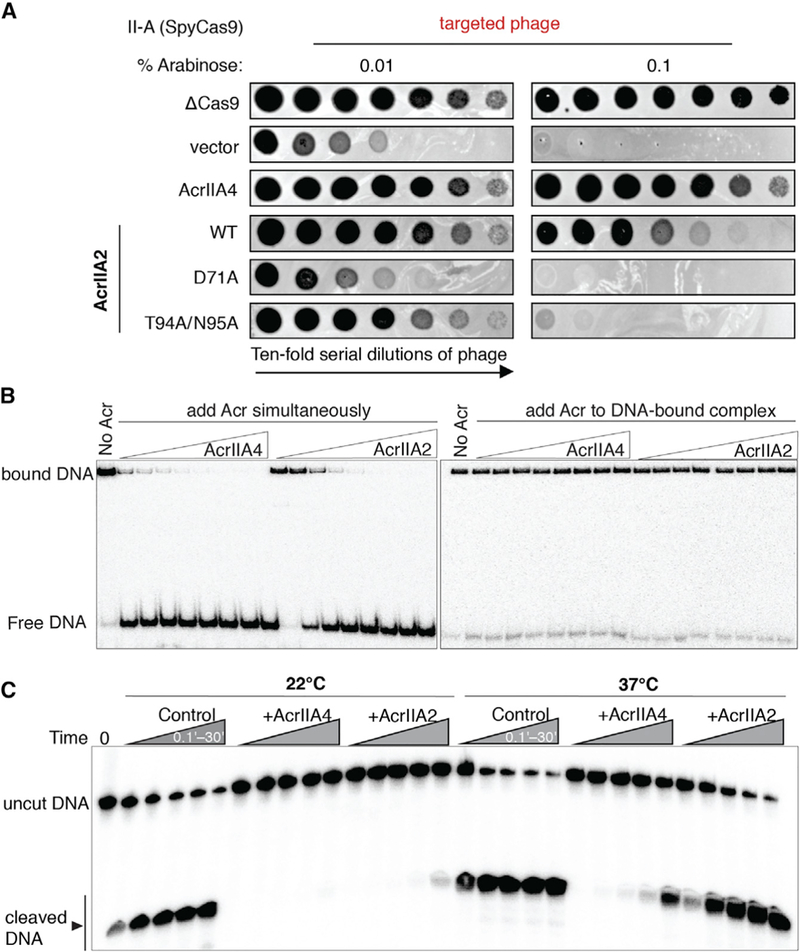
(A) Phage-plaquing experiment where P. aeruginosa JBD30 phage is titrated in 10-fold dilutions (black circles) on a lawn of P. aeruginosa (hazy background) expressing the indicated anti-CRISPR proteins and intermediate (0.01 % arabinose)orhigh (0.1% arabinose) levels of a type II-A CRISPR-Cas9 system programmed to target phage DNA. Plaquing of P. aeruginosa phages targeted by SpyCas9 in the presence of AcrIIA2 mutants reveals a nearly complete inactivation of AcrIIA2’s inhibitory effect on SpyCas9 by mutation of D71A.
(B) Competition EMSA assays showing that AcrIIA2 efficiently competes with target DNA for binding to SpyCas9-sgRNA complex (left), but not the preformed DNA-bound complex (right).
(C) Radiolabeled cleavage assays conducted using purified SpyCas9-sgRNA complex to assess AcrIIA2 capacity for inhibiting cleavage of both target and non-target DNA strands by SpyCas9 at room temperature and normal body temperature.
