Table 2.
Fine-tuning of catalyst amount, reaction temperature, and cellulose nanofiber quantity a.
| Entry | Conditions | TOCN | Yield (%) b | syn:antic | ee for syn (%) c |
|---|---|---|---|---|---|
| 1 | 5 mol% catalyst, rt | − | 37 | 87:13 | 29 |
| + | 74 | 93:7 | 39 | ||
| 2 | 15 mol% catalyst, rt | − | 58 | 69:31 | 26 |
| + | 77 | 69:31 | 39 | ||
| 3 | 7.5 mol% catalyst, 0 °C | − | 9 | 77:23 | 19 |
| + | 23 | 74:26 | 39 | ||
| 4 d | 7.5 mol% catalyst, 40 °C | − | 66 | 94:6 | 32 |
| + | 91 | 94:6 | 42 | ||
| 5 | Substrate/TOCN (mg/mg) 74.6/25 | + | 51 | 92:8 | 41 |
| 6 | Substrate/TOCN (mg/mg) 74.6/50 | + | 66 | 94:6 | 43 |
| 7 | Substrate/TOCN (mg/mg) 74.6/100 | + | 88 | 90:10 | 41 |
| 8 | Substrate/TOCN (mg/mg) 74.6/150 | + | 63 | 71:29 | 41 |
| 9 | Substrate/TOCN (mg/mg) 74.6/200 | + | 43 | 83:17 | 43 |
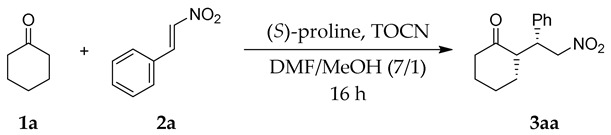
a Unless otherwise noted, the reaction was performed using cyclohexanone (1a) (4 mL, excess), trans-β-nitrostyrene (2a) (74.6 mg, 0.50 mmol), (S)-proline (7.5 mol%), and TOCN-Na (100 mg dry weight) in a mixture of DMF (14 mL) and MeOH (2 mL). Aqueous medium of TOCN suspension was replaced with MeOH by repetitive centrifugation prior to reaction; b Isolated yield; c Determined by chiral stationary phase SFC analysis; d Stirred for 11 h.
