Abstract
Background
Early readmissions among older adults hospitalized for acute myocardial infarction (AMI) are costly and difficult to predict. Aging-related functional impairments may inform risk prediction, but are unavailable in most studies. Our objective was to therefore develop and validate an AMI readmission risk model for older patients that considered functional impairments and was suitable for use before hospital discharge.
Methods and Results
SILVER-AMI is a prospective cohort study of 3006 patients age ≥75 hospitalized with AMI at 94 U.S. hospitals. Participants underwent in-hospital assessment of functional impairments including cognition, vision, hearing, and mobility. Other variables plausibly associated with readmissions, were also collected. The outcome was all-cause readmission at 30 days. We used backward selection and Bayesian model averaging to derive (N=2004) a risk model that was subsequently validated (N=1002). Mean age was 81.5 years, 44.4% were women, and 10.5% were nonwhite. Within 30 days, 547 participants (18.2%) were readmitted. Readmitted participants were older, had more comorbidities, and had a higher prevalence of functional impairments, including activities of daily living disability (17.0% vs. 13.0%, P=0.013) and impaired functional mobility (72.5% vs. 53.6%, p<0.001). The final risk model included 8 variables: functional mobility, ejection fraction, COPD, arrhythmia, acute kidney injury, first diastolic blood pressure, P2Y12 inhibitor use, and general health status. Functional mobility was the only functional impairment variable retained, but was the strongest predictor. The model was well calibrated (Hosmer-Lemeshow p-value >0.05) with moderate discrimination (C-statistic: 0.65 derivation cohort, 0.63 validation cohort). Functional mobility significantly improved performance of the risk model (net reclassification improvement index=20%, p<0.001).
Conclusions
In our final risk model, functional mobility, previously not included in readmission risk models, was the strongest predictor of 30-day readmission among older adults after AMI. The modest discrimination indicates much of the variability in readmission risk among this population remains unexplained by patient-level factors.
Clinical Trial Registration:
ClinicalTrials.gov. Identifier: NCT01755052.
INTRODUCTION
Nearly one in five older adults hospitalized for acute myocardial infarction (AMI) is readmitted to the hospital within 30 days of discharge (1). Early readmissions have considerable impact on patients’ quality of life, as well as on healthcare expenditures (2). In this context, over the past decade there has been considerable attention by payors and hospitals towards reducing readmissions after AMI in this population. For example, in 2012 the Centers for Medicare & Medicaid Services (CMS) began instituting financial penalties for hospitals with excessive risk-adjusted readmission rates within 30 days of hospitalization for AMI (1).
However, predicting hospital readmissions among older adults, with the goal of tailoring interventions in the early post-discharge setting, remains challenging; the causes of post-AMI readmissions are heterogeneous, and only a minority of readmissions are related to the sequelae of the index AMI (3). Non-disease specific impairments in important functional domains, including cognition, vision, hearing, and muscle strength, are highly prevalent in older adults and may influence post-AMI readmissions in several ways (4,5). For example, cognitive impairment may affect medication adherence; vision impairment may result in fall-related injuries; and muscle weakness may diminish ability to adhere with recommendations for physical activity, such as cardiac rehabilitation. However, to date these functional impairments have not been rigorously ascertained or studied in the context of hospital readmission for older adults, and no AMI risk models have been developed using data from a large network of hospitals across the U.S. Existing AMI readmission risk models have either been derived from claims data for use at the hospital level to compare performance between hospitals (6), or have been developed within single healthcare systems and therefore may have failed to include broadly representative samples of older adults (7).
Accordingly, our goal was to develop and validate a readmission risk model suitable for pre-discharge use in older patients hospitalized for AMI that considered traditional demographic and clinical variables as well as functional impairments. We used data from the ComprehenSIVe Evaluation of Risk in Older Adults with AMI (SILVER-AMI) study, a prospective multi-center longitudinal study of 3041 patients age ≥75 hospitalized with AMI. SILVER-AMI included abstraction of detailed clinical information (including variables from prior AMI risk models), as well as an assessment of non-disease specific impairments in important functional domains at the time of hospitalization. Measurement of these impairments is a key distinguishing feature of SILVER-AMI compared with previous AMI cohort studies, and allowed their consideration alongside traditional risk factors. We hypothesized that consideration of functional impairments would inform readmission risk.
METHODS
All data and supporting materials have been provided with the published article.
Study Participants
The design of SILVER-AMI has been described previously (8). Briefly, patients age ≥75 years were enrolled if they met criteria for the Third Universal Definition of AMI (9), as verified by physician investigators at the Yale Coordinating Center. Patients were deemed ineligible if they developed AMI secondary to another cause (e.g. postoperative AMI and/or initial troponin elevation was 24 hours after admission), if they were transferred after >24 hours’ admission at an outside hospital, if they were incarcerated, or if they were unable to provide informed consent (e.g. due to cognitive impairment) with no proxy consent available. A flow diagram including eligible patients screened is provided in the Appendix (eFigure 1). Of 9049 patients who met initial inclusion criteria, 5054 were eligible after also applying exclusion criteria. Subsequently, 3151 patients provided informed consent (62.3% of individuals meeting inclusion and exclusion criteria). At the time of hospitalization, enrolled patients underwent a baseline interview including demographics, pre-hospital symptoms, and health status measures (SF-12, Seattle Angina Questionnaire), as well as a comprehensive functional assessment. Timing of the assessment was left to the discretion on the site investigators and was scheduled so as not to conflict with diagnostic testing or procedures. The in-hospital visit was complemented with a detailed medical record review performed by a trained site research coordinator which included details of initial presentation (blood pressure, heart rate), presence of comorbid diseases, laboratory results, and in-hospital adverse events. Medical records were also provided to the Yale Coordinating Center, where two physicians reviewed AMI eligibility criteria and readmission events and a research nurse obtained information about medications, cardiac procedures, and other details of the hospitalization. All centers participating in SILVER-AMI (Figure 1) obtained institutional review board approval, and all participants gave written informed consent. A total of 3041 participants were enrolled at 94 U.S. study sites/hospitals from 1/11/2013–10/28/2016 (with the last follow-up assessment completed on 6/14/17). Among those enrolled (N=3041) versus screened but not enrolled (N=6008), mean age was slightly older (81.6 vs. 81.2, P<0.001), male sex was more common (55.7% vs. 51.2%, P<.001), and nonwhite race was similar (10.7% vs. 10.2%, P=0.856). The majority of enrollment sites were non-academic hospitals (71%), and more than half were located in suburban or rural areas (53%). For purposes of our study, which modeled readmission risk post-discharge, we excluded participants who died in-hospital (N=35), leaving a sample of 3006 for analysis. From this sample, we randomly selected 2004 participants to serve as a derivation cohort and 1002 to serve as the validation cohort. This allocation of the overall sample allowed sufficient power to derive and validate the risk prediction model.
Figure 1. SILVER-AMI study sites.
SILVER-AMI included 94 hospitals throughout the U.S.
Outcome
A primary outcome of the SILVER-AMI study was all-cause readmission within 30 days of hospital discharge, which included any overnight hospital stay (including “observation status”). Readmissions were identified through a two-stage process. During enrollment, the participant identified the hospitals he or she utilized for medical care and signed the appropriate medical release forms. When the 6-month follow-up window closed, the research coordinator contacted the hospitals that were identified at the time of enrollment to assess and collect readmission records. Separately, as part of the 6-month follow-up interview, the participant also reported hospital readmissions to the Yale Coordinating Center. The Yale Coordinating Center then reconciled the hospital records collected by the coordinator against self-reported events to ensure that no readmissions were missing from the assessment. If necessary, additional records were collected to capture all events (e.g. a readmission occurring at an out-of-area hospital during travel). Readmissions (occurrence and causes) were double-adjudicated by physician investigators at the Yale Coordinating Center; discrepancies between investigators were resolved by consensus including a third physician when necessary. Mortality events were ascertained through interviews with family members and verified with death certificates, hospital records, or obituaries.
Selection of Predictors
For risk model development, we initially selected 72 candidate variables (eTable 1) based on (1) elements from existing AMI readmission risk models (3,6,10–12), (2) major functional impairments plausibly related to readmission in an older population, including cognitive, sensory, and physical function, and (3) other clinical variables that, per the clinical judgment of the study investigators, may potentially influence readmission (such as symptom burden, patient-reported health status, and in-hospital complications). The following functional domains were considered: general cognitive function (assessed using the Telephone Interview for Cognitive Status [TICS]) (13), verbal fluency (based on the Controlled Word Association Task [COWAT]) (14), vision impairment (based on the Visual Functioning Questionnaire [VFQ-25]) (15), hearing impairment (based on a single global question about impairments imposed by hearing) (16), unintentional weight loss (defined as >10 lbs. in prior year), activities of daily living disability (in bathing, dressing, rising from a chair, or ambulating) (17), depressive symptoms (assessed using the PHQ-8) (18), upper extremity strength (measured with a handheld dynamometer, B&L Engineering, Santa Ana, CA) (19), fall history, and functional mobility based on the Timed Up and Go (TUG) (20) which involved evaluation of chair rise and gait speed over a distance of 10 feet (eTable 2). For functional mobility, while multiple assessments have been developed, we chose TUG as it integrates several basic mobility skills (reflective of everyday activity) and simultaneously can be performed with minimal equipment in a small space. TUG also lends itself to an ordinal scale that includes a value for those physically unable to participate, thereby boosting its utility in this older patient population. We selected cutpoints for all functional impairments based on previously validated thresholds described in the literature (13,15,16,18,21,22); if there was ambiguity regarding definitive thresholds, a consensus was reached among study team members based on the best available evidence. For in-hospital complications of acute kidney injury, we used the KDIGO criteria (increase in serum Cr ≥0.3 mg/dL from baseline or ≥1.5 times baseline) (23).
Statistical Analysis
We generated descriptive statistics in the overall cohort, using means for continuous variables and percentages for categorical variables. For categorical variables, we chose thresholds based on clinical relevance and distributions. From our initial list, we omitted variables with >20% missingness (including variables that were added to data collection forms after enrollment had begun), and those with extremely low (<5%) or high (>95%) prevalence. Under the assumption that data were missing-at-random, we multiply imputed the data 20 times. The allocation of the overall sample (N=3006) into derivation (n=2004) and validation (n=1002) cohorts was based on the following. After considering other cardiac risk prediction models, we assumed a conservative rate of 30-day readmission of 10% and a final model of approximately 10 predictors. Wanting a minimum of 100 events in the validation cohort, we randomly selected 1002 observations for this purpose. The remaining observations comprised the derivation data used to select the multivariable risk prediction model. Because we started with a large number of potential predictors, the first step in multivariable model selection was to reduce the number of candidate variables. Per recommendations of White et al. (24), we reduced the number of candidate variables by applying multivariable logistic regression with backwards selection to an aggregate dataset of the 20 imputations, retaining the 30 variables with the strongest adjusted associations with the outcome. We subsequently applied Bayesian model averaging with multivariable logistic regression to these final candidates in each of the multiply imputed datasets. The final predictors were those exhibiting a positive posterior probability in at least half of the imputations. The final predictors were subsequently examined for linearity and used in a multivariable model fit to each imputation using generalized estimating equations to adjust for the clustering of patients within hospitals, with the final coefficients calculated using Rubin’s rules (25). We note that because Bayesian model averaging was used to select variables rather than the corresponding p-values, some model terms may not exhibit p-values below 0.05 (26).
Discrimination and calibration of the final model were respectively evaluated in both derivation and validation cohorts with the C-statistic and the Hosmer-Lemeshow goodness of fit statistic. Observed and predicted probabilities of the outcome were calculated for quintiles of the risk score obtained by applying the final model to the validation data. The incremental value of adding functional impairments to improvement in discrimination of our model was evaluated with category-free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices (27).
Analyses were performed in SAS Version 9.4, with the exception of the Bayesian Model Averaging, which used the R package named BMA (28). For bedside prognostication, we subsequently developed a web-based calculator derived from model effect estimates.
RESULTS
Baseline Characteristics
The mean age of the study sample was 81.5 years; 44.4% of participants were women, and 10.5% were of nonwhite race. Slightly over one-quarter of the sample (26.3%) presented with ST elevation MI (STEMI). Over half (53.4%) had a known history of coronary disease, and 40.6% had undergone previous coronary revascularization. Functional impairments were observed most commonly in mobility (TUG ≥15 seconds or unable to complete TUG) (71.2%), weak grip strength (60.1%), unintentional weight loss (21.9%), and multiple falls within the prior year (19.7%). The majority of participants (59.0%) experienced at least one in-hospital complication (which included bleeding, acute kidney injury, decompensated heart failure, arrhythmia, or hyperglycemia).
Readmission at 30 days
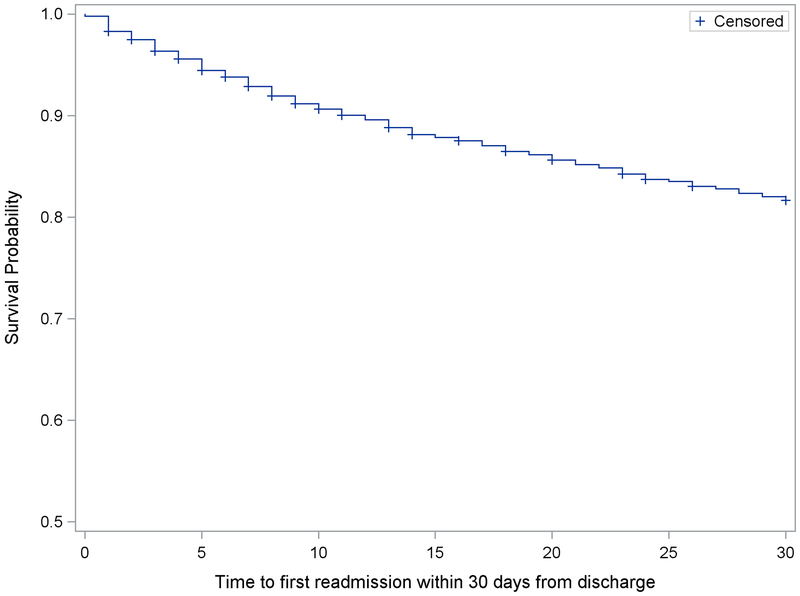
Within 30 days of discharge, 547 unique patients (18.2% of study sample) experienced at least one readmission. Overall, there were 626 readmissions: 483 participants were readmitted once, 53 were readmitted twice, and 11 were readmitted 3 or more times. The majority of readmissions (61.0%) were cardiac related (Table 1). The most common cause of readmission was congestive heart failure (18.2%), followed by bleeding (11.2%) and arrhythmia (8.2%). The rate of readmissions was relatively constant over 30 days; median time to first readmission was 10 days (Figure 2). Within the same post-discharge period, there were 59 deaths (2.6% of study sample).
Table 1:
Causes of 30-day hospital readmission
| Total (N=626) | |
|---|---|
| Cardiovascular | 382 (61.0%) |
| Congestive Heart Failure (CHF) | 114 (18.2%) |
| Arrhythmia | 51 (8.2%) |
| Non-cardiac chest pain | 45 (7.2%) |
| Non ST-Elevated Myocardial Infarction (NSTEMI) | 45 (7.2%) |
| Unstable Angina | 32 (5.1%) |
| Elective Procedure | 29 (4.6%) |
| Stroke | 15 (2.4%) |
| Other Cardiovascular including hypertensive disease | 13 (2.1%) |
| ST-Elevated Myocardial Infarction (STEMI) | 11 (1.8%) |
| Valvular Heart Disease | 9 (1.4%) |
| Transient Ischemic Attack (TIA) | 6 (1.0%) |
| Thrombotic Event | 6 (1.0%) |
| Cardiac Syncope | 4 (0.6%) |
| Peripheral Vascular Disease (PVD) (aorta, carotids, or extremities) | 2 (0.3%) |
| Non-Cardiovascular | 244 (39.0%) |
| Bleeding Episode | 70 (11.2%) |
| Other Non-Cardiovascular | 38 (6.1%) |
| Sepsis/Septic Shock | 23 (3.7%) |
| Skin and soft tissue infections | 15 (2.4%) |
| Pneumonia including aspiration pneumonitis | 14 (2.2%) |
| Pleural Effusion/Pneumothorax | 14 (2.2%) |
| Renal Disorders (kidney injury, or electrolyte/acid-base abnormalities) | 12 (1.9%) |
| Urinary Tract Infection and urinary system complaints | 12 (1.9%) |
| Fall/Fracture | 12 (1.9%) |
| COPD/Asthma | 11 (1.8%) |
| Syncope | 7 (1.1%) |
| Weakness/fatigue/failure to thrive | 6 (1.0%) |
| Dehydration | 4 (0.6%) |
| Diabetes, including blood glucose abnormalities | 3 (0.5%) |
| Primary cancer of trachea, bronchus, lung and pleura | 1 (0.2%) |
| Clostridium Difficile-associated infection | 1 (0.2%) |
| Vomiting | 1 (0.2%) |
Table describes all readmissions among study sample; 547 unique participants experienced readmission (483 readmitted once, 53 readmitted twice, and 11 readmitted ≥3 times).
Figure 2. Timing of 30-day readmissions.
Kaplan-Meier curve for survival free from hospital readmission within 30 days of discharge. Among patients readmitted, median time to readmission was 10 days.
In bivariate analyses, participants who were readmitted were, on average, older (mean age 82.1 vs. 81.4 years, P=0.011), less likely to be married or living with a partner (45.5% vs. 51.4%, P=0.014), and had a higher burden of comorbidities including prior arrhythmia (30.3% vs. 23.7%, P=0.001), prior heart failure (25.6% vs. 17.2%, P<0.001), and COPD (18.1% vs. 13.3%, P=0.004) (Table 2). Several functional impairments were also more common, including activities of daily living disability (17.0% vs. 13.0%, P=0.013), weak grip strength (64.4% vs. 59.2%, P=0.007), and impaired mobility (72.5% vs. 53.6%, P<0.001).
Table 2:
Participant characteristics: readmitted vs. not readmitted at 30 days (N=3006)
| 30-day readmission (N=547) Mean (SD) or N (%) |
No readmission (N=2459) Mean (SD) or N (%) |
P value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 82.1 ± 5.4 | 81.4 ± 4.9 | 0.011 |
| Male sex (%) | 302 (55.2%) | 1369 (55.7%) | 0.844 |
| Nonwhite race (%) | 65 (11.9%) | 252 (10.2%) | 0.273 |
| Married/living as married or with partner (%) | 249 (45.5%) | 1265 (51.4%) | 0.014 |
| Medical History | |||
| Hypertension (%) | 475 (86.8%) | 2091 (85.0%) | 0.281 |
| Dyslipidemia (%) | 347 (63.4%) | 1551 (63.1%) | 0.874 |
| Arrhythmia (%) | 166 (30.3%) | 583 (23.7%) | 0.001 |
| Heart failure (%) | 140 (25.6%) | 423 (17.2%) | <0.001 |
| Prior myocardial infarction (%) | 155 (28.3%) | 664 (27.0%) | 0.526 |
| Prior revascularization procedure (%) | 216 (39.5%) | 1004 (40.8%) | 0.563 |
| Peripheral arterial disease (%) | 84 (15.4%) | 279 (11.3%) | 0.009 |
| Valvular disease (%) | 90 (16.5%) | 259 (10.5%) | <0.001 |
| Stroke (%) | 99 (18.1%) | 369 (15.0%) | 0.071 |
| Diabetes mellitus (%) | 214 (39.1%) | 902 (36.7%) | 0.285 |
| COPD (%) | 99 (18.1%) | 327 (13.3%) | 0.004 |
| Current or ever smoker (%) | 308 (56.3%) | 1358 (55.2%) | 0.582 |
| Presentation Characteristics | |||
| ST elevation MI (%) | 127 (23.2%) | 664 (27.0%) | 0.069 |
| Chest pain as primary symptom (%) | 208 (38.0%) | 1003 (40.8%) | 0.260 |
| ≥6 hours from symptoms to presentation (%) | 244 (44.6%) | 1027 (41.8%) | 0.206 |
| Body mass index, mean ± SD | 27.6 ± 5.47 | 27.5 ± 5.30 | 0.693 |
| Killip Class II-IV (%) | 91 (16.6%) | 301 (12.2%) | 0.006 |
| First systolic BP, mmHg, mean ± SD | 140.5 ± 31.3 | 147.1 ± 30.6 | <0.001 |
| First diastolic BP, mmHg, mean ± SD | 75.5 ± 18.4 | 78.6 ± 17.5 | <0.001 |
| First heart rate, bpm, mean ± SD | 85.6 ± 23.9 | 83.1 ± 22.4 | 0.030 |
| Initial hemoglobin, mean ± SD | 12.5 ± 2.2 | 12.9 ± 2.1 | <0.001 |
| Initial WBC count, mean ± SD | 10.1 ± 5.4 | 9.5 ± 4.8 | 0.017 |
| Peak troponin/3 times ULN, median (IQR) | 65.1 (15.4–235.0) | 51.0 (12.5–216.0) | 0.039 |
| eGFR, mean ± SD | 51.8 ± 20.5 | 55.3 ± 19.8 | <0.001 |
| TIMI Score (NSTEMI), mean ± SD | 4.6 ± 1.2 | 4.6 ± 1.2 | 0.549 |
| TIMI Score (STEMI), mean ± SD | 6.7 ± 1.9 | 6.0 ± 1.5 | <0.001 |
| GRACE ACS score, mean ± SD | 151.0 ± 23.0 | 144.2 ± 22.2 | <0.001 |
| In-hospital diagnostics, therapies, and complications | |||
| Left ventricular ejection fraction | <0.001 | ||
| Normal (≥50%) | 224 (41.0%) | 1303 (53.0%) | |
| Mildly reduced (40–49%) | 127 (23.2%) | 470 (19.1%) | |
| Moderately reduced (30–39%) | 79 (14.4%) | 314 (12.8%) | |
| Severely reduced (<30%) | 55 (10.1%) | 157 (6.4%) | |
| Medications within first 24 hours | |||
| Aspirin | 517 (94.5%) | 2359 (95.9%) | 0.194 |
| Antiplatelet agent (P2Y12 inhibitor) | 303 (55.4%) | 1570 (63.8%) | <0.001 |
| Beta blocker | 407 (74.4%) | 1958 (79.6%) | <0.009 |
| ACE inhibitor or ARB | 236 (43.1%) | 1122 (45.6%) | 0.307 |
| Statin | 400 (73.1%) | 1872 (76.1%) | 0.158 |
| Intravenous antithrombotic agent | 0.305 | ||
| No agent | 102 (18.6%) | 413 (16.8%) | |
| Single agent (heparin or bivalirudin) | 385 (70.4%) | 1813 (73.7%) | |
| Two agents (heparin or bivalirudin plus GP IIb/IIIa | 59 (10.8%) | 233 (9.5%) | |
| Revascularization status | <0.001 | ||
| No cardiac catheterization | 119 (21.8%) | 339 (13.8%) | |
| Cardiac catheterization only | 96 (17.6%) | 398 (16.2%) | |
| Cardiac catheterization with PCI | 262 (47.9%) | 1438 (58.5%) | |
| Coronary artery bypass graft surgery | 70 (12.8%) | 284 (11.5%) | |
| In-hospital complication: bleeding | 166 (30.3%) | 607 (24.7%) | 0.006 |
| In-hospital complication: acute kidney injury | 163 (29.8%) | 529 (21.5%) | <0.001 |
| In-hospital complication: heart failure | 104 (19.0%) | 310 (12.6%) | <0.001 |
| Functional impairments | |||
| Cognitive impairment (TICS) | 0.165 | ||
| No impairment (TICS ≥27) | 432 (79.0%) | 2019 (82.1%) | |
| Mild impairment (TICS 23–36) | 65 (11.9%) | 249 (10.1%) | |
| Moderate or severe impairment (TICS ≤22) | 42 (7.7%) | 150 (6.1%) | |
| Verbal fluency (Total COWAT S words), mean ± SD | 9.2 ± 4.7 | 9.8 ± 4.8 | 0.006 |
| Clinically significant vision impairment (VFQ-25) | 65 (11.9%) | 191 (7.8%) | 0.019 |
| Clinically significant hearing impairment | 72 (13.2%) | 333 (13.5%) | 0.351 |
| Unintentional weight loss (>10 lbs. in 1 year) | 143 (26.1%) | 528 (21.5%) | 0.012 |
| ADL disability (any) | 93 (17.0%) | 319 (13.0%) | 0.013 |
| Multiple falls (> 1 within past year) | 115 (21.0%) | 478 (19.4%) | 0.375 |
| Weak grip strength | 352 (64.4%) | 1455 (59.2%) | 0.007 |
| Functional mobility (based on Timed Up and Go) | <0.001 | ||
| Completed in ≤15 seconds | 105 (19.2%) | 760 (30.9%) | |
| Completed in >15 and ≤25 seconds | 97 (17.7%) | 522 (21.2%) | |
| Completed in >25 seconds | 111 (20.3%) | 372 (15.1%) | |
| Unable to complete | 134 (24.5%) | 426 (17.3%) | |
| Other measures | |||
| Short-form 12: general health question (4 categories) | <0.001 | ||
| Excellent or very good | 123 (22.5%) | 719 (29.2%) | |
| Good | 183 (33.5%) | 923 (37.5%) | |
| Fair | 153 (28.0%) | 607 (24.7%) | |
| Poor | 85 (15.5%) | 207 (8.4%) | |
| Depressive symptoms (PHQ-8 ≥10) | 102 (18.6%) | 320 (13.0%) | <0.001 |
ULN = upper limit of normal (based on local hospital reference value); eGFR = estimated glomerular filtration rate; TICS = Telephone Interview for Cognitive Status;COWAT = Controlled Oral Word Association Test; VFQ-25 = Visual Function Questionnaire 25; PHQ=8 = Patient Health Questionnaire 8
Data missing for fewer than 5% of variables except for left ventricular ejection fraction (9.2%) and Timed Up and Go (15.9%)
Multivariable results
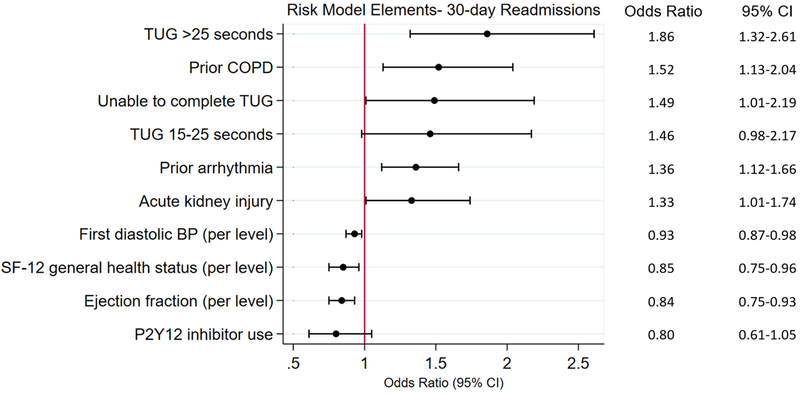
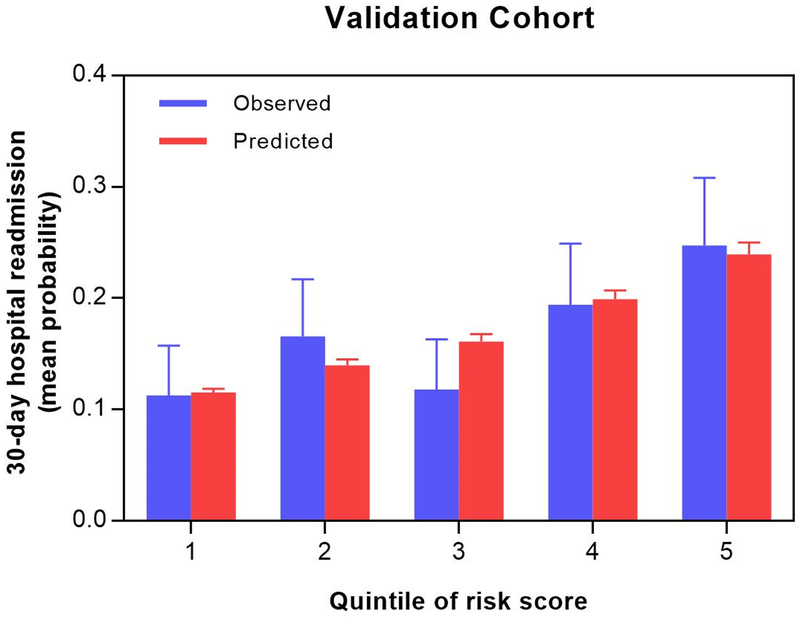
After application of Bayesian model averaging to the derivation cohort, eight variables were associated with readmission in the final prediction model: reduced ejection fraction, impaired functional mobility, poor patient reported health status (based on a single question: “in general, would you say your health is…”), prior arrhythmia, acute kidney injury (based on KDIGO criteria), low initial diastolic blood pressure, COPD, and lack of early P2Y12 inhibitor use. The strongest predictor of readmission was functional mobility (OR for TUG 15–25 seconds = 1.46, 95% CI 0.98–2.17; OR for TUG ≥25 seconds = 1.86, 95% CI 1.32–2.61; OR for TUG unable to complete = 1.49, 95% CI 1.01–2.19), followed by COPD (OR 1.52, 95% CI = 1.13–2.04) and prior arrhythmia (OR 1.36, 95% CI = 1.12–1.66) (Figure 3). Posterior effect probabilities from Bayesian Model Averaging are shown in eTable 3. Discrimination of this model was moderate (C statistic: 0.65 for derivation cohort, 0.63 for validation cohort). The model demonstrated consistently good calibration as defined by P values >0.05 for the Hosmer-Lemeshow statistic across all multiply imputed datasets. Figure 4 presents the means and confidence intervals of the observed and predicted probabilities of readmission in the validation cohort for quintiles based on risk scores using the coefficients of the model developed in the derivation cohort. Inclusion of TUG in the risk model improved the category-free NRI by 20% (41% of events correctly reclassified, 21% of non-events correctly reclassified; p<0.001) and the IDI by 13% (p=.004). We subsequently used beta coefficients from the regression equation (eTable 4) to develop a web-based risk calculator for 30-day readmission, shown in Figure 5 (and available at www.silverscore.org).
Figure 3. Risk model elements: 30 day readmission.
After Bayesian model averaging with multivariable logistic regression, eight variables were retained in the final risk model. Functional mobility based on Timed Up and Go (TUG) with reference: TUG <15 seconds. Diastolic BP based on categories (in mmHg): <50, 50–59, 60–79, 80–89, 90–99, >100 with reference: <50. SF-12 general health status treated as four-level variable based on single question (“In general, would you say your health status is (1) excellent or very good; (2) good; (3) fair; (4) poor”) with reference: poor. Ejection fraction treated as four-level categorical variable (≥50%, 40–49%, 30–39%, <30%) with reference: <30%.
Figure 4. Model calibration, validation cohort (by quintile).
Shown are observed (blue) and predicted (red) 30-day readmission rates, by quintiles of predicted readmission risk within the validation cohort. Among these quintiles, the SILVER-AMI readmission risk model was well calibrated (Hosmer-Lemeshow P > 0.05).
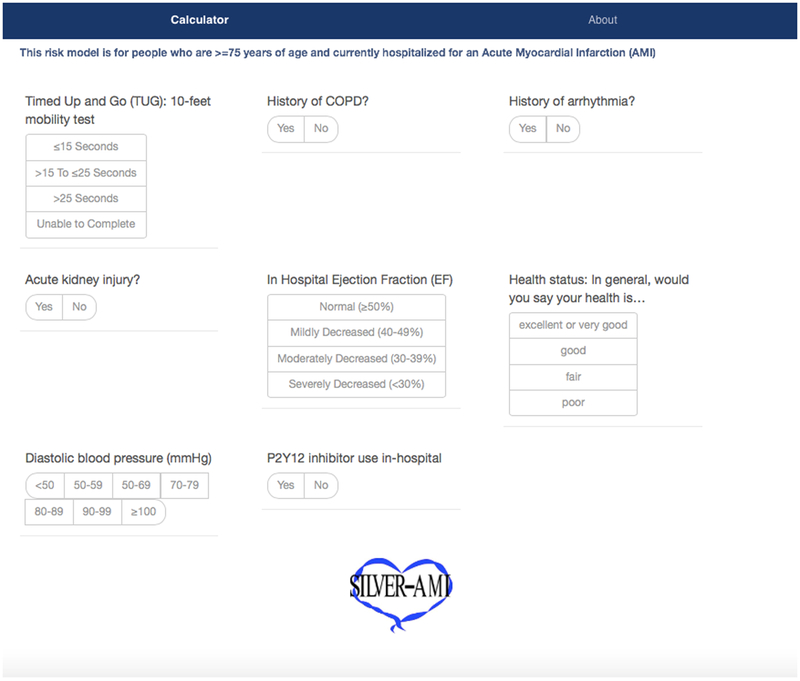
Figure 5. SILVER-AMI 30-day readmission calculator.
A web-based calculator for predicted readmission risk among patients age ≥75 hospitalized for AMI is available at www.silverscore.org.
DISCUSSION
Hospital readmissions after AMI among older adults are common, challenging to predict, and detrimental. While geriatric-oriented functional impairments may plausibly influence these readmissions, prior large-scale studies have generally failed to evaluate them. In this context, SILVER-AMI was designed to assess a broad array of non-cardiovascular functional impairments among older adults hospitalized with AMI, while simultaneously recording information on more “traditional” clinical variables including presentation characteristics and comorbidities. Among our sample, 18% of participants experienced hospital readmission within 30 days, which approximates other reports in older adults hospitalized for AMI (1,29). Several functional impairments were associated with these readmissions in bivariate analyses including impaired functional mobility, vision impairment, ADL disability, and weak grip strength. While impaired mobility remained the only functional impairment associated with 30-day readmission after multivariable adjustment, the most impaired mobility category (TUG >25 seconds) was the strongest predictor – nearly doubling the odds of readmission (versus TUG <15 seconds). Our final, validated prediction model included functional mobility, as well as self-reported health status and more traditional clinical variables (e.g. COPD, acute kidney injury, ejection fraction). This model was well-calibrated, with modest discrimination.
To our knowledge, this is the first risk model developed and validated specifically for use at the patient level (as opposed to hospital level) in an older AMI population that considers functional impairments and that is broadly representative of patients hospitalized across the U.S. Previous investigators have aimed to incorporate variables that may capture readmission risk beyond those available in administrative datasets (e.g. age, comorbidities), albeit in younger cohorts enrolled in localized geographic settings (12,30). For example, McManus et al. used the TRACE-CORE acute coronary syndrome registry, which captured a range of clinical, psychosocial, and sociodemographic characteristics, to develop a model for 30-day readmission risk in patients age ≥65 (12). They enrolled patients from 6 hospitals in the Northeastern and Southeastern United States and found that low health literacy was an independent predictor of readmission, along with more traditional variables, including serum sodium, prior coronary intervention, chronic kidney disease, and current smoking. Nguyen et al. used data from electronic health records at 6 hospitals in north Texas to validate a post-AMI readmission risk model that included brain natriuretic peptide (BNP) as well as renal function, age, diabetes, sex, early PCI, and low systolic blood pressure (30). Both studies enrolled relatively young cohorts (mean age=73 years in the McManus et al. study, and 66 years in the Nguyen et al. study) from relatively few sites, limiting their potential relevance and generalizability to older populations.
While the readmission risk model we have developed and validated considers a wide range of potential risk factors, is practical for clinical use, and included patients from a diverse network of 94 hospitals across the United States (with a mix of academic medical centers, community hospitals, and regional referral centers located in urban, rural, and suburban areas), it exhibits modest discrimination, similar to the majority of AMI readmission risk models (7,10). This highlights the limitations of considering only patient-level information (as opposed to environmental factors reflecting the health system or local communities) in estimating readmission risk. In addition, we did not collect data on stressors of hospitalization, including immobility, sleep disruption, and poor nutrition, which may contribute to the state of heightened risk for readmission described by the term “post-hospital syndrome” (31,32). Reliable assessment of these stressors is challenging, but may contribute important information to readmission risk stratification. In addition, a recent study by Krumholz et al. using Centers for Medicare & Medicaid Services (CMS) data demonstrated that hospital quality is an important contributor to readmission rates independent of patient-level factors (33). Specifically, the authors found that when the same patients were admitted with similar diagnoses to hospitals in the best-performing quartile compared with the worst-performing quartile of hospital readmission performance, they had a significantly higher risk of 30-day readmission after hospitalization at the worst-performing facilities. This underscores the limitations of relying on patient-level information to quantify risk for readmission.
Our study was designed in the context of an emerging literature that demonstrates that functional impairments are a generalized marker of risk in older adults (4,34). Impaired mobility is among the most rigorously studied and has been shown to increase adverse events in several AMI cohorts (4,35). For example, a study of participants enrolled in the TRIUMPH registry found that impaired mobility (using a definition of gait speed <0.8 meters per second) nearly doubled the risk of readmission or death within 1 year of hospitalization for AMI (35). Matsuzawa et al. demonstrated that among patients hospitalized with AMI, those in the lowest tertile of gait speed experienced a tenfold increase in the risk of cardiovascular events at long term follow-up (~8 years) compared with those in the highest tertile of gait speed (4). We found that impaired mobility (integrating information about gait speed, lower extremity strength, and balance) was strongly predictive of readmission: participants who took >25 seconds to complete TUG had nearly twice the odds of readmission at 30 days compared with those whom completed TUG in <15 seconds. Impaired mobility was also the strongest individual predictor of readmission in our multivariable model and improved model discrimination by 20%. Mechanistically, mobility impairment shares considerable overlap with the frailty syndrome (35), which is defined as a state of increased physiologic vulnerability to stressors, and patients with mobility impairment may therefore be especially vulnerable to the stressors of hospitalization, contributing to risk for readmission. A simple functional mobility measure such as TUG is rapidly performed and easily reproducible, which provides a potential advantage in clinical practice over more detailed frailty assessments (36).
Other functional impairments have been less well studied in the context of AMI but may plausibly be related to readmission through multiple mechanisms, such as fall-related injury (cognitive, vision, and hearing impairment), susceptibility to infection (unintentional weight loss), poor self-care (cognitive impairment), or poor medication adherence (cognitive and vision impairment). In the heart failure literature, cognitive impairment has been studied extensively (37–39); for example, Patel et al. found that cognitive impairment doubled the risk of readmission or mortality within 6 months of heart failure hospitalization, and was the strongest predictor among 55 candidate variables evaluated (37). Cognitive impairment is thought to influence risk through impaired self-care, whereby patients are unable to manage their own disease (e.g. reliably take diuretics) due to the complex nature of these tasks (39).
Despite these potential mechanisms, our study did not find that impairments in functional domains other than mobility influenced the risk of readmission after multivariable adjustment, even though the prevalence of impairments in these domains (activities of daily living disability, weak grip strength, vision impairment, impaired verbal fluency) was higher among readmitted participants. Conversely, other more traditional clinical risk factors (COPD, history of arrhythmia, acute kidney injury, ejection fraction, diastolic blood pressure) emerged as influential determinants of post-hospital risk in our multivariable model. It is possible that the nature of our readmissions (the majority of which were cardiovascular, and very few of which were exclusively “geriatric” such as injurious falls) partially explains this finding. For example, acute kidney injury and ejection fraction both have well-described associations with hospitalization for heart failure (40,41), which was the most common cause of readmission within our cohort. While cognitive impairment has known associations with heart failure readmissions, the overall degree of cognitive impairment among our sample was relatively mild – and perhaps not sufficient to adversely impact self-care tasks that may influence readmission events. Notably, we also found that lower self-reported health status, based on the SF-12, was associated with higher readmission risk. Health status (with instruments such as the SF-12) has been collected in prior AMI research studies and associated with adverse outcomes (42,43). Our findings emphasize that patient-reported health status is an important prognostic measure that is easy to collect and perhaps should be used routinely in risk assessment for older adults.
Our findings must be interpreted in the context of our study design. We excluded those with severe cognitive impairment or delirium and no proxy available, as these individuals were unlikely to be able to complete the detailed study assessments. Further, slightly over one-third of patients meeting eligibility criteria declined informed consent, which may limit the generalizability of our findings. In addition, while our model was validated with a split sample, which provides a distinct advantage over other many risk models that are only internally validated, we cannot make conclusions on how our model may perform in external datasets. There were two risk factors for readmission in our final model – low diastolic BP and lack of P2Y12 inhibitor use – for which we did not find previous associations in the literature. While there is clinical plausibility between both of these factors and readmissions (for example, low diastolic BP as a marker of advanced illness, and lack of P2Y12 inhibitor use as a risk factor for recurrent ischemic events), they require confirmation in other datasets. A third limitation is that for some risk factors we used values obtained at admission rather than discharge (e.g. laboratory values, vital signs). We made this decision since admission values were universally available at the same time point, but failure to include discharge values may have failed to reflect changes that occurred during the hospitalization. Fourth, rates of vision and hearing impairment were also lower than in several other epidemiologic studies of aging (44,45), which suggests that the SILVER-AMI cohort may have been healthier than the general population; this is a known phenomenon in voluntary research studies including prospective cohorts (46,47). While it is important to consider these issues, SILVER-AMI included over 3000 patients recruited from 94 hospitals, with a mix of academic, community-based, and regional referral centers. To our knowledge, this is the largest study to date that includes a detailed assessment of functional impairments in the context of hospitalization for AMI. Finally, we studied a single outcome (30-day readmission), and are unable to comment on the prognostic utility of functional impairments for other events including mortality, health status decline, and longer term readmissions. We plan to investigate these outcomes in subsequent analyses.
In conclusion, we developed and validated a risk model for hospital readmission within 30 days after AMI among patients age ≥75 that considered functional impairments, health status, and more traditional clinical characteristics. Among the considered variables, functional mobility was the strongest predictor of 30-day readmission and significantly improved risk model performance. Our validated risk model was well-calibrated and can be used to calculate predicted risk. However, discrimination was modest, indicating that much of the variability in readmission risk among this older adult population remains unexplained by patient-level characteristics. Future studies that incorporate factors beyond patient-level characteristics (such as hospital stressors or health system performance) may lead to improved discrimination.
Supplementary Material
What is known:
After hospitalization for acute myocardial infarction (AMI), hospital readmissions among older adults (age ≥75) within 30 days of discharge are common.
Current AMI risk models have limited discrimination in older adults, possibly because they failed to consider relevant functional impairments common with aging.
What this study adds:
We derived and validated a risk model for 30-day readmission after AMI hospitalization in older adults that explicitly considered functional impairments.
One impairment (functional mobility) was retained in the final risk model, as well as ejection fraction, COPD, arrhythmia, acute kidney injury, first diastolic blood pressure, P2Y12 inhibitor use, and general health status.
This model had good calibration but only modest discrimination, indicating much of the variability in readmission risk among this population remains unexplained by patient-level factors.
Acknowledgements
We would like to thank Jenny Summapund for assistance with the preparation of this manuscript for submission.
Sources of Funding
This research was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (R01HL115295). This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). The project described used REDCap which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant UL1 TR00000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Dodson is supported by a Patient Oriented Career Development Award (K23 AG052463) from the National Institute of Aging, and a Mentored Clinical and Population Research Award from the American Heart Association. Dr. Hajduk was supported by NIA training grant T32 AG019134. Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. Dr. Nanna is supported by an NIH training grant 5T32HL069749–15.
Footnotes
Disclosures
No authors report relevant disclosures.
REFERENCES
- 1.Dharmarajan K, Hsieh AF, Lin Z, Lin Z, Bueno H, Ross J, Horwitz L, Barreto-Filho JA, Kim N, Bernheim S, Suter L, Drye E, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Dharmarajan K, Krumholz HM. Strategies to reduce 30-day readmissions in older patients hospitalized with heart failure and acute myocardial infarction. Curr Geriatr Reports. 2014;3:306–315. doi: 10.1007/s13670-014-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Nakayama N, Kiyokuni M, Sumita S, Ebina T, Kosuge M, Hibi K, Tsukahara K, Iwahashi N, Endo M, Maejima N, Saka K, Hashiba K, Okada K, Taguri M, Morita S, Sugiyama S, Ogawa H, Sashika H, Umemura S, Kimura K. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Michael Gharacholou S, Lopes RD, Alexander KP, Mehta RH, Stebbins AL, Pieper KS, James SK, Armstrong PW, Granger CB. Age and outcomes in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: Findings from the APEX-AMI trial. Arch Intern Med. 2011;171:559–567. doi: 10.1001/archinternmed.2011.36. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Mattera JA, Normand SL. An Administrative Claims Measure Suitable for Profiling Hospital Performance Based on 30-Day All-Cause Readmission Rates Among Patients With Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243 LP–252. 10.1161/CIRCOUTCOMES.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith LN, Makam AN, Darden D, Mayo H, Das SR, Halm EA, Nguyen OK. Acute myocardial infarction readmission risk prediction models. Circ Cardiovasc Qual Outcomes. 2018;11:e003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodson JA, Geda M, Krumholz HM, Lorenze N, Murphy TE, Allore HG, Charpentier P, Tsang SW, Acampora D, Tinetti ME, Gill TM, Chaudhry SI. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14 10.1186/s12913-014-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 10.Desai MM, Stauffer BD, Feringa HHH, Schreiner GC. Statistical models and patient predictors of readmission for acute myocardial infarction a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507. 10.1161/CIRCOUTCOMES.108.832949. [DOI] [PubMed] [Google Scholar]
- 11.Burke RE, Schnipper JL, Williams MV, Robinson EJ, Vasilevskis EE, Kripalani S, Metlay JP, Fletcher GS, Auerbach AD, Donze JD. The HOSPITAL Score Predicts Potentially Preventable 30-Day Readmissions in Conditions Targeted by the Hospital Readmissions Reduction Program. Med Care. 2017:285–290. doi: 10.1097/MLR.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McManus DD, Saczynski JS, Lessard D, Waring ME, Allison J, Parish DC, Golderberg RJ, Ash A, Kiefe CI. Reliability of predicting early hospital readmission after discharge for an acute coronary syndrome using claims-based data. Am J Cardiol. 2016;117:501–507. 10.1016/j.amjcard.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 14.Rodríguez-Aranda C, Martinussen M. Age-related differences in performance of phonemic verbal fluency measured by Controlled Oral Word Association Task (COWAT): A meta-analytic study. Dev Neuropsychol. 2006;30:697–717. 10.1207/s15326942dn3002_3. [DOI] [PubMed] [Google Scholar]
- 15.Mangione CM. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 16.Hayman KJ, Kerse N, Dyall L, Kepa M, Teh R, Wham C, Wright-St Clair V, Wiles J, Keeling S, Connolly MJ, Wilkinson TJ, Moyes S, Broad JB, Jatrana S. Life and Living in Advanced Age: A Cohort Study in New Zealand -Te Puāwaitanga o Nga Tapuwae Kia Ora Tonu, LiLACS NZ: Study protocol. BMC Geriatr. 2012;12:33 10.1186/1471-2318-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb D, White L. Midlife hand grip strength as a predictor of old age disability. J Am Med Assoc. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 21.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C-Y, Chen L-Y. Grip Strength in Older Adults: Test-Retest Reliability and Cutoff for Subjective Weakness of Using the Hands in Heavy Tasks. Arch Phys Med Rehabil. 2010;91:1747–1751. 10.1016/j.apmr.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Lameire N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 26.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian Model Averaging: A Tutorial. Stat Sci. 1999;14(4):382–417. doi: 10.2307/2676803. [DOI] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 28.Raftery A, Hoeting J, Volinsky C, Painter I, Yeung KY. Baysean Model Averaging: version 3.18.6. Available at: http://Www.r-project.org. Accessed August 22, 2018.
- 29.Tisminetzky M, McManus DD, Erskine N, Saczynski JS, Yarzebski J, Granillo E, Gore J, Goldberg RJ. Thirty-day hospital readmissions in patients with non-ST-segment elevation acute myocardial infarction. Am J Med. 2015;128:760–765. doi: 10.1016/j.amjmed.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen OK, Makam AN, Clark C, Zhang S, Das SR, Halm EA. Predicting 30-Day Hospital Readmissions in Acute Myocardial Infarction: The AMI “READMITS” (Renal Function, Elevated Brain Natriuretic Peptide, Age, Diabetes Mellitus, Nonmale Sex, Intervention with Timely Percutaneous Coronary Intervention, and Low Systo. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krumholz HM. Post-hospital syndrome — an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldwater DS, Dharmarajan K, McEwen BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13. doi: 10.12788/jhm.2986. [DOI] [PubMed] [Google Scholar]
- 33.Krumholz HM, Wang K, Lin Z, Dharmarajan K, Horwitz LI, Ross JS, Drye EE, Bernheim SM, Normand ST. Hospital-Readmission Risk — Isolating Hospital Effects from Patient Effects. N Engl J Med. 2017;377:1055–1064. doi: 10.1056/NEJMsa1702321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodson JA, Arnold SV, Gosch KL, Gill TM, Spertus JA, Krumholz HM, Rich MW, Chaudhry SI, Forman DE, Masoudi FA, Alexander KP. Slow Gait Speed and Risk of Mortality or Hospital Readmission Following Myocardial Infarction in the TRIUMPH Registry. J Am Geriatr Soc. 2016;64:596–601. 10.1111/jgs.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhry SI, Gill TM. Geriatric assessment to improve risk stratification in older patients undergoing coronary revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:491–492. 10.1161/CIRCOUTCOMES.111.962647. [DOI] [PubMed] [Google Scholar]
- 37.Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance novel marker of post discharge risk among patients hospitalized for heart failure. Circ Hear Fail. 2015;8:8–16. 10.1161/CIRCHEARTFAILURE.114.001438. [DOI] [PubMed] [Google Scholar]
- 38.Dodson JA, Truong T-TN, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126:120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–515. 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 40.Thakar CV, Parikh PJ, Liu Y. Acute Kidney Injury (AKI) and Risk of Readmissions in Patients With Heart Failure. Am J Cardiol. 2012;109:1482–1486. doi: 10.1016/j.amjcard.2012.01.362. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 42.Dodson JA, Arnold SV, Reid KJ, Gill TM, Rich MW, Masoudi FA, Spertus JA, Krumholz HM, Alexander KP. Physical function and independence 1 year after myocardial infarction: Observations from the Translational Research Investigating Underlying disparities in recovery from acute myocardial infarction: Patients’ Health status registry. Am Heart J. 2012;163:790–796. doi: 10.1016/j.ahj.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold SV, Alexander KP, Masoudi FA, Ho PM, Xiao L, Spertus JA. The effect of age on functional and mortality outcomes after acute myocardial infarction. J Am Geriatr Soc. 2009;57:209–217. 10.1111/j.1532-5415.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 44.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care. JAMA. 2003;289:1976. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]
- 45.Ryskulova A, Turczyn K, Makuc DM, Cotch MF, Klein RJ, Janiszewski R. Self-reported age-related eye diseases and visual impairment in the United States: Results of the 2002 National Health Interview Survey. Am J Public Health. 2008;98:454–461. doi: 10.2105/AJPH.2006.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 47.Sica GT. Bias in Research Studies. Radiology. 2006;238:780–789. 10.1148/radiol.2383041109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.