Abstract
Background
Retinoblastoma is the most common primary intraocular malignancy of childhood. Systemic chemotherapy is a common treatment for intraocular retinoblastoma, and laser treatment is used as adjuvant therapy during or immediately after chemotherapy courses in selected cases.
Objectives
To compare the effectiveness and safety of adding focal laser therapy to systemically‐delivered chemotherapy in treating intraocular retinoblastoma.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 9), MEDLINE Ovid (1946 to 20 October 2016), Embase Ovid (1980 to 20 October 2016), LILACS (Latin American and Caribbean Health Sciences Literature Database) (1982 to 20 October 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch); searched 20 October 2016, ClinicalTrials.gov (www.clinicaltrials.gov); searched 20 October 2016, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en); searched 20 October 2016. We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We searched for randomised controlled trials (RCTs) of systemic chemotherapy with versus without adjuvant laser therapy for postequatorial retinoblastoma.
Data collection and analysis
We planned to use standard methodological procedures expected by Cochrane. We planned to meta‐analyse the primary outcome, that is the proportion of eyes with recurrence of tumours within three years from treatment
Main results
No studies met the inclusion criteria for this review.
Authors' conclusions
No evidence from randomised controlled trials was found to support or refute laser therapy in addition to systemic chemotherapy for postequatorial retinoblastoma.
Plain language summary
Focal laser treatment in addition to chemotherapy for retinoblastoma
What was the aim of this review? The aim of this Cochrane Review was to find out if focal laser therapy in addition to treatment by systemic chemotherapy for retinoblastoma reduces the risk of tumour recurrence.
Key messages There were no data comparing systemic chemotherapy alone to systemic chemotherapy with laser therapy.
What was studied in the review? Retinoblastoma is the most common primary cancer in children to arise from within the eye. Treatments for retinoblastoma have evolved significantly throughout the years from eye removal (i.e. enucleation); to radiotherapy and, currently, the main treatment used is chemotherapy. The addition of laser therapy at the same time as, or immediately after, chemotherapy is administered may improve tumour control.
What are the main results of the review? The review authors did not find any completed studies that could be included in this review.
How up‐to‐date is this review? The review authors searched for studies published up to 20 October 2016.
Background
Description of the condition
Retinoblastoma is the most common intraocular malignancy of childhood, with a constant incidence worldwide of 1:15,000 to 1:20,000 live births (Kivela 2009). In most cases the tumour is initiated by a mutation in the RB1 gene, a tumour suppressor gene located on chromosome 13q14, and mutations in both RB1 alleles are a prerequisite for developing the cancer (Knudson 1971). In heritable retinoblastoma, in which in most cases both eyes are clinically affected, mutation in one RB1 allele is constitutional, whereas a somatic mutation in the second allele initiates tumour growth in the sensory retinal cells. In non‐heritable RB1‐related retinoblastoma, both mutations are somatic, which gives rise to unilateral disease in most cases. Children with the heritable form of retinoblastoma are also prone to developing additional non‐ocular tumours (Eng 1993), and are at higher risk of developing trilateral retinoblastoma, that is, ocular retinoblastoma in conjunction with an intracranial midline primitive neuroectodermal tumour (De Jong 2015).
The median age of diagnosis of unilateral retinoblastoma is 24 months and of bilateral retinoblastoma is 10 months (MacCarthy 2009). A white pupillary reflex, also termed leukocoria, is the most common presenting clinical sign in both developing and developed countries (Abramson 1998; Bowman 2008; Menon 2009). Additional signs are strabismus, usually when the macula is involved, and less frequently red eye, inflammation and additional non‐specific signs (Abramson 1998). Early detection and urgent referral to specialised retinoblastoma centres is of utmost importance in order to salvage life, eye and vision. If untreated, retinoblastoma will spread outside the globe, via the central nervous system, and haematogenously, inevitably leading to death. In developing countries, in which there is a lack of educational strategies and infrastructure is poor, retinoblastoma patients’ survival rate is estimated to be 40% or less (Dimaras 2012; Kivela 2009). In developed countries, while these were the survival rates in the early 20th century, currently the five‐year survival is estimated to be over 95% (Shields 2004).
Treatment strategies for retinoblastoma have evolved significantly throughout the years. Traditionally, retinoblastoma was treated by removal of the eyeball (i.e. enucleation), a definite cure when the tumour is contained within the globe. In advanced cases with extraocular tumour extension, enucleation alone is not curative, and further treatment is warranted. Currently, enucleation is reserved only for advanced stage intraocular disease or as salvage treatment after failure of other conservative modalities.
In seeking a treatment modality with better outcomes and less morbidity, external beam radiotherapy (EBRT) was found to be an effective alternative, and by the mid‐20th century had largely replaced enucleation as the mainstay treatment for most retinoblastoma cases (Reese 1949; Stallard 1952). In order to better predict outcomes of children with retinoblastoma treated with EBRT, the Reese‐Ellsworth (R‐E) classification for intraocular retinoblastoma was developed (Reese 1964). It soon became an essential tool in its management, and also enabled comparison of study results from different centres. Unfortunately, after nearly half a century of extensive use of EBRT for retinoblastoma, it was recognised that radiation significantly increases the risk of developing a secondary cancer in survivors of hereditary retinoblastoma (Fletcher 2004; Kleinerman 2005). As a result, radiotherapy was widely abandoned and replaced by chemotherapy as the primary treatment for intraocular retinoblastoma. To date, it is reserved only as a last resort when all other modalities have failed.
Description of the intervention
Systemically administered chemotherapy for retinoblastoma is used as adjuvant treatment when high‐risk histopathological features are found after enucleation, to treat systemic retinoblastoma spread and as primary treatment for intraocular disease. Kupfer 1953 described the first use of chemotherapy for the latter indication and attempts to combine EBRT and chemotherapy were soon published (Reese 1958). However, it was abandoned and for many years reserved only for extraocular disease. The use of systemic chemotherapy as a primary treatment modality for intraocular disease was revived in the 1990s in London using potent chemotherapeutic agents, namely vincristine, etoposide and carboplatin (VEC). The VEC regimen was first given in combination with EBRT and resulted in 70% of eyes salvaged (Kingston 1996). In subsequent studies, systemic chemotherapy with additional focal therapy was used as an alternative to EBRT and resulted in a high eye‐salvage rate (Gallie 1996; Murphree 1996; Shields 1996). Soon after it was first introduced, the VEC regimen became the standard protocol, given through a central venous access line every three weeks for six cycles. In order to better predict the outcomes of children with retinoblastoma treated with chemotherapy, a new classification scheme, the International Classification of Retinoblastoma (ICRB), was introduced in the early 2000s to replace the R‐E classification, which became less relevant (Murphree 2005). One of the main features integrated into the ICRB scheme was the presence of retinoblastoma seeds (vitreal or subretinal, or both), as it was recognised that seeding is a predictor for failure after chemotherapy. Despite some discrepancies in its interpretation, the ICRB is currently used throughout the world (Novetsky 2009).
Early use of systemic chemotherapy in conjunction with radiation was reported to cause serious side effects and therefore abandoned (Reese 1958). In the VEC regimen era, possible chemotherapy‐related side effects include febrile episodes, temporary alopecia, bone marrow suppression, peripheral neuropathy and allergic reactions to the substances used, but the incidences of which are poorly documented. Carboplatin was suggested to cause ototoxicity (Lambert 2008), and etoposide to induce secondary acute myelogenous leukaemia (Gombos 2007; Turaka 2012); both on rare occasions. In an attempt to avoid potential systemic complications of systemically‐delivered chemotherapy, new methods of delivery were developed, namely intra‐arterial and intravitreal chemotherapy. The former method of delivery was developed in Japan (Yamane 2004), and was refined in the USA (Abramson 2008). The latter was developed in Sweden (Ericson 1961; Seregard 1995), and is extensively used in Japan and Switzerland (Kaneko 2003; Munier 2012). There is increasing enthusiasm towards the use of these selective modalities, and in some centres they have replaced systemic chemotherapy as a primary treatment for intraocular retinoblastoma (Abramson 2015). Despite these trends, the indications for use and whole spectrum of side effects and complications are not yet fully understood. Worldwide, systemically‐delivered chemotherapy remains a major therapeutic option for unilateral and especially bilateral retinoblastoma.
Focal laser is the treatment of choice for small postequatorial tumours. It is also used in combination or after administration of chemotherapy for larger tumours and for tumour recurrence (retinal or subretinal) after successful initial treatment by various modalities. Though it was initially performed by means of light photocoagulation, currently most centres use an 810 nm modified diode laser, termed transpupillary thermotherapy (TTT). Lagendijk 1982 performed the first description of TTT as treatment for intraocular retinoblastoma, which used a microwave applicator and successfully treated two patients with recurrent disease. It has since been used successfully as a primary sole treatment for ICRB group A eyes, and has resulted in a high rate of tumour control. Shields 1999 used TTT to primarily treat 188 tumours in a mean base diameter of 3 mm and elevation of 2 mm, and showed that a complete regression was achieved in 86% of the tumours. In a case series of 91 tumours that measured less than 1.5 disc diameters (DD), Abramson 2004a showed that 92% of the tumours were cured with TTT alone. The procedure is performed using indirect ophthalmoscopy through dilated pupils, with the laser beam exiting the head piece and aimed at the intraocular tumour. The laser is applied for a prolonged period of time (minimum nine seconds per application) as compared to laser photocoagulation. The number of applications, spot size, power energy and treatment end points vary between different centres (Abramson 2004a; Brichard 2002; Levy 1998; Lumbroso 2002; Shields 2002).
How the intervention might work
Systemic chemotherapy
Historically, chemotherapy was considered to be ineffective for intraocular retinoblastoma, and therefore was not used for this indication (White 1983). However, several advances in the field of chemotherapy for paediatric cancers have driven physicians and scientists to revisit this paradigm: carboplatin, a cisplatin analogue with an improved side effect profile, was found to be an effective chemotherapeutic agent for other neuroectodermal solid tumours (i.e. neuroblastoma) (Gaynon 1994), was shown to cross the blood‐brain barrier (Riccardi 1992), and together with etoposide was found to be effective for extraocular retinoblastoma (Doz 1995). Kingston and colleagues’ above‐mentioned pilot study using VEC in conjunction with EBRT, salvaging most R‐E group V eyes (Kingston 1996), paved the way for additional clinical trials and also empirical experimental studies. Murphree and colleagues measured carboplatin concentrations in enucleated retinoblastoma eyes after systemic administration of the agent and found it was at significant levels (Murphree 1996). In addition, carboplatin was found to effectively inhibit tumour growth in both in vivo and in vitro experimental models (Harbour 1996; Murray 1997).
Systemic chemotherapy and focal laser treatment
While some use chemotherapy as the sole primary treatment for intraocular retinoblastoma (Gombos 2002), others claim it to be insufficient to combat the disease; they have stated that it merely reduces tumour size, thus enabling further focal therapies to be applied to achieve full tumour control (Murphree 1996; Shields 1996; Shields 2005). The use of chemotherapy in this manner, termed chemoreduction, has roots in treatments of other solid tumours (Dropcho 1992; Follézou 1989; Larner 1995). In a study by Shields and colleagues, chemotherapy for intraocular retinoblastoma resulted in complete tumour response in 46% of the children and a partial response that warranted additional focal treatments in the remaining children (Shields 1996). None of the children required enucleation, although EBRT was necessary in 29% of eyes because of diffuse vitreous seeds. Wilson 2001 treated 20 children with retinoblastoma with eight cycles of vincristine and carboplatin, and found that in 92% of cases tumours progressed after chemotherapy and required adjuvant supplemental focal therapies. Shields 2005 performed a prospective non‐randomised study in which 28 of 68 tumours were treated by means of systemic chemotherapy alone and 40 of 68 were treated with chemotherapy combined with foveal‐sparing TTT. The study findings indicated that recurrence in the group that received chemotherapy alone occurred in 35% of tumours at four years' follow‐up versus 17% in the combined treatment group. In support of the combined use of TTT and chemotherapy, not only the insufficient role of chemotherapy alone is stated but also the beneficial interaction of heat energy and chemotherapy. The use of thermal energy in conjunction with chemotherapy has been shown to be synergistic (Da Silva 1987; Herman 1994), and to enhance the cytotoxic effect of carboplatin in vivo (Tapazoglou 1991). In addition, in a transgenic murine retinoblastoma cell line, the cytocidal interaction of heat and carboplatin were found to be superior to each of the monotherapies (Murray 1997). Lumbroso 2002 treated 51 children (103 tumours) with TTT that was administered shortly after injection of carboplatin. More than 96% of tumours regressed after a median follow‐up time of 30 months. The use of systemic chemotherapy alone for intraocular retinoblastoma was advocated, as focal laser therapy was found to cause large scotomas (Abramson 2004b). The laser scar may also increase in size (Lee 2004). Also, rates of vitreous or vitreous base relapse are thought to increase with a longer duration of TTT (Gombos 2006). Schefler and colleagues assessed children treated with chemotherapy and repetitive TTT ablations (Schefler 2007). Nearly 90% of eyes achieved control at three years' follow‐up. However, over 60% of children developed iris atrophy with laser treatment. Gombos and colleagues retrospectively reviewed retinoblastoma patients treated with primary systemic chemotherapy alone and found that in 72% of cases there was no need for additional treatment (Gombos 2002).
Why it is important to do this review
To date there has been no systematic review of randomised trials to summarise the evidence of the effectiveness and safety of focal laser therapy in children with retinoblastoma treated with systemic chemotherapy. Also, there is no consensus as to the preferred timing of laser therapy application, if used, after chemotherapy administration. While in some practices focal laser treatment is added only if required, in others it is used at a prespecified timing in regard to the chemotherapy courses given (i.e. before or after a specific predetermined chemotherapy course). In addition, there is clinical uncertainty as for which group classification (R‐E or ICRB) focal laser treatment is best used as an adjunct to systemic chemotherapy.
As retinoblastoma is a sight‐ and life‐threatening disease, it is important to establish the preferred practice in terms of efficacy and safety of focal laser therapy added to systemically‐delivered chemotherapy.
Objectives
To compare the effectiveness and safety of adding focal laser therapy to systemically‐delivered chemotherapy in treating intraocular retinoblastoma.
Methods
Criteria for considering studies for this review
Types of studies
We planned to only include randomised controlled trials (RCTs).
Types of participants
Participants diagnosed with intraocular postequatorial retinoblastoma in either one or both eyes. There were no restrictions regarding age, gender, ethnicity, co‐morbidities of participants or the number of trial participants.
Types of interventions
We planned to include trials that compared chemotherapy plus laser treatment versus chemotherapy alone. There were no restrictions on the type of laser used (i.e. laser beam wavelength).
Types of outcome measures
Primary outcomes
The proportion of eyes with recurrence of tumours within three years from treatment.
Secondary outcomes
The proportion of eyes that required external beam radiotherapy (EBRT) or enucleation at any time point
The proportion of enucleated eyes at any time point with high‐risk histopathological characteristics for tumour progression and metastasis (i.e. invasion of the postlaminar optic nerve, choroid, sclera and anterior chamber) (Khelfaoui 1996)
The proportion of enucleated eyes with extraocular tumour spread at any time point
Metastatic spread: the proportion of participants with distant metastatic spread at any time point
Survival: the proportion of participants that died due to metastatic spread at any time point
Total number of additional treatments given
Time from last treatment (i.e. chemotherapy or laser treatment) to tumour relapse
Visual acuity of 6/60 or better versus worse than 6/60 measured at least 12 months after initial treatment
Adverse outcomes
We planned to compare complications and adverse events between treatment groups that occurred throughout follow‐up for all included trials. Complications included those from chemotherapy or use of laser therapy, or both. Adverse effects of interest included but were not limited to the following.
Retinal scar
Retinal tear
Development of vitreous seeds
Iris atrophy
Neovascularisation at the disc, retina elsewhere, iris or anterior chamber angle
Chorio‐retinal ischaemia
Systemic adverse effects of any kind
Death
Follow‐up
We planned to not place any restrictions on the duration of follow‐up.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases. There were no study design, language or publication year restrictions. The date of the search was 20 October 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 20 October 2016) (Appendix 1);
MEDLINE Ovid (1946 to 20 October 2016) (Appendix 2);
Embase Ovid (1980 to 20 October 2016) (Appendix 3);
LILACS (Latin American and Caribbean Health Science Information Database (1982 to 20 October 2016) (Appendix 4);
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 20 October 2016) (Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 20 October 2016) (Appendix 6);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 20 October 2016) (Appendix 7).
Searching other resources
We searched the citation lists of reports from studies that potentially met the inclusion criteria to look for additional trials. We did not conduct manual searches of conference proceedings or abstracts specifically for this review.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts that resulted from the searches by using internet‐based software (Covidence 2015). They classified each record as either 'definitely relevant', 'possibly relevant' or 'irrelevant'. They obtained full‐text copies of records classified as 'definitely relevant' or 'possibly relevant' after discussion between the review authors. They classified each full‐text report as either 'included', 'awaiting assessment' or 'excluded'. A third review author resolved any disagreements regarding full‐text assessments. For studies written in languages not understood by the review authors, we planned to use Google Translate or request translation of the full‐text report in order to determine eligibility. We planned to contact the primary study investigators to clarify the eligibility of studies classified as 'awaiting assessment'. We documented the reasons for excluding studies identified by both review authors as 'excluded'. Also, we listed the excluded studies and their reasons for exclusion in the Characteristics of excluded studies table. The review authors were not masked to the report authors, institutions and trial results during these assessments. We created a PRISMA diagram to illustrate the study selection process (Moher 2009).
Data extraction and management
Two review authors planned to independently extract data using a prepiloted online form and the web‐based software Covidence (Covidence 2015). One review author planned to enter data into Review Manager 5 (RevMan 5) (Review Manager 5 2014), and the second review author would review the accuracy of the work performed by the first review author. Specific study and participant data items of interest are shown in Appendix 8.
Assessment of risk of bias in included studies
Two review authors planned to assess the risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This assessment would include assessing sequence generation, allocation concealment, completeness of data, selective outcome reporting and other potential sources of bias. We planned to construct a 'Risk of bias' table, in which we would identify whether any of these types of bias were present in each included study, the risk that the bias compromised the results of the study, and supporting evidence for these judgments. Two review authors planned to conduct these assessments and would resolve any disagreements by consensus or arbitration by a third review author.
Measures of treatment effect
We planned to present dichotomous data as risk ratios with 95% confidence intervals (CIs).
Primary outcome: proportion of eyes with recurrence of tumours within three years from treatment
Secondary outcome: proportion of eyes (enucleated or treated with EBRT, eyes with high‐risk histopathological features and eyes with extraocular tumour spread), proportion of participants (distant metastatic spread and death), visual acuity (better versus worse than 6/60)
Adverse outcomes
We planned to present continuous data as mean differences with 95% CIs.
Secondary outcome: number of additional treatments, visual acuity and visual field when assessed as means or mean deviation
Unit of analysis issues
In our analyses, we planned to compare eyes treated with chemotherapy and additional laser therapy versus those treated with chemotherapy alone. Trials may randomise tumours, eyes or participants to the intervention or comparator. We planned to record details of study design with respect to treatment modality (chemotherapy with/without laser therapy), treatment of one or more tumours, one or both eyes of the participant. If a study randomly allocated participants to treatment but included and reported all tumours or both eyes, we planned to analyse the data as 'clustered data', that is, we planned to adjust for within‐person correlation. If insufficient information were available in the article, we planned to contact the study authors for clarification.
Dealing with missing data
We anticipated that missing data would be present within the included studies. We planned to analyse studies using an available‐case analysis. We planned to record the percentage of missing data from each intervention group in all included studies, and examine the reasons, to determine whether it met the assumption of data being missing at random. We planned to consider studies that exhibited an unequal rate (greater than 20%) of missing data between intervention groups as at risk of attrition bias.
Assessment of heterogeneity
We planned to assess heterogeneity and inconsistency among trials statistically using the I² statistic value to assess if variability in effect was due to sampling error (Higgins 2003). We also planned to assess diversity between the included studies by reviewing participant characteristics and trial methodology.
Assessment of reporting biases
We planned to assess selective outcome reporting when we judged individual studies for risk of bias. We planned to assess reporting biases by examining funnel plots when we included 10 or more RCTs in meta‐analyses.
Data synthesis
When we did not detect any substantial clinical or methodological heterogeneity, we planned to combine the results in a meta‐analysis. If there were three or fewer eligible RCTs, then we planned to use a fixed‐effect model for the meta‐analysis. If more than three trials met the inclusion criteria, we planned to use a random‐effects model. If substantial heterogeneity was present and the direction of effect was inconsistent across the included studies, we planned not to combine the data in a meta‐analysis but to present a descriptive summary.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis according to tumour grouping (i.e. Reese‐Elsworth Classification I‐V (R‐E I‐V) and International Classification of Retinoblastoma groups A‐E (ICRB A‐E)).
Sensitivity analysis
We planned to conduct one sensitivity analysis, and exclude studies that were at high risk of bias in one or more domain.
'Summary of findings' table
Two review authors planned to independently assess the overall quality of the evidence for each outcome using the GRADE classification (GRADEpro 2014). We planned to include the following outcomes in the 'Summary of findings' table.
The proportion of eyes with recurrence of tumours within three years from treatment
The proportion of eyes that required EBRT or enucleation at any time point
The proportion of enucleated eyes at any time point with high‐risk histopathological characteristics for tumour progression and metastasis
The proportion of enucleated eyes with extraocular tumour spread at any time point
The proportion of participants with distant metastatic spread at any time point
The proportion of participants that died due to metastatic spread at any time point
Adverse outcomes
Results
Description of studies
Results of the search
The electronic searches yielded a total of 323 references (Figure 1). The Cochrane Information Specialist removed 50 duplicate records, screened the remaining 273 records, and removed 30 references that were not relevant to the scope of this review. We screened the remaining 243 references and discarded 238 reports as not relevant. We reviewed four full‐text reports of four studies for possible inclusion in the review and contacted the authors of one conference abstract. After this assessment, we excluded all five references as none met the inclusion criteria for this review (see Characteristics of excluded studies for details). Three out of the five were non‐RCTs, one was a report of a randomised trial; however randomisation was of chemotherapy type rather than of chemotherapy versus chemotherapy plus laser treatment, and the conference abstract was a duplicate report of the latter trial. We did not identify any ongoing studies from our searches of the clinical trials registries.
1.
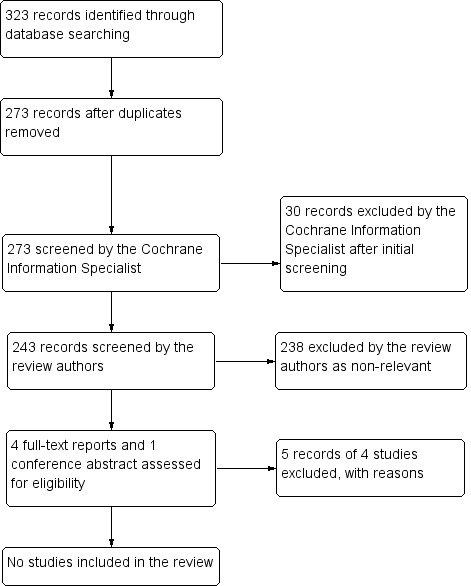
Study flow diagram
Included studies
We did not find any studies that met our inclusion criteria.
Excluded studies
We excluded five records of four studies (Friedman 2000; Levy 1998; Lumbroso‐Le Rouic 2016; Shields 1997) and details can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We included no trials in the review.
Effects of interventions
We included no trials in the review.
Discussion
Summary of main results
There are currently no RCTs reporting the effectiveness and safety of laser treatment in addition to systemic chemotherapy for intraocular postequatorial retinoblastoma.
Overall completeness and applicability of evidence
Not applicable.
Quality of the evidence
We did not identify any trials for inclusion in this review.
Potential biases in the review process
The review authors may not be aware of individuals or organisations who have conducted or may be conducting relevant RCTs, therefore it is possible that relevant RCTs have not been identified.
Agreements and disagreements with other studies or reviews
There is no new evidence provided by this review.
Authors' conclusions
Implications for practice.
There is currently no high‐quality evidence for the outcomes of laser treatment in addition to systemic chemotherapy for intraocular postequatorial retinoblastoma. Practitioners need to take this into account when considering treatment options for intraocular postequatorial retinoblastoma.
Implications for research.
Currently there are no clinical trials offering standardised evidence of the safety and effectiveness of laser treatment in addition to systemic chemotherapy for intraocular postequatorial retinoblastoma. Future research should be conducted in the form of RCTs to assess the long‐term efficacy and safety of laser therapy in addition to chemotherapy for postequatorial retinoblastoma. As retinoblastoma is a rare malignancy, it is likely that only a multicentre collaborative effort will enable the conducting of such a study.
Acknowledgements
Cochrane Eyes and Vision have created the electronic search strategies for this review and will execute them. We are extremely grateful for the contributions the late Dr Judith Kingston made to earlier drafts of this protocol. We thank Jennifer Evans and Anupa Shah for their assistance throughout the editorial process. We also thank Cochrane Childhood Cancer for suggesting peer reviewers for this protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Retinal Neoplasms] explode all trees #2 retinoblastoma* #3 retina* near/3 (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma* or adenocarcinoma* or sarcoma*) #4 MeSH descriptor: [Eye Enucleation] explode all trees #5 enucleat* or envicerat* #6 (eye* or globe) near/2 (remov* or extract*) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Antineoplastic Agents] explode all trees #9 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] this term only #10 MeSH descriptor: [Combined Modality Therapy] explode all trees #11 chemotherap* or chemoreduct* or chemothermotherap* or chemoprophyla* #12 MeSH descriptor: [Vincristine] this term only #13 MeSH descriptor: [Etoposide] this term only #14 MeSH descriptor: [Carboplatin] this term only #15 vincristine* or etoposide* or carboplatin* #16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Lasers] explode all trees #18 MeSH descriptor: [Light Coagulation] this term only #19 laser* #20 photocoagulat* #21 transpupillary near/2 thermotherap* #22 TTT #23 #17 or #18 or #19 or #20 or #21 or #22 #24 #7 and #16 and #23
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp Retinal Neoplasms/ 14. retinoblastoma$.tw. 15. (retina$ adj3 (cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or carcinoma$ or adenocarcinoma$ or sarcoma$)).tw. 16. Eye Enucleation/ 17. (enucleat$ or envicerat$).tw. 18. ((eye$ or globe) adj2 (remov$ or extract$)).tw. 19. or/13‐18 20. exp Antineoplastic Agents/ 21. Antineoplastic Combined Chemotherapy Protocols/ 22. exp Combined Modality Therapy/ 23. (chemotherap$ or chemoreduct$ or chemothermotherap$ or chemoprophyla$).tw. 24. Vincristine/ 25. Etoposide/ 26. Carboplatin/ 27. (vincristine$ or etoposide$ or carboplatin$).tw. 28. or/20‐27 29. exp Lasers/ 30. Light Coagulation/ 31. laser$.tw. 32. photocoagulat$.tw. 33. (transpupillary adj2 thermotherap$).tw. 34. TTT.tw. 35. or/29‐34 36. 12 and 19 and 28 and 35
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp eye tumor/ 34. retinoblastoma$.tw. 35. (retina$ adj3 (cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or carcinoma$ or adenocarcinoma$ or sarcoma$)).tw. 36. Enucleation/ 37. (enucleat$ or envicerat$).tw. 38. ((eye$ or globe) adj2 (remov$ or extract$)).tw. 39. or/33‐38 40. exp chemotherapy/ 41. antineoplastic agent/ 42. multimodality cancer therapy/ 43. chemoprophylaxis/ 44. (chemotherap$ or chemoreduct$ or chemothermotherap$ or chemoprophyla$).tw. 45. vincristine/ 46. etoposide/ 47. carboplatin/ 48. (vincristine$ or etoposide$ or carboplatin$).tw. 49. or/40‐48 50. exp laser/ 51. exp laser coagulation/ 52. laser$.tw. 53. photocoagulat$.tw. 54. (transpupillary adj2 thermotherap$).tw. 55. TTT.tw. 56. or/50‐55 57. 39 and 49 and 56 58. 32 and 57
Appendix 4. LILACS search strategy
retinoblastoma or enucleat$ or evicerat$ or retinal cancer$ or retinal neoplasm$ or retinal tumor$ or retinal tumour$ or retinal malignan$ or retinal carcinoma$ or retinal adenocarcinoma$ or retinal sarcoma and chemotherapy or antineoplastic or chemoreduction or chemothermotherapy or chemoprophylaxis or vincristine or etoposide or carboplatin and laser$ or photocoagulat$ or coagulat$ or thermotherap$
Appendix 5. ISRCTN search strategy
(retinoblastoma OR enucleation OR evicerate OR retinal cancer OR retinal neoplasm OR retinal tumor OR retinal tumour OR retinal malignant OR retinal carcinoma OR retinal adenocarcinoma OR retinal sarcoma) AND (chemotherapy OR antineoplastic OR chemoreduction OR chemothermotherapy OR chemoprophylaxis OR vincristine OR etoposide OR carboplatin)
Appendix 6. ClinicalTrials.gov search strategy
(retinoblastoma OR enucleation OR evicerate OR retinal cancer OR retinal neoplasm OR retinal tumor OR retinal tumour OR retinal malignant OR retinal carcinoma OR retinal adenocarcinoma OR retinal sarcoma) AND (chemotherapy OR antineoplastic OR chemoreduction OR chemothermotherapy OR chemoprophylaxis OR vincristine OR etoposide OR carboplatin)
Appendix 7. WHO ICTRP search strategy
(retinoblastoma OR enucleation OR evicerate OR retinal cancer OR retinal neoplasm OR retinal tumor OR retinal tumour OR retinal malignan OR retinal carcinoma OR retinal adenocarcinoma OR retinal sarcoma) = CONDITION AND (chemotherapy OR antineoplastic OR chemoreduction OR chemothermotherapy OR chemoprophylaxis OR vincristine OR etoposide OR carboplatin) = INTERVENTION
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Exclusions after randomisation Losses to follow‐up Number randomised/analysed How were missing data handled? e.g. available‐case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or unit of randomisation/unit of analysis |
|
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If the study only reports these data for the participants who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (N = ) Comparator (N = ) (See MECIR 65 and 70) |
|
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports (See MECIR R70) |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants month/year (mm/yr) to mm/yr | Full study name: (if applicable) Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | ||
| Declarations of interest (See MECIR 69) |
||
| Abbreviations MECIR: Methodological Expectations of Cochrane Intervention Reviews; RCT: randomised controlled trial | ||
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Friedman 2000 | Prospective but non‐randomised single‐arm clinical trial |
| Levy 1998 | Comparative study in which cohort 1 consisted of participants treated with intravenous etoposide and carboplatin only and cohort 2 consisted of participants treated similarly to cohort 1 followed by intravenous carboplatin in conjunction with diode laser. No randomisation |
| Lumbroso‐Le Rouic 2016 | Randomised study comparing participants receiving intravenous vincristine and carboplatin versus etoposide and carboplatin. Following chemotherapy, participants also received laser therapy, among other treatments, at the discretion of the treating clinician. However, adjuvant therapy was given in a non‐randomised fashion. |
| Shields 1997 | A prospective but non‐randomised clinical trial comparing participants treated with intravenous vincristine, etoposide and carboplatin with/without adjuvant treatments (i.e. diode laser and other modalities) |
Contributions of authors
IDF did a literature search and wrote the first review draft. All co‐authors performed a literature search, examined the review and provided intellectual input. AWS assessed the Methods section of the protocol and provided comments. MAR edited the draft significantly.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
IDF: none known KPJ: none known AWS: none known MSS: none known MAR: none known
New
References
References to studies excluded from this review
Friedman 2000 {published data only}
- Friedman DL, Himelstein B, Shields CL, Shields JA, Needle M, Miller D, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. Journal of Clinical Oncology 2000;18(1):12‐7. [DOI] [PubMed] [Google Scholar]
Levy 1998 {published data only}
- Levy C, Doz F, Quintana E, Pacquement H, Michon J, Schlienger P, et al. Role of chemotherapy alone or in combination with hyperthermia in the primary treatment of intra ocular retinoblastoma: preliminary results. British Journal of Ophthalmology 1998;82(10):1154‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumbroso‐Le Rouic 2016 {published and unpublished data}
- Aerts I, Livia LL, Hajage D, Christine LG, Savignoni A, Algret, N, et al. Conservative treatment of intraocular retinoblastoma: a prospective phase II randomized trial of neoadjuvant chemotherapy followed by local treatments and chemothermotherapy. Journal of Clinical Oncology 2013;30(15 Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbroso‐Le Rouic L, Aerts I, Hajage D, Lévy‐Gabriel C, Savignoni A, Algret N, et al. Conservative treatment of retinoblastoma: a prospective phase II randomized trial of neoadjuvant chemotherapy followed by local treatments and chemothermotherapy. Eye 2016;30(1):46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1997 {published data only}
- Shields CL, Shields JA, Needle M, Potter P, Kheterpal S, Hamada A, et al. Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology 1997;104(12):2101‐11. [DOI] [PubMed] [Google Scholar]
Additional references
Abramson 1998
- Abramson DH, Frank CM, Susman M, Whalen MP, Dunkel IJ, Boyd NW 3rd. Presenting signs of retinoblastoma. Journal of Pediatrics 1998;132(3 Pt 1):505‐8. [DOI] [PubMed] [Google Scholar]
Abramson 2004a
- Abramson DH, Schefler AC. Transpupillary thermotherapy as initial treatment for small intraocular retinoblastoma: technique and predictors of success. Ophthalmology 2004;111(5):984‐91. [DOI] [PubMed] [Google Scholar]
Abramson 2004b
- Abramson DH, Melson MR, Servodidio C. Visual fields in retinoblastoma survivors. Archives of Ophthalmology 2004;122(9):1324‐30. [DOI] [PubMed] [Google Scholar]
Abramson 2008
- Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology 2008;115(8):1398‐404. [DOI] [PubMed] [Google Scholar]
Abramson 2015
- Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmology 2015;133(11):1341‐7. [DOI] [PubMed] [Google Scholar]
Bowman 2008
- Bowman RJ, Mafwiri M, Luthert P, Luande J, Wood M. Outcome of retinoblastoma in east Africa. Pediatric Blood and Cancer 2008;50(1):160‐2. [DOI] [PubMed] [Google Scholar]
Brichard 2002
- Brichard B, Bruycker JJ, Potter P, Neven B, Vermylen C, Cornu G. Combined chemotherapy and local treatment in the management of intraocular retinoblastoma. Medical and Pediatric Oncology 2002;38(6):411‐5. [DOI] [PubMed] [Google Scholar]
Covidence 2015 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Version accessed prior to 18 April 2016. Melbourne: Veritas Health Innovation, 2015.
Da Silva 1987
- Silva VF, Raaphorst GP, Goyal R, Feeley M. Drug cytotoxicity at elevated temperature. In vitro study on the U‐87MG glioma cell line. Journal of Neurosurgery 1987;67(6):885‐8. [DOI] [PubMed] [Google Scholar]
De Jong 2015
- Jong MC, Kors WA, Graaf P, Castelijns JA, Moll AC, Kivelä T. The incidence of trilateral retinoblastoma: a systematic review and meta‐analysis. American Journal of Ophthalmology 2015;160(6):1116‐26.e.5. [DOI] [PubMed] [Google Scholar]
Dimaras 2012
- Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet 2012;379(9824):1436‐46. [DOI] [PubMed] [Google Scholar]
Doz 1995
- Doz F, Neuenschwander S, Plantaz D, Courbon B, Gentet JC, Bouffet E, et al. Etoposide and carboplatin in extraocular retinoblastoma: a study by the Société Française d'Oncologie Pédiatrique. Journal of Clinical Oncology 1995;13(4):902‐9. [DOI] [PubMed] [Google Scholar]
Dropcho 1992
- Dropcho EJ, Rosenfeld SS, Morawetz RB, Vitek J, Brothers M, Gorum T, et al. Preradiation intracarotid cisplatin treatment of newly diagnosed anaplastic gliomas. The CNS Cancer Consortium. Journal of Clinical Oncology 1992;10(3):452‐8. [DOI] [PubMed] [Google Scholar]
Eng 1993
- Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, et al. Mortality from second tumors among long‐term survivors of retinoblastoma. Journal of the National Cancer Institute 1993;85(14):1121‐8. [DOI] [PubMed] [Google Scholar]
Ericson 1961
- Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmologica 1961;39:569‐76. [DOI] [PubMed] [Google Scholar]
Fletcher 2004
- Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. Journal of the National Cancer Institute 2004;96(5):357‐63. [DOI] [PubMed] [Google Scholar]
Follézou 1989
- Follézou JY, Fauchon F, Chiras J. Intra‐arterial infusion of carboplatin in the treatment of malignant gliomas: a phase II study. Neoplasma 1989;36(3):349‐52. [PubMed] [Google Scholar]
Gallie 1996
- Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Archives of Ophthalmology 1996;114(11):1321‐8. [DOI] [PubMed] [Google Scholar]
Gaynon 1994
- Gaynon PS. Carboplatin in pediatric malignancies. Seminars in Oncology 1994;21(5 Suppl 12):65‐76. [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gombos 2002
- Gombos DS, Kelly A, Coen PG, Kingston JE, Hungerford JL. Retinoblastoma treated with primary chemotherapy alone: the significance of tumour size, location, and age. British Journal of Ophthalmology 2002;86(1):80‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gombos 2006
- Gombos DS, Cauchi PA, Hungerford JL, Addison P, Coen PG, Kingston JE. Vitreous relapse following primary chemotherapy for retinoblastoma: is adjuvant diode laser a risk factor?. British Journal of Ophthalmology 2006;90(9):1168‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gombos 2007
- Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor?. Ophthalmology 2007;114(7):1378‐83. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 18 April 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harbour 1996
- Harbour JW, Murray TG, Hamasaki D, Cicciarelli N, Hernández E, Smith B, et al. Local carboplatin therapy in transgenic murine retinoblastoma. Investigative Ophthalmology and Visual Science 1996;37(9):1892‐8. [PubMed] [Google Scholar]
Herman 1994
- Herman TS, Teicher BA. Summary of studies adding systemic chemotherapy to local hyperthermia and radiation. International Journal of Hyperthermia 1994;10(3):443‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kaneko 2003
- Kaneko A, Suzuki S. Eye‐preservation treatment of retinoblastoma with vitreous seeding. Japanese Journal of Clinical Oncology 2003;33(12):601‐7. [PubMed] [Google Scholar]
Khelfaoui 1996
- Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer 1996;77(6):1206‐13. [PubMed] [Google Scholar]
Kingston 1996
- Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Archives of Ophthalmology 1996;114(11):1339‐43. [DOI] [PubMed] [Google Scholar]
Kivela 2009
Kleinerman 2005
- Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, et al. Risk of new cancers after radiotherapy in long‐term survivors of retinoblastoma: an extended follow‐up. Journal of Clinical Oncology 2005;23(10):2272‐9. [DOI] [PubMed] [Google Scholar]
Knudson 1971
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences 1971;68(4):820‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kupfer 1953
- Kupfer C. Retinoblastoma treated with intravenous nitrogen mustard. American Journal of Ophthalmology 1953;36(12):1721‐3. [DOI] [PubMed] [Google Scholar]
Lagendijk 1982
- Lagendijk JJ. A microwave heating technique for the hyperthermic treatment of tumours in the eye, especially retinoblastoma. Physics in Medicine and Biology 1982;27(11):1313‐24. [DOI] [PubMed] [Google Scholar]
Lambert 2008
- Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin‐based chemotherapy. Pediatric Blood and Cancer 2008;50(2):223‐6. [DOI] [PubMed] [Google Scholar]
Larner 1995
- Larner JM, Phillips CD, Dion JE, Jensen ME, Newman SA, Jane JA. A phase 1‐2 trial of superselective carboplatin, low‐dose infusional 5‐fluorouracil and concurrent radiation for high‐grade gliomas. American Journal of Clinical Oncology 1995;18(1):1‐7. [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee TC, Lee SW, Dinkin MJ, Ober MD, Beaverson KL, Abramson DH. Chorioretinal scar growth after 810‐nanometer laser treatment for retinoblastoma. Ophthalmology 2004;111(5):992‐6. [DOI] [PubMed] [Google Scholar]
Lumbroso 2002
- Lumbroso L, Doz F, Urbieta M, Levy C, Bours D, Asselain B, et al. Chemothermotherapy in the management of retinoblastoma. Ophthalmology 2002;109(6):1130‐6. [DOI] [PubMed] [Google Scholar]
MacCarthy 2009
- MacCarthy A, Birch JM, Draper GJ, Hungerford JL, Kingston JE, Kroll ME, et al. Retinoblastoma in Great Britain 1963‐2002. British Journal of Ophthalmology 2009;93(1):33‐7. [DOI] [PubMed] [Google Scholar]
Menon 2009
- Menon BS, Alagaratnam J, Juraida E, Mohamed M, Ibrahim H, Naing NN. Late presentation of retinoblastoma in Malaysia. Pediatric Blood and Cancer 2009;52(2):215‐7. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munier 2012
- Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. British Journal of Ophthalmology 2012;96(8):1078‐83. [DOI] [PubMed] [Google Scholar]
Murphree 1996
- Murphree AL, Villablanca JG, Deegan WF 3rd, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Archives of Ophthalmology 1996;114(11):1348‐56. [DOI] [PubMed] [Google Scholar]
Murphree 2005
- Murphree LA. Intraocular retinoblastoma: the case for a new group classification. Ophthalmology Clinics of North America 2005;18(1):45‐53. [DOI] [PubMed] [Google Scholar]
Murray 1997
- Murray TG, Cicciarelli N, McCabe CM, Ksander B, Feuer W, Schiffman J, et al. In vitro efficacy of carboplatin and hyperthermia in a murine retinoblastoma cell line. Investigative Ophthalmology and Visual Science 1997;38(12):2516‐22. [PubMed] [Google Scholar]
Novetsky 2009
- Novetsky DE, Abramson DH, Kim JW, Dunkel IJ. Published international classification of retinoblastoma (ICRB) definitions contain inconsistencies‐‐an analysis of impact. Ophthalmic Genetics 2009;30(1):40‐4. [DOI] [PubMed] [Google Scholar]
Reese 1949
- Reese AB, Merriam GR Jr, Martin HE. Treatment of bilateral retinoblastoma by irradiation and surgery; report on 15‐year results. American Journal of Ophthalmology 1949;32(2):175‐90. [DOI] [PubMed] [Google Scholar]
Reese 1958
- Reese AB, Hyman GA, Tapley ND, Forrest AW. The treatment of retinoblastoma by X‐ray and triethylene melamine. AMA Archives of Ophthalmology 1958;60(5):897‐906. [DOI] [PubMed] [Google Scholar]
Reese 1964
- Reese AB, Ellsworth RM. Management of retinoblastoma. Annals of the New York Academy of Sciences 1964;114(2):958‐62. [PubMed] [Google Scholar]
Review Manager 5 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riccardi 1992
- Riccardi R, Riccardi A, Rocco C, Carelli G, Tartaglia RL, Lasorella A, et al. Cerebrospinal fluid pharmacokinetics of carboplatin in children with brain tumors. Cancer Chemotherapy and Pharmacology 1992;30(1):21‐4. [DOI] [PubMed] [Google Scholar]
Schefler 2007
- Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology 2007;114(1):162‐9. [DOI] [PubMed] [Google Scholar]
Seregard 1995
- Seregard S, Kock E, af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. British Journal of Ophthalmology 1995;79(2):194‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1996
- Shields CL, Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Archives of Ophthalmology 1996;114(11):1330‐8. [DOI] [PubMed] [Google Scholar]
Shields 1999
- Shields CL, Santos MC, Diniz W, Gündüz K, Mercado G, Cater JR, et al. Thermotherapy for retinoblastoma. Archives of Ophthalmology 1999;117(7):885‐93. [DOI] [PubMed] [Google Scholar]
Shields 2002
- Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Singh A, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. American Journal of Ophthalmology 2002;133(5):657‐64. [DOI] [PubMed] [Google Scholar]
Shields 2004
- Shields CL, Meadows AT, Leahey AM, Shields JA. Continuing challenges in the management of retinoblastoma with chemotherapy. Retina 2004;24(6):849‐62. [DOI] [PubMed] [Google Scholar]
Shields 2005
- Shields CL, Mashayekhi A, Cater J, Shelil A, Ness S, Meadows AT, et al. Macular retinoblastoma managed with chemoreduction: analysis of tumor control with or without adjuvant thermotherapy in 68 tumors. Archives of Ophthalmology 2005;123(6):765‐73. [DOI] [PubMed] [Google Scholar]
Stallard 1952
- Stallard HB. Irradiation of retinoblastoma (glioma retinae). Lancet 1952;1(6717):1046‐9. [DOI] [PubMed] [Google Scholar]
Tapazoglou 1991
- Tapazoglou E, Cohen JD, Schmitt CL, Khatana A, Sapareto SA, Robins HI. Whole body hyperthermia and carboplatin: cytotoxicity for murine leukaemia and normal marrow. British Journal of Cancer 1991;64(3):528‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turaka 2012
- Turaka K, Shields CL, Meadows AT, Leahey A. Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatric Blood and Cancer 2012;59(1):121‐5. [DOI] [PubMed] [Google Scholar]
White 1983
- White L. The role of chemotherapy in the treatment of retinoblastoma. Retina 1983;3(3):194‐9. [DOI] [PubMed] [Google Scholar]
Wilson 2001
- Wilson MW, Rodriguez‐Galindo C, Haik BG, Moshfeghi DM, Merchant TE, Pratt CB. Multiagent chemotherapy as neoadjuvant treatment for multifocal intraocular retinoblastoma. Ophthalmology 2001;108(11):2106‐14. [DOI] [PubMed] [Google Scholar]
Yamane 2004
- Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. International Journal of Clinical Oncology 2004;9(2):69‐73. [DOI] [PubMed] [Google Scholar]


