Abstract
Background
This review is an update of "Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults" last updated in Issue 4, 2009. Ketoprofen is a non‐selective nonsteroidal anti‐inflammatory drug (NSAID) used to treat acute and chronic painful conditions. Dexketoprofen is the (S)‐enantiomer, which is believed to confer analgesia. Theoretically dexketoprofen is expected to provide equivalent analgesia to ketoprofen at half the dose, with a consequent reduction in gastrointestinal adverse events. This review is one of a series on oral analgesics for acute postoperative pain. Individual reviews have been brought together in two overviews to provide information about the relative efficacy and harm of the different interventions.
Objectives
To assess the efficacy and safety of single dose oral ketoprofen and oral dexketoprofen compared with placebo for acute postoperative pain, using methods that permit comparison with other analgesics evaluated in the same way, and criteria of efficacy recommended by an in‐depth study at the individual patient level.
Search methods
For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase from 2009 to 28 March 2017. We also searched the reference lists of retrieved studies and reviews, and two online clinical trial registries.
Selection criteria
Randomised, double‐blind, placebo‐controlled trials of single dose orally administered ketoprofen or dexketoprofen in adults with moderate to severe acute postoperative pain.
Data collection and analysis
Two review authors independently considered studies for inclusion in the review, examined issues of study quality and potential bias, and extracted data. For dichotomous outcomes, we calculated risk ratio (RR) and number needed to treat for an additional beneficial outcome (NNT) or harmful outcome (NNH) with 95% confidence intervals (CI) for ketoprofen and dexketoprofen, compared with placebo, where there were sufficient data. We collected information on the number of participants with at least 50% of the maximum possible pain relief over six hours, the median time to use of rescue medication, and the proportion of participants requiring rescue medication. We also collected information on adverse events and withdrawals. We assessed the quality of the evidence using GRADE, and created 'Summary of findings' tables.
Main results
This updated review included 24 studies; six additional studies added 1001 participants involved in comparisons of ketoprofen or dexketoprofen and placebo, with a 12% increase in participants taking ketoprofen and a 65% increase for dexketoprofen. Most participants (70%) were women. Dental studies typically involved young participants (mean age 20 to 30 years); other types of surgery involved older participants (mean age 37 to 68 years). Overall, we judged the studies at high risk of bias only for small size, which can lead to an overestimation of benefit.
Ketoprofen doses ranged between 6.5 mg and 150 mg. The proportion of participants achieving at least 50% pain relief over six hours with the usual ketoprofen oral dose of 50 mg was 57%, compared to 23% with placebo, giving an NNT of 2.9 (95% CI 2.4 to 3.7) (RR 2.5, 95% CI 2.0 to 3.1; 594 participants; 8 studies; high quality evidence). Efficacy was significantly better in dental studies (NNT 1.8) than other surgery (NNT 4.2). The proportion of participants using rescue medication within six hours was lower with ketoprofen (32%) than with placebo (75%), giving a number needed to treat to prevent use of rescue medication (NNTp) of 2.3 (95% CI 1.8 to 3.1); 263 participants; 4 studies; high quality evidence). Median time to remedication estimates were poorly reported. Reports of any adverse event were similar with ketoprofen (18%) and placebo (11%) (RR 1.6, 95% CI 0.98 to 2.8; 342 participants; 5 studies; high quality evidence). No study reported any serious adverse events (very low quality evidence).
Dexketoprofen doses ranged between 5 mg and 100 mg. The proportion of participants achieving at least 50% pain relief over six hours with the usual dexketoprofen oral dose of 20 mg or 25 mg was 52%, compared to 27% with placebo, giving an NNT of 4.1 (95% CI 3.3 to 5.2) (RR 2.0, 95% CI 1.6 to 2.2; 1177 participants; 8 studies; high quality evidence). Efficacy was significantly better in dental studies (NNT 2.7) than other surgery (NNT 5.7). The proportion of participants using rescue medication within six hours was lower with dexketoprofen (47%) than placebo (69%), giving an NNTp of 4.7 (95% CI 3.3 to 8.0); 445 participants; 5 studies; high quality evidence). Median time to remedication estimates were poorly reported. Reports of any adverse event were similar with dexketoprofen (14%) and placebo (10%) (RR 1.4, 95% CI 0.89 to 2.2; 536 participants, 6 studies; high quality evidence). No study reported any serious adverse events (very low quality evidence).
Authors' conclusions
Ketoprofen at doses of 25 mg to 100 mg is an effective analgesic in moderate to severe acute postoperative pain with an NNT for at least 50% pain relief of 2.9 with a 50 mg dose. This is similar to that of commonly used NSAIDs such as ibuprofen (NNT 2.5 for 400 mg dose) and diclofenac (NNT 2.7 for 50 mg dose). Dexketoprofen is also effective with an NNT of 4.1 in the dose range 10 mg to 25 mg. Differential efficacy between dental surgery and other types of surgery seen for both drugs is unusual. Both drugs were well tolerated in single doses.
Plain language summary
Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults
Bottom line
This review found that most people with moderate or severe pain after an operation get good pain relief from taking ketoprofen 50 mg or dexketoprofen 25 mg.
Background
Acute pain is short‐lived pain often felt soon after injury, including after operations. Most people who have an operation have moderate or severe pain afterwards. Painkillers are tested in people with acute pain, often following the removal of wisdom teeth. This pain is usually treated with painkillers taken by mouth. We believe these results can be applied to other acute painful conditions.
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are painkillers that usually provide good pain relief to a high proportion of people with moderate or severe pain after an operation when taken by mouth by people who are able to swallow. This review updated the evidence on two closely related NSAIDs, ketoprofen and dexketoprofen. Ketoprofen has two forms, one of which, dexketoprofen, is the form that produces pain relief.
Study characteristics
In March 2017, we found 24 studies involving 5220 people. The main comparison was between usual oral doses of ketoprofen 50 mg and placebo, and dexketoprofen 25 mg and placebo. The studies tested single doses after wisdom tooth extraction, and after other types of surgery, mainly hip replacement and gynaecological operations. Studies included adults over a range of ages, and 7 out of 10 participants were women. The main outcome was participants having at least half of the maximum possible pain relief over the first six hours after taking the tablets.
Key results
For ketoprofen, there were 594 participants in eight studies in the comparison with placebo (a dummy tablet). About 6 in 10 achieved at least half of the maximum possible pain relief with ketoprofen 50 mg compared with 2 in 10 with placebo. The number of participants who needed more painkillers within six hours was 5 in 10 with ketoprofen compared with 8 in 10 with placebo.
For dexketoprofen, there were 1177 participants in eight studies in the comparison with placebo. About 5 in 10 achieved at least half of the maximum possible pain relief with dexketoprofen 25 mg compared with 3 in 10 with placebo. The number of participants who needed more painkillers within six hours was 5 in 10 with dexketoprofen compared with 7 in 10 with placebo.
About 1 or 2 in 10 people had any side effects with ketoprofen, dexketoprofen, or placebo. Serious side effects were uncommon. Few people dropped out of the studies for any reason.
Quality of the evidence
The quality of the evidence was judged to be high for most outcomes. This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low.
Summary of findings
Summary of findings for the main comparison. Ketoprofen 25 mg compared with placebo for acute postoperative pain.
| Ketoprofen 25 mg compared with placebo for acute postoperative pain | ||||||
|
Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence (GRADE) | Comments |
| Participants with ≥ 50% pain relief over 6 hours | 620 in 1000 | 120 in 1000 | RR 4.9 (3.5 to 6.9) NNT 2.0 (1.8 to 2.3) |
8 studies 535 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 5.3 hours (4.6 hours) |
1.6 hours (2.5 hours) |
Not estimated | 2 studies 188 participants (5 studies 277 participants) |
Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 460 in 1000 | 79 in 1000 | RR 0.60 (0.52 to 0.69) NNTp 3.0 (2.4 to 4.1) |
6 studies 402 participants |
Moderate | Modest numbers of participants and events. |
| Participants with ≥ 1 adverse event following a single dose | 100 in 1000 | 91 in 1000 | RR 1.2 (0.68 to 2.0) NNH not calculated |
7 studies 490 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 8 studies 535 participants |
Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
Summary of findings 2. Ketoprofen 50 mg compared with placebo for acute postoperative pain.
| Ketoprofen 50 mg compared with placebo for acute postoperative pain | ||||||
|
Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 50 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence (GRADE) | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 570 in 1000 | 230 in 1000 | RR 2.5 (2.0 to 3.1) NNT 2.9 (2.4 to 3.7) |
8 studies 594 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | Approximately 5 hours (3.4 hours) | Approximately 3 hours (2.5 hours) | Not estimated | 1 study 77 participants (5 studies, 342 participants) |
Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 320 in 1000 | 750 in 1000 | RR 0.42 (0.33 to 0.52) NNTp 2.3 (1.8 to 3.1) |
4 studies 263 participants |
High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 180 in 1000 | 110 in 1000 | RR 1.6 (0.98 to 2.8) NNH not calculated |
5 studies 342 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 688 participants |
Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
Summary of findings 3. Dexketoprofen 10 mg‐12.5 mg compared with placebo for acute postoperative pain.
| Dexketoprofen 10 mg‐12.5 mg compared with placebo for acute postoperative pain | ||||||
|
Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 10 mg‐12.5 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence (GRADE) | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 440 in 1000 | 180 in 1000 | RR 2.4 (1.8 to 3.3) NNT 3.9 (3.0 to 5.7) |
5 studies 480 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 3.6 hours (4.9 hours) | 1.4 hours (3.6 hours) | Not estimated | 1 study 122 participants (3 studies 253 participants) |
Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 490 in 1000 | 680 in 1000 | RR 0.73 (0.61 to 0.86) NNTp 5.3 (3.5 to 11) |
4 studies 373 participants |
High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 68 in 1000 | 96 in 1000 | RR 0.70 (0.36 to 1.4) NNH not calculated |
4 studies 380 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 6 studies 574 participants |
Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
Summary of findings 4. Dexketoprofen 20 mg or 25 mg compared with placebo for acute postoperative pain.
| Dexketoprofen 20 mg or 25 mg compared with placebo for acute postoperative pain | ||||||
|
Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 20 mg or 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence (GRADE) | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 520 in 1000 | 270 in 1000 | RR 2.0 (1.6 to 2.2) NNT 4.1 (3.3 to 5.2) |
8 studies 1177 participants |
High quality | Good quality studies, important outcome available, robust numbers |
| Median (mean) time to use of rescue medication | 4.7 hours (5.2 hours) | 1.8 hours (3.6 hours) | Not estimated | 3 studies 281 participants (3 studies, 251 participants) |
Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 470 in 1000 | 690 in 1000 | RR 0.66 (0.56 to 0.78) NNTp 4.7 (3.3 to 8.0) |
5 studies 445 participants |
High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 160 in 1000 | 100 in 1000 | RR 1.4 (0.89 to 2.2) NNH not calculated |
6 studies 536 participants |
High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 1271 participants |
Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for one additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This review is an update of an earlier review (Barden 2009), and includes new studies. We have updated the methods to conform with current standards, including the use of 'Risk of bias' and 'Summary of findings' tables, and the GRADE system to assess the quality of evidence.
Description of the condition
Acute pain occurs as a result of tissue damage either accidentally due to an injury, or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. Acute pain in hospitals is common, with perhaps 40% to 80% of patients experiencing severe pain at some time (Gregory 2016). Prevalence of severe pain is inversely related to the use of analgesics, at least in Italian hospitals (Visentin 2005), although concentrated efforts to eliminate pain can reduce severe pain to 1% or less (Aldington 2011).
The management of postoperative pain and inflammation is a critical component of patient care. This is one of a series of reviews whose aim is to increase awareness of the range of analgesics that are potentially available, and present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
The series covers all analgesics licensed for acute postoperative pain in the UK, and dipyrone, which is commonly used in Spain, Portugal, and Latin‐American countries. Individual reviews have been brought together in two overviews to provide information about the relative efficacy and harm of the different interventions (Moore 2015a; Moore 2015b).
Description of the intervention
Acute pain trials
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants is small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about one hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years (McQuay 2012). Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following four to six hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication, it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials, the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Knowing the relative efficacy of different analgesic drugs at various doses can be helpful (Moore 2015a).
Ketoprofen, (RS)2‐(3‐benzoylphenyl)‐propionic acid, is one of the propionic acid class of nonsteroidal anti‐inflammatory drugs (NSAIDs) and has analgesic and antipyretic effects. In some countries, the optically pure S(+)‐enantiomer (dexketoprofen) is available; its trometamol salt is said to be particularly rapidly reabsorbed from the gastrointestinal tract, having a rapid onset of effects. Racemic ketoprofen is used as an analgesic and an anti‐inflammatory agent, and is one of the most potent in vitro inhibitors of prostaglandin synthesis, but is also implicated as having an association with higher risk of serious gastrointestinal bleeding events than other NSAIDs (Hernández‐Diaz 2000; Laporte 2004). The analgesic effect is due to the S(+)‐enantiomer (dexketoprofen), while the R(‐)‐enantiomer is devoid of analgesic activity (Barbanoj 2001). Because the R(‐)‐enantiomer appears to have ulcerogenic activity, at least in rats (Barbanoj 2001; Herrero 2003), the implication is that use of dexketoprofen alone should produce equivalent analgesia to double‐dose ketoprofen (or the same effect as ketoprofen, at half the dose), but at lower risk of harm.
Ketoprofen is available by prescription in a range of strengths from 25 mg to 200 mg capsules; tablet strength varies to some extent in different countries. Some strengths may be available as modified‐release formulations. Dexketoprofen is available as 25 mg tablets. Injectable, topical, and suppository formulations are also available for ketoprofen, and injectable and topical forms for dexketoprofen. In 2015, in England, there were about 25,400 prescriptions for ketoprofen and 2000 for dexketoprofen in primary care (PACT 2016). Ketoprofen is generally prescribed for arthritis‐related inflammatory pains or severe dental pain. It is rarely used for postoperative pain. Dexketoprofen use is less well documented; while it is used in postoperative pain its license typically limits its use to one week or so. Licensed indications vary between countries.
Both drugs are sold by many suppliers worldwide. For acute pain, doses recommended are ketoprofen 25 mg to 50 mg, and dexketoprofen 25 mg.
How the intervention might work
NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclo‐oxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclo‐oxygenase‐dependent prostanoid formation (Hawkey 1999). Since NSAIDs do not depress respiration and do not impair gastrointestinal motility as do opioids (BNF 2016), they are clinically useful for treating pain after minor surgery and day surgery, and have an opiate‐sparing effect after more major surgery (Grahame‐Smith 2002).
Ketoprofen is one of the most potent in vitro inhibitors of prostaglandin synthesis. Dexketoprofen is the S(+)‐enantiomer of ketoprofen. This S(+)‐enantiomer is responsible for the analgesic effect seen with racaemic ketoprofen, while the R(‐)‐enantiomer is devoid of analgesic activity, but appears to have ulcerogenic activity, at least in rats (Barbanoj 2001; Herrero 2003). The implication is that use of dexketoprofen alone should produce the same analgesic effect as ketoprofen, but at half the dose, potentially lowering the risk of harm.
Why it is important to do this review
Since the original review was published, the standards required for Cochrane systematic reviews have been substantially updated, particularly with regard to assessing risk of bias within studies and assessing our confidence in the evidence across studies. New studies are also available. Together, these factors could influence the results and interpretation of the review, so we considered an update was timely.
Objectives
To assess the efficacy and safety of single dose oral ketoprofen and oral dexketoprofen compared with placebo for acute postoperative pain, using methods that permit comparison with other analgesics evaluated in the same way, and criteria of efficacy recommended by an in‐depth study at the individual patient level.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), with at least 10 participants randomly allocated to each treatment group, and double‐blind assessment of participant outcomes. We included multiple dose studies if appropriate data from the first dose were available, and cross‐over studies provided that data from the first period were presented separately or could be obtained.
We excluded:
review articles, case reports, and clinical observations;
studies of experimental pain;
studies of less than four hours' duration or studies that did not present data over four to six hours post dose.
For postpartum pain, we planned to include studies if the pain investigated was due to episiotomy or Caesarean section irrespective of the presence of uterine cramps, but to exclude studies investigating pain due to uterine cramps alone. In the event, there were no studies of postpartum pain.
We required full journal publication, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis.
Types of participants
We included studies of adults (aged over 15 years) with established postoperative pain of moderate to severe intensity following day surgery or inpatient surgery. For studies using a visual analogue scale (VAS) (see 'Glossary'; Appendix 1), we considered that pain intensity of greater than 30 mm equated to pain of at least moderate intensity (Collins 1997).
Types of interventions
Ketoprofen or dexketoprofen, administered as a single oral dose for the relief of acute postoperative pain, and compared with placebo.
Types of outcome measures
Primary outcomes
Participants achieving at least 50% pain relief over four to six hours after taking the medication.
Secondary outcomes
Median (or mean) time to use of rescue medication.
Number of participants using rescue medication over four to six hours after taking the medication.
Number of participants with: any adverse event; any serious adverse event (as reported in the study); withdrawal due to an adverse event, at the end of the (single dose) study period.
Other withdrawals: withdrawals for reasons other than lack of efficacy (participants using rescue medication) or an adverse event at the end of the (single dose) study period.
Quality of the evidence
We used the GRADE system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 2). Two review authors (HG, SD) independently rated the quality of each outcome.
We paid particular attention to inconsistency, where point estimates varied widely across studies or confidence intervals (CIs) of studies showed minimal or no overlap (Guyatt 2011), and potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008a).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, where there were so few data that the results were highly susceptible to the random play of chance, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we report the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We have included 'Summary of findings' tables as set out in the Cochrane Pain, Palliative and Supportive Care Group (PaPaS) author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 11, Higgins 2011). The tables include, where possible, outcomes at least 50% pain relief over four to six hours, median (and mean) time to use of rescue medication, participants using rescue medication over six hours, participants with at least one adverse event following a single dose, and participants with a serious adverse event following a single dose.
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015).
High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differenta is low.
Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differenta is moderate.
Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differenta is high.
Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differenta is very high.
a Substantially different: a large enough difference that it might affect a decision.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, without language restriction.
Cochrane Central Register of Controlled Trials (CENTRAL) (2009, Issue 3 for the original review, and via CRSO from 2009 to 28 March 2017, for this update).
MEDLINE via Ovid (from 1946 to August 2009 for the original review, and from 2009 to 28 March 2017 for this update).
Embase via Ovid (from 1974 to August 2009 for the original review, and from 2009 to 28 March 2017 for this update).
Oxford Pain Relief Database (Jadad 1996a) for the original review. This database is no longer updated.
The search strategies for CENTRAL, MEDLINE, and Embase are in Appendix 3, Appendix 4, and Appendix 5, respectively.
Searching other resources
We searched clinicaltrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials. In addition, we checked reference lists of reviews and retrieved articles for additional studies.
Data collection and analysis
Selection of studies
Two review authors (HG, SD) independently determined eligibility by reading the abstract of each study identified by the search, and independently eliminated studies that clearly did not satisfy inclusion criteria. They obtained full copies of the remaining studies and read them to determine eligibility; a third review author (RAM) would have adjudicated in the event of disagreement, but was not required. We did not anonymise the studies before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart, to show the status of identified studies (Chapter 11, Higgins 2011). We included studies in the review irrespective of whether measured outcome data were reported in a 'usable' way.
Data extraction and management
Two review authors (HG, SD) independently extracted data using a standard form and the third review author (RAM) checked for agreement before entry into Review Manager 5 (RevMan 2014). We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We collected information about the included studies (e.g. study methods, study population, baseline pain intensity) in sufficient detail to complete a 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors (HG, SD) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in Review Manager 5 (RevMan 2014), and assessed criteria for inclusion using the Oxford Quality Score (Jadad 1996b).
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Blinding of outcome assessment (checking for possible detection bias). In this review, outcomes were self‐assessed, so that the same considerations apply to detection bias as performance bias.
Size of study (checking for possible biases confounded by small size (Dechartes 2013; Dechartres 2014; Moore 1998; Nüesch 2010; Thorlund 2011). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference, and number needed to treat for an additional beneficial outcome (NNT) and pooled percentages as absolute measures of effect with 95% CI.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with treatment than with control (placebo or active), we used the term the number needed to treat to prevent an additional harmful event (NNTp).
When significantly more adverse outcomes occurred with treatment compared with control (placebo or active), we used the term the number needed to treat for an additional harmful event (NNH).
Unit of analysis issues
We accepted only randomisation of the individual participant. For multiple dose studies, we used data for the first dose only. There were no cross‐over studies.
Dealing with missing data
The only likely issue with missing data in these studies was from imputation using last observation carried forward when a participant requested rescue medication. We have previously shown that this does not affect results for up to six hours after taking study medication (Moore 2005).
Assessment of heterogeneity
We examined heterogeneity using L'Abbé plots (L'Abbé 1987), a visual method for assessing differences in results of individual studies, and using the I2 statistic.
Assessment of reporting biases
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher in this condition; Moore 2008b).
Data synthesis
For efficacy analyses, we used the number of participants in each treatment group who were randomised, received medication, and provided at least one postbaseline assessment. For safety analyses, we used the number of participants randomised to each treatment group who took the study medication.
For each study, we planned to convert the mean total pain relief (TOTPAR), or summed pain intensity difference (SPID), VAS TOTPAR, or VAS SPID (see 'Glossary'; Appendix 1) values for the active and placebo groups to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). We would then calculate the proportion of participants in each treatment group who achieved at least 50%maxTOTPAR using verified equations (Moore 1996; Moore 1997a; Moore 1997b), convert these proportions into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group.
We accepted the following pain measures for the calculation of TOTPAR or SPID (in order of priority: see Appendix 1).
5‐point categorical pain relief scales with comparable wording to 'none', 'slight', 'moderate', 'good', and 'complete'.
4‐point categorical pain intensity scales with comparable wording to 'none', 'mild', 'moderate', and 'severe'.
VAS for pain relief.
VAS for pain intensity.
We used this information for active and placebo groups to calculate RR and NNT.
We also calculated 'response' using the number of participants reporting 'very good or excellent' on a 5‐point categorical global scale with the wording 'poor', 'fair', 'good', 'very good', and 'excellent' for the number of participants achieving at least 50% pain relief (Collins 2001).
For each treatment group, we extracted the number of participants using rescue medication and the number reporting treatment‐emergent adverse events.
We calculated RR estimates with 95% CIs using the Mantel‐Haenszel method and a fixed‐effect model in Review Manager 5 (RevMan 2014). We calculated NNT and NNH with 95% CIs using the pooled number of events and the method of Cook and Sackett (Cook 1995). We have assumed a statistically significant difference from control when the 95% CI of the RR did not include the number one. We required a minimum of two studies and 200 participants (in the comparison) for any pooled analysis.
We did not plan to pool data from individual studies for time to use of rescue medication, but have calculated a mean value weighted by participant numbers where possible.
Subgroup analysis and investigation of heterogeneity
We planned to analyse different doses separately, where there were sufficient data, and determine significant differences between different doses using the z test (Tramèr 1997).
Sensitivity analysis
We planned to carry out sensitivity analyses for pain model (dental versus other) and formulation, although there were insufficient data to assess formulation. We also carried out a posthoc sensitivity analysis to assess the impact of a single study in hallux valgus surgery (bunionectomy), which used patient‐controlled analgesia (PCA) for rescue analgesia.
Results
Description of studies
Results of the search
New searches identified 150 potentially relevant articles in CENTRAL, 125 in MEDLINE, and 276 in Embase. After deduplication and screening of titles and abstracts, we obtained the full texts of four new studies. We also obtained the full text of two of the three studies that were previously identified and placed in the 'Studies awaiting assessment' table (Akural 2009; Balzanelli 1996). The remaining study awaiting assessment is Japanese and remains unobtainable (Yatomi 1979). Figure 1 shows the flow of study acquisition and use.
1.
Study flow diagram.
Details of individual studies are in the Characteristics of included studies, Characteristics of excluded studies, and Studies awaiting classification tables.
Included studies
In this updated review, we included the 18 studies from the earlier review, the three studies identified by new searches (McQuay 2016; Moore 2015c; Moore 2016), and the two studies that were awaiting assessment and for which full texts were available (Akural 2009; Balzanelli 1996). One further study had been omitted from the earlier review, due to mislabelling of the downloaded PDF, and is now included (Sunshine 1988).
Of the 24 studies included in this update, 14 used ketoprofen only, seven used dexketoprofen only, and three used both ketoprofen and dexketoprofen. One of these studies also included a combination of ketoprofen with paracetamol, and three also included a combination of dexketoprofen with tramadol.
The six additional studies added 1001 participants involved in comparisons of ketoprofen or dexketoprofen and placebo. Three were large studies (McQuay 2016; Moore 2015c; Moore 2016). The total number of participants who took medication was 5220, of whom 1084 received ketoprofen alone (dose range 6.25 mg to 150 mg; mostly 25 mg and 50 mg), 1120 received dexketoprofen alone (dose range 5 mg to 100 mg; mostly 12.5 mg and 25 mg), and 1156 received placebo. In the previous version of this review, the numbers were: ketoprofen 968 participants and dexketoprofen 681 participants, making the increase in participants treated with the drugs 12% for ketoprofen and 65% more for dexketoprofen.
All studies included placebo controls, and all except two (Balzanelli 1996; Harrison 1996), included mostly small numbers of participants treated in active comparator arms with licensed doses of other analgesics (see below). There were insufficient data for comparison of ketoprofen or dexketoprofen with other active comparators, except the combination of dexketoprofen plus tramadol, which has been evaluated in a separate review (Derry 2016).
The mean age reported was between early 20s (typically dental studies) and late 40s to late 60s (typically other types of surgery). Most studies reported the sex of participants, and where it was reported women (70%), predominated.
Study with patient‐controlled analgesia
One study, in hallux valgus (bunion) surgery, used PCA rescue analgesia (Vidal 1999). This study had much lower response rates for at least 50% of maximum pain relief in both the active and placebo treatment arms than the other studies in non‐dental pain, at almost 0% for ketoprofen and placebo and 30% and 2% for dexketoprofen and placebo. The PCA device was programmed to deliver a bolus of morphine 2 mg with a 15‐minute lockout. Any participant taking rescue morphine within the first hour was withdrawn from the study; for participants remedicating thereafter, pain intensity was assessed as that of the last observation carried forward and pain relief rated as 'none' in later assessments. It is unclear whether this low response rate in Vidal 1999 is due to chance, the nature of the surgery (which is known to be very painful over several days), or the easy availability of rescue medication with PCA, which may have encouraged participants to use it earlier and influence postoperative pain scores and responses.
As the Vidal 1999 study matched all the study inclusion criteria and used morphine as a rescue treatment in the same way as other studies use oral rescue analgesia, it was included. Sensitivity analyses were planned to evaluate any impact of potential study differences on overall estimates, and because bunion surgery is an uncommon pain model without the proven sensitivity of third molar extraction (Bulley 2009). The amount of information precluded formal sensitivity analyses on bunion surgery, and the meaning of any such analyses would be quite unclear in this case. For these reasons, while information from this study is included, the main analyses are presented without it.
Ketoprofen
Seventeen studies fulfilled the inclusion criteria (Akural 2009; Arnold 1990; Balzanelli 1996; Cooper 1984; Cooper 1988; McGurk 1998; Mehlisch 1984; Olson 1999; Olson 2001; Schreiber 1996; Seymour 1996; Seymour 2000; Sunshine 1988: Sunshine 1993; Sunshine 1998; Turek 1988; Vidal 1999).
The studies used the following treatments.
Ketoprofen 6.25 mg (Sunshine 1998), n = 35.
Ketoprofen 12.5 mg (Seymour 1996; Seymour 2000; Sunshine 1998), n = 138.
Ketoprofen 25 mg (Arnold 1990; Cooper 1984; Cooper 1988; Mehlisch 1984; Olson 1999; Olson 2001; Seymour 1996; Sunshine 1998), n = 281.
Ketoprofen 50 mg (Cooper 1984; McGurk 1998; Mehlisch 1984; Olson 1999; Schreiber 1996; Sunshine 1988; Sunshine 1993; Turek 1988; Vidal 1999), n = 349.
Ketoprofen 80 mg (Balzanelli 1996), n = 30.
Ketoprofen 100 mg (Akural 2009; Arnold 1990; Cooper 1984; Cooper 1988; Mehlisch 1984; Sunshine 1993), n = 181.
Ketoprofen 150 mg (Sunshine 1988: Turek 1988), n = 70.
Ketoprofen 100 mg plus paracetamol 1000 mg (Akural 2009), n = 20.
Paracetamol 500 mg (Seymour 1996), n = 41.
Paracetamol 1000 mg (Akural 2009; Seymour 1996), n = 71.
Paracetamol 650 mg plus codeine 60 mg (Sunshine 1988; Turek 1988), n = 67.
Ibuprofen 200 mg (Seymour 2000; Sunshine 1998), n = 94.
Ibuprofen 400 mg (Arnold 1990; Cooper 1988; Olson 2001), n = 119.
Aspirin 650 mg (Cooper 1984), n = 31.
Codeine 90 mg (Mehlisch 1984), n = 27.
Dipyrone 500 mg (liquid) (Olson 1999), n = 27.
Dexketoprofen 12.5 mg (McGurk 1998; Schreiber 1996; Vidal 1999), n = 143.
Dexketoprofen 25 mg (McGurk 1998; Schreiber 1996; Vidal 1999), n = 140.
Dexketoprofen 50 mg (McGurk 1998), n = 44.
Formulation
One study administered ketoprofen in liquid formulation (Olson 1999). All other studies administered ketoprofen as a capsule or tablet. One study administered the lysine salt of ketoprofen (Balzanelli 1996), and one administered "buffered ketoprofen" (Seymour 2000). Some of these formulations (liquid, lysine salt, and buffered) are likely to be absorbed faster than standard formulations, which can enhance efficacy in NSAIDs (Derry 2015; Moore 2014).
Type of surgery
Ten studies enrolled participants with dental pain following extraction of at least one impacted third molar (Akural 2009; Balzanelli 1996; Cooper 1984; Cooper 1988; McGurk 1998; Mehlisch 1984; Olson 2001; Seymour 1996; Seymour 2000; Sunshine 1998), and seven studies enrolled participants with pain following other types of surgery (general surgery (Arnold 1990; Sunshine 1988); postepisiotomy pain (Olson 1999); knee or ankle surgery (Schreiber 1996); Caesarean section (Sunshine 1993); elective surgery (Turek 1988); hallux valgus surgery (Vidal 1999)).
Study duration
Study duration was six hours in 12 studies (Arnold 1990; Cooper 1984; Cooper 1988; McGurk 1998; Mehlisch 1984; Olson 1999; Olson 2001; Seymour 1996; Seymour 2000; Sunshine 1988; Sunshine 1998; Turek 1988), eight hours in one (Akural 2009), 24 hours in one (Vidal 1999), three days in two (Balzanelli 1996; Schreiber 1996), and up to seven days in one (Sunshine 1993). These latter four studies included multiple dose phases, but reported results for the first dose separately for at least some relevant outcomes (Balzanelli 1996; Schreiber 1996; Sunshine 1993; Vidal 1999).
Dexketoprofen
Ten studies using dexketoprofen fulfilled the inclusion criteria (Cooper 1998; Gay 1996; Harrison 1996; Jackson 2004; McGurk 1998; McQuay 2016; Moore 2015c; Moore 2016; Schreiber 1996; Vidal 1999).
The studies used the following treatments.
Dexketoprofen 5 mg (Gay 1996), n = 41.
Dexketoprofen 10 mg (Gay 1996), n = 42.
Dexketoprofen 12.5 mg (Harrison 1996; McGurk 1998; Moore 2015c; Schreiber 1996; Vidal 1999), n = 252.
Dexketoprofen 20 mg (Gay 1996), n = 41.
Dexketoprofen 25 mg (Cooper 1998; Harrison 1996; Jackson 2004; McGurk 1998; McQuay 2016; Moore 2015c; Moore 2016; Schreiber 1996; Vidal 1999), n = 650.
Dexketoprofen 50 mg (McGurk 1998), n = 43.
Dexketoprofen 100 mg (Cooper 1998), n = 51.
Dexketoprofen 12.5 mg plus tramadol 37.5 mg (Moore 2015c), n = 60.
Dexketoprofen 12.5 mg plus tramadol 75 mg (Moore 2015c), n = 62.
Dexketoprofen 25 mg plus tramadol 37.5 mg (Moore 2015c), n = 63.
Dexketoprofen 25 mg plus tramadol 75 mg (McQuay 2016; Moore 2015c; Moore 2016), n = 372.
Tramadol 37.5 mg (Moore 2015c), n = 59.
Tramadol 75 mg (Moore 2015c), n = 59.
Tramadol 100 mg (McQuay 2016; Moore 2016), n = 311.
Ibuprofen 400 mg (Gay 1996; Moore 2015c), n = 101.
Paracetamol 1000 mg (Cooper 1998), n = 50.
Rofecoxib 50 mg (Jackson 2004), n = 37.
Ketoprofen 50 mg (McGurk 1998; Schreiber 1996; Vidal 1999), n = 144.
For the purposes of analysis, we combined data for ketoprofen 80 mg and 100 mg, for dexketoprofen 10 mg and 12.5 mg, and for dexketoprofen 20 mg and 25 mg, as we judged the small differences in dose were unlikely to have a clinically significant impact on results. Dexketoprofen was administered as the trometamol salt formulation that is likely to be absorbed faster than standard formulations (Barbanoj 2001), which can enhance efficacy in NSAIDs (Derry 2015; Moore 2014).
Type of surgery
Six studies enrolled participants with dental pain following extraction of at least one impacted third molar (Cooper 1998; Gay 1996; Harrison 1996; Jackson 2004; McGurk 1998; Moore 2015c); three studies enrolled participants with pain following orthopaedic surgery (hallux valgus surgery (Vidal 1999, see 'Ketoprofen' section above), knee or ankle surgery (Schreiber 1996), and hip surgery (McQuay 2016)); and one study enrolled participants with pain following abdominal hysterectomy for benign conditions (Moore 2016).
Study duration
Most studies had a duration of six hours (Cooper 1998; Gay 1996; Harrison 1996; McGurk 1998), or 24 hours (Jackson 2004; Moore 2015c; Vidal 1999), but three had a duration of three days (Moore 2016; Schreiber 1996), or five days (McQuay 2016). Four studies included multiple dose phases (McQuay 2016; Moore 2016; Schreiber 1996; Vidal 1999), but reported results for the first dose separately for at least some relevant outcomes.
Excluded studies
We excluded 15 studies from the earlier review (Avila 1991; Bagan 1998; Berti 2000; Gallardo 1982; Giudice 1987; Jimenez‐Martinez 2004; Kantor 1984; Letarget 1998; Lobo 1983; Olmedo 2001; Perez 2002; Schreiber 1998; Sunshine 1986; Tufano 1981; Zapata 2000), and one additional study for this update (Esparza‐Villalpando 2016). Reasons for exclusion are in the Characteristics of excluded studies table.
Risk of bias in included studies
Oxford quality scores were high, with four studies scoring 3/5, 13 scoring 4/5, and seven scoring 5/5. These high scores are indicative of low risk of bias.
Allocation
All studies were described as randomised, but only nine provided an adequate description of the randomisation process, and eight an adequate description of the allocation process. Where adequate descriptions were not provided, we judged the study at unknown risk of bias, although the likelihood is that the methods were adequate but the reporting was not.
Blinding
All studies were described as double‐blind, and all except three provided an adequate description of the method used to maintain blinding of both participants and personnel. Where an adequate description was not provided, we judged the study at unknown risk of bias, although the likelihood is that the methods were adequate but the reporting was not.
Other potential sources of bias
Twenty of the included studies included treatment arms with fewer than 50 participants, and we judged these at high risk of bias due to size. In six studies, all treatment arms had between 50 and 200 participants and we judged them at unclear risk of bias, but only two were substantially over the 50‐participant threshold (McQuay 2016; Moore 2016).
Full details of the risk of bias assessments are in Characteristics of included studies table, and Figure 2 provides a summary.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Details of outcomes in individual studies are in Appendix 6 (efficacy) and Appendix 7 (adverse events and withdrawals).
Participants achieving at least 50% pain relief with ketoprofen over four to six hours
Ketoprofen 6.25 mg versus placebo
Only one study, with 70 participants in the comparison, provided data (Sunshine 1998); 10/35 participants experienced at least 50% pain relief over six hours with ketoprofen 6.25 mg and 3/35 with placebo. No analysis was undertaken.
Ketoprofen 12.5 mg versus placebo
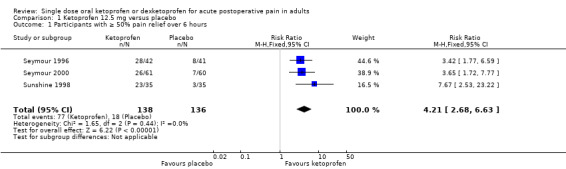
Three studies with 274 participants provided data (Seymour 1996; Seymour 2000; Sunshine 1998) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.
The proportion of participants experiencing at least 50% pain relief over six hours with ketoprofen 12.5 mg was 56% (77/138, range 43% to 67%).
The proportion of participants experiencing at least 50% pain relief over six hours with placebo was 13% (18/136, range 9% to 20%).
The RR for treatment compared with placebo 4.2 (95% CI 2.7 to 6.6).
The NNT for at least 50% pain relief over six hours was 2.4 (95% CI 1.9 to 3.1).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
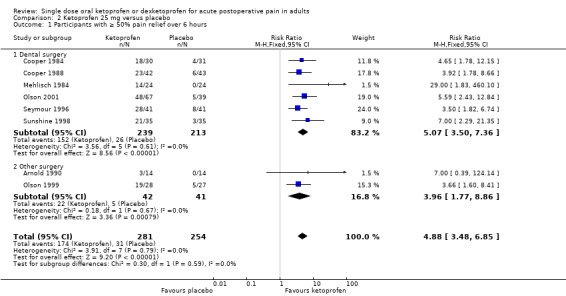
Ketoprofen 25 mg versus placebo
Eight studies with 535 participants provided data (Arnold 1990; Cooper 1984; Cooper 1988; Mehlisch 1984; Olson 1999; Olson 2001; Seymour 1996; Sunshine 1998) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.
The proportion of participants experiencing at least 50% pain relief over six hours with ketoprofen 25 mg was 62% (174/281, range 21% to 72%).
The proportion of participants experiencing at least 50% pain relief over six hours with placebo was 12% (31/254, range 0% to 20%).
The RR for treatment compared with placebo was 4.9 (95% CI 3.5 to 6.9).
The NNT for at least 50% pain relief over six hours was 2.0 (95% CI 1.8 to 2.3).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
Ketoprofen 50 mg versus placebo
Nine studies with 688 participants provided data (Cooper 1984; McGurk 1998; Mehlisch 1984; Olson 1999; Schreiber 1996; Sunshine 1988; Sunshine 1993; Turek 1988; Vidal 1999). One study, in bunionectomy, had very low event rates with ketoprofen 50 mg and placebo (Vidal 1999). Analysis with this study removed made a minor difference to the overall results, but as the results were so different from other single dose studies, the following analyses are those with that study omitted.
Omitting Vidal 1999, eight studies with 594 participants gave the following results (Analysis 3.1; Figure 3).
3.1. Analysis.
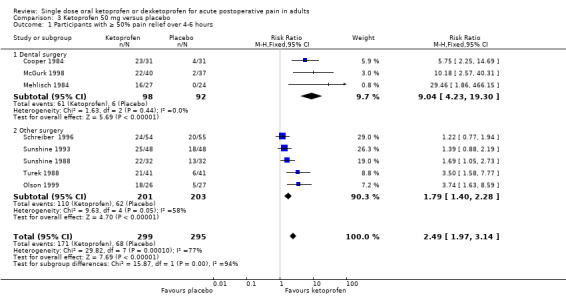
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
3.
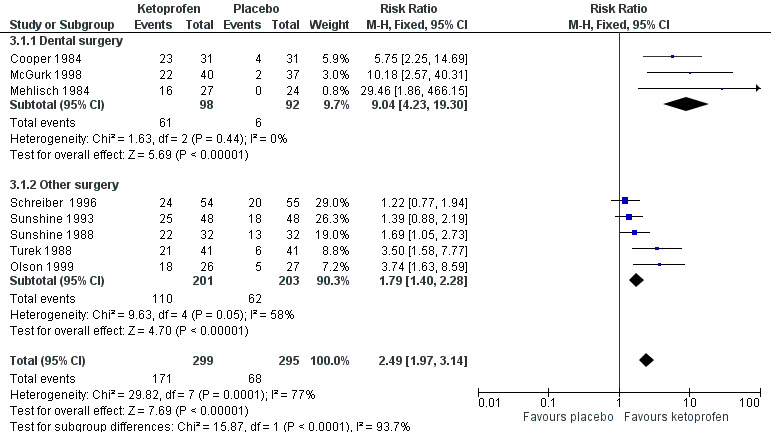
Forest plot of comparison: 3 Ketoprofen 50 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over four to six hours.
The proportion of participants experiencing at least 50% pain relief over four to six hours with ketoprofen 50 mg was 57% (171/299, range 44% to 74%).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 23% (68/295, range 2% to 41%).
The RR for treatment compared with placebo was 2.5 (95% CI 2.0 to 3.1).
The NNT for at least 50% pain relief over four to six hours was 2.9 (95% CI 2.4 to 3.7).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
Ketoprofen 80 mg or 100 mg versus placebo
Six studies with 381 participants provided data (Arnold 1990; Balzanelli 1996; Cooper 1984; Cooper 1988; Mehlisch 1984; Sunshine 1993) (Analysis 4.1).
4.1. Analysis.
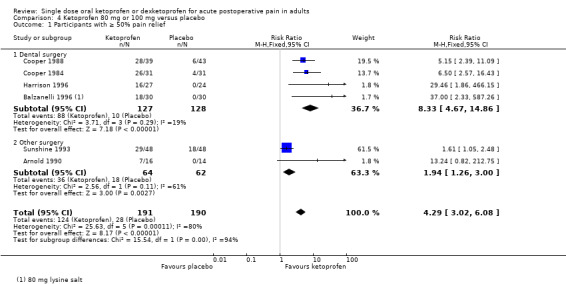
Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief.
The proportion of participants experiencing at least 50% pain relief over six hours with ketoprofen 80 mg or 100 mg was 65% (124/191, range 44% to 84%).
The proportion of participants experiencing at least 50% pain relief over six hours with placebo was 15% (28/190, range 0% to 38%).
The RR for treatment compared with placebo was 4.3 (95% CI 3.0 to 6.1).
The NNT for at least 50% pain relief over six hours was 2 (95% CI 2 to 2).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
Ketoprofen 150 mg versus placebo
Two studies with 143 participants in the comparison, provided data (Sunshine 1988; Turek 1988); 44/70 participants achieved at least 50% pain relief with ketoprofen 150 mg, and 19/73 with placebo. No analysis was undertaken.
| Summary of results A: number of participants with ≥ 50% pain relief over 4 to 6 hours with ketoprofen (note: not including data fromVidal 1999) | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNT (95% CI) |
| 12.5 mg | 3 | 274 | 56 | 13 | 4.2 (2.7 to 6.6) | 2.4 (1.9 to 3.1) |
| 25 mg | 8 | 535 | 62 | 12 | 4.9 (3.5 to 6.9) | 2.0 (1.8 to 2.3) |
| 50 mg | 8 | 594 | 57 | 23 | 2.5 (2.0 to 3.1) | 2.9 (2.4 to 3.7) |
| 80 mg or 100 mg | 6 | 381 | 65 | 15 | 4.3 (3.0 to 6.1) | 2.0 (1.7 to 2.4) |
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
Sensitivity analysis of primary outcome
Pain model
There were sufficient data to compare dental and other types of surgery for ketoprofen 50 mg only (Figure 4).
4.
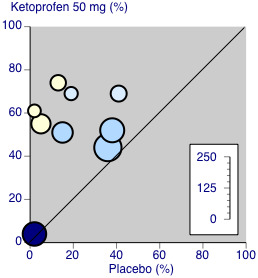
Ketoprofen 50 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.
Three studies (190 participants) used ketoprofen 50 mg in dental surgery (Cooper 1984; McGurk 1998; Mehlisch 1984). Overall, 62% (61/98, range 55% to 74%) of participants achieved 50% pain relief with ketoprofen and 6% (6/92, range 0% to 13%) with placebo. The RR for treatment compared with placebo was 9.0 (95% CI 4.2 to 19), and the NNT for at least 50% pain relief over six hours was 1.8 (95% CI 1.5 to 2.2).
Excluding Vidal 1999, five studies (404 participants) used ketoprofen 50 mg in other types of surgery (Olson 1999; Schreiber 1996; Sunshine 1988; Sunshine 1993; Turek 1988). Overall, 55% (110/201, range 44% to 69%) of participants achieved 50% pain relief with ketoprofen and 31% (62/203, range 15% to 41%) with placebo. The RR for treatment compared with placebo was 1.8 (95% CI 1.4 to 2.3) and the NNT for at least 50% pain relief over four to six hours was 4.2 (95% CI 3.0 to 6.7).
The difference between the NNTs was statistically significant (z = 4.32, P < 0.00006), but based on small numbers, particularly for the dental studies. The extent of clinical heterogeneity between these studies is illustrated in Figure 4.
Post hoc analysis of dental studies alone shows a dose response trend over the range of doses used and available data (Summary of results B). There was a significantly better result with 80 mg or 100 mg than 12.5 mg (z = 2.7108, P < 0.01).
| Summary of results B: number of participants with ≥ 50% pain relief over 6 hours with ketoprofen in dental studies | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNT (95% CI) |
| 12.5 mg | 3 | 274 | 56 | 13 | 4.2 (2.7 to 6.6) | 2.4 (1.9 to 3.1) |
| 25 mg | 6 | 452 | 64 | 12 | 5.1 (3.5 to 7.4) | 2.0 (1.7 to 2.3) |
| 50 mg | 3 | 190 | 62 | 6.5 | 9.0 (4.2 to 19) | 1.8 (1.5 to 2.2) |
| 80/100 mg | 4 | 255 | 69 | 8 | 8.3 (4.7 to 15) | 1.6 (1.4 to 1.9) |
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
Formulation
Three studies used probably faster‐acting formulations in dental surgery (Balzanelli 1996; Seymour 2000), or women with episiotomy pain (Olson 1999). Different pain models and doses used meant that it was impossible to form any conclusions about differences in efficacy in different formulations.
Participants achieving at least 50% pain relief with dexketoprofen over four to six hours
Dexketoprofen 5 mg versus placebo
Only one study, with 82 participants in the comparison, provided data (Gay 1996); 18/41 participants experienced at least 50% pain relief over six hours with dexketoprofen 5 mg and 7/39 with placebo.
Dexketoprofen 10 mg or 12.5 mg versus placebo
Six studies with 574 participants provided data; one study used dexketoprofen 10 mg (Gay 1996) and five studies used dexketoprofen 12.5 mg (Harrison 1996; McGurk 1998; Moore 2015c; Schreiber 1996; Vidal 1999). One study, in bunionectomy, had lower event rates with dexketoprofen 12.5 mg (30%) and placebo (2%) (Vidal 1999). Analysis with this study removed made a minor difference to the overall results, but as the results were so different from other single dose studies, the following analyses are those with that study omitted.
Omitting Vidal 1999, five studies with 480 participants gave the following results (Analysis 5.1).
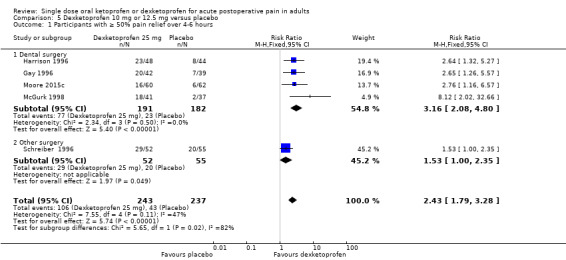
5.1. Analysis.
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
The proportion of participants experiencing at least 50% pain relief over four to six hours with dexketoprofen 10 mg or 12.5 mg was 44% (106/243).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 18% (43/237).
The RR for treatment compared with placebo was 2.4 (95% CI 1.8 to 3.3).
The NNT for at least 50% pain relief over four to six hours was 3.9 (95% CI 3.0 to 5.7).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
Dexketoprofen 20 mg or 25 mg versus placebo
Nine studies with 1271 participants provided data; one study used dexketoprofen 20 mg (Gay 1996) and eight studies used dexketoprofen 25 mg (Cooper 1998; Harrison 1996; McGurk 1998; McQuay 2016; Moore 2015c; Moore 2016; Schreiber 1996; Vidal 1999). One study, in bunionectomy, had low event rates with dexketoprofen 25 mg and placebo (Vidal 1999). Analysis with this study removed made a minor difference to the overall results, but as the results were so different from other single dose studies, the following analyses are those with that study omitted.
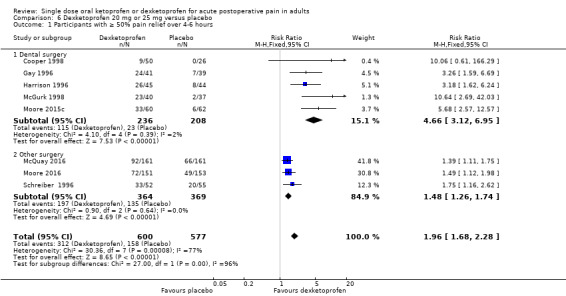
Omitting Vidal 1999, eight studies with 1177 participants gave the following results (Analysis 6.1; Figure 5).
6.1. Analysis.
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
5.
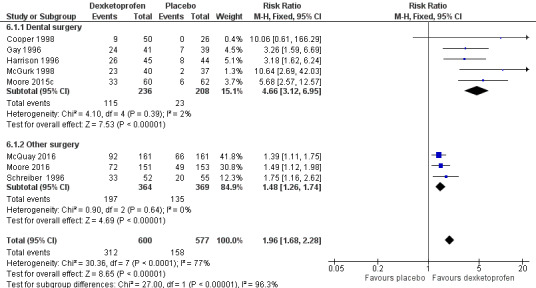
Forest plot of comparison: 6 Dexketoprofen 20 mg or 25 mg versus placebo, outcome: 6.1 Participants with at least 50% pain relief over four to six hours.
The proportion of participants experiencing at least 50% pain relief over four to six hours with dexketoprofen 20 mg or 25 mg was 52% (312/600, range 18% to 63%).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 27% (158/577, range 0% to 41%).
The RR for treatment compared with placebo was 2.0 (95% CI 1.6 to 2.2).
The NNT for at least 50% pain relief over four to six hours was 4.1 (95% CI 3.3 to 5.2).
We judged the quality of the evidence as high. Study methods were robust and there were adequate numbers of participants and a large treatment effect consistent with other doses.
Dexketoprofen 50 mg or 100 mg versus placebo
In one study, with 82 participants in the comparison, 24/42 participants experienced at least 50% pain relief over six hours with dexketoprofen 50 mg and 2/37 with placebo (McGurk 1998).
In another study, with 77 participants in the comparison, 17/51 participants experienced at least 50% pain relief over six hours with dexketoprofen 100 mg and 0/26 with placebo (Cooper 1998).
No analyses were undertaken for these doses.
| Summary of results C: number of participants with ≥ 50% pain relief over 4 to 6 hours with dexketoprofen (note: not including data fromVidal 1999) | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNT (95% CI) |
| 10/12.5 mg | 5 | 480 | 44 | 18 | 2.4 (1.8 to 3.3) | 3.9 (3.0 to 5.7) |
| 20/25 mg | 8 | 1177 | 52 | 27 | 2.0 (1.6 to 2.2) | 4.1 (3.3 to 5.2) |
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
Sensitivity analysis of primary outcome
Pain model
Dexketoprofen 10 mg or 12.5 mg (Analysis 5.1)
Four studies (373 participants) used dexketoprofen 10 mg or 12.5 mg in dental surgery (Gay 1996; Harrison 1996; McGurk 1998; Moore 2015c). Overall, 40% (77/191, range 27% to 48%) of participants achieved 50% pain relief with dexketoprofen and 13% (23/182) with placebo. The RR for treatment compared with placebo was 3.2 (95% CI 2.1 to 4.8), and the NNT for at least 50% pain relief over six hours was 3.6 (95% CI 2.8 to 5.2).
One study (107 participants) used dexketoprofen 12.5 mg in other types of surgery (Schreiber 1996); 56% (29/52) of participants achieved 50% pain relief with dexketoprofen and 36% (20/55) with placebo. The NNT for at least 50% pain relief over four hours was 5.2.
There were insufficient data to determine whether there was a significant difference at this dose.
Dexketoprofen 20 mg or 25 mg (Analysis 6.1; Figure 6)
Five studies (444 participants) used dexketoprofen 20 mg or 25 mg in dental surgery (Cooper 1998; Gay 1996; Harrison 1996; McGurk 1998; Moore 2015c). Overall, 49% (115/236, range 18% to 59%) of participants achieved 50% pain relief with dexketoprofen and 11% (23/208, range 0% to 18%) with placebo. The RR for treatment compared with placebo was 4.7 (95% CI 3.1 to 7.0), and the NNT for at least 50% pain relief over six hours was 2.7 (95% CI 2.2 to 3.3).
Three studies (733 participants) used dexketoprofen 20 mg or 25 mg in other types of surgery (McQuay 2016; Moore 2016; Schreiber 1996). Overall, 54% (197/364, range 48% to 63%) of participants achieved 50% pain relief with dexketoprofen and 37% (135/369, range 32% to 41%) with placebo. The RR for treatment compared with placebo was 1.5 (95% CI 1.3 to 1.7), and the NNT for at least 50% pain relief over four to six hours was 5.7 (95% CI 4.1 to 9.6).
There was no overlap in the CIs of the NNTs indicating a statistically significant difference (z = 3.77, P < 0.0002).
| Summary of results D: number of participants with ≥ 50% pain relief over 4 to 6 hours with dexketoprofen in dental and other types of surgery (note not including data fromVidal 1999) | ||||||
| Dose and type of surgery | Studies | Participants | Dexketoprofen (%) | Placebo (%) | RR (95% CI) | NNT (95% CI) |
| 10 mg or 12.5 mg Dental |
4 | 373 | 40 | 13 | 3.2 (2.1 to 4.8) | 3.6 (2.8 to 5.2) |
| 12.5 mg Other surgery |
1 | 201 | 56 | 36 | Not calculated | 5.2 |
| 20 mg or 25 mg Dental |
5 | 444 | 49 | 11 | 4.7 (3.1 to 7.0) | 2.7 (2.2 to 3.3) |
| 20 mg or 25 mg Other surgery |
3 | 733 | 54 | 37 | 1.5 (1.3 to 1.7) | 5.7 (4.1 to 9.6) |
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
Comparison of ketoprofen and dexketoprofen
Since the analgesic effect of ketoprofen is due to the S(+)‐enantiomer (Barbanoj 2001), it might be hypothesised that dexketoprofen alone should produce equivalent analgesia to twice the dose of ketoprofen. There was insufficient information where like could be compared with like to reach any definitive conclusions. Problems included the mix of dental and other types of surgery, small numbers in some subgroups, few studies, and small numbers that compared the two drugs directly in the same trial.
Use of rescue medication with ketoprofen
Time to use of rescue medication
Six studies reported the median time to use of rescue medication (Akural 2009; McGurk 1998; Olson 2001; Seymour 1996; Seymour 2000; Sunshine 1993). The study using ketoprofen 50 mg and 100 mg in participants who had undergone Caesarean section (Sunshine 1993) had notably longer times to use of rescue medication in both active (seven to nine hours) and placebo (six hours) treatment arms than the dental studies. Based on very limited data (fewer than 200 participants in each comparison), the median time to use of rescue medication in the dental studies was around five hours for ketoprofen 25 mg and 50 mg, and two hours for placebo. We judged the quality of the evidence as very low due to the small number of participants in each comparison. Seven studies reported the mean time to use of rescue medication (Arnold 1990; Cooper 1984; Cooper 1988; Mehlisch 1984; Olson 1999; Turek 1988; Vidal 1999). Based on very limited data (fewer than 200 participants in each comparison), the mean time to use of rescue medication in dental studies was 4 to 4.5 hours with ketoprofen 25 mg to 100 mg, and 2.5 hours with placebo. In non‐dental studies, it was about six hours for ketoprofen 25 mg and 50 mg, and five hours for placebo in episiotomy pain, and two hours for both ketoprofen 50 mg and placebo in bunionectomy and other elective surgery. The study in bunionectomy pain used morphine PCA as rescue analgesia (Vidal 1999). We judged the quality of the evidence as very low due to the small number of participants in each comparison.
Number of participants using rescue medication
Two studies (198 participants) using ketoprofen 12.5 mg reported proportion of participants using rescue medication, both at six hours (Seymour 1996; Seymour 2000). The mean proportion using rescue medication with ketoprofen was 80% (79/99) and with placebo was 98% (97/99), giving an NNTp of 5.5 (95% CI 3.8 to 10) (Analysis 1.2). We judged the quality of the evidence as very low due to the small number of studies and participants.
Six studies (402 participants) using ketoprofen 25 mg reported proportion of participants using rescue medication, all at six hours (Arnold 1990; Cooper 1988; Mehlisch 1984; Olson 1999; Olson 2001; Seymour 1996). The mean proportion using rescue medication with ketoprofen was 46% (99/216) and with placebo was 79% (147/186), giving an NNTp of 3.0 (95% CI 2.4 to 4.1) (Analysis 2.2). We judged the quality of the evidence as moderate due to the moderate number of participants. There was some heterogeneity from one small study, but it did not affect the overall result.
Six studies (468 participants) using ketoprofen 50 mg reported proportion of participants using rescue medication, at four at six hours (McGurk 1998; Mehlisch 1984; Olson 1999; Turek 1988), and two at eight hours (Schreiber 1996; Sunshine 1993). Overall, the mean proportion using rescue medication with ketoprofen was 39% (93/236) and with placebo was 70% (162/232), giving an NNTp of 3.3 (95% CI 2.6 to 4.6). For six hours only, the mean proportion using rescue medication with ketoprofen was 32% (43/134) and with placebo was 75% (97/129), giving an NNTp of 2.3 (95% CI 1.9 to 3.1) (Analysis 3.2). We judged the quality of the evidence as high. There was some heterogeneity, but it did not affect the overall result.
Four studies (259 participants) using ketoprofen 100 mg reported proportion of participants using rescue medication, at three at six hours (Arnold 1990; Cooper 1988; Mehlisch 1984), and one at eight hours (Sunshine 1993). Overall, the mean proportion using rescue medication with ketoprofen was 44% (57/130) and with placebo was 81% (104/129), giving an NNTp of 2.7 (95% CI 2.1 to 2.9). For six hours only, the mean proportion using rescue medication with ketoprofen was 43% (35/82) and with placebo was 85% (69/81), giving an NNTp of 2.4 (95% CI 1.8 to 3.4) (Analysis 4.2). We judged the quality of the evidence as low; the results were consistent, but there were small numbers of studies and participants.
One study (81 participants) reported that 18/39 participants used rescue medication with ketoprofen 150 mg and 35/42 with placebo at six hours (Turek 1988).
1.2. Analysis.
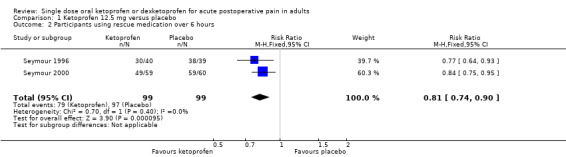
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
2.2. Analysis.
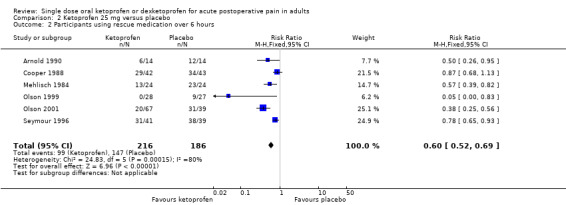
Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
3.2. Analysis.
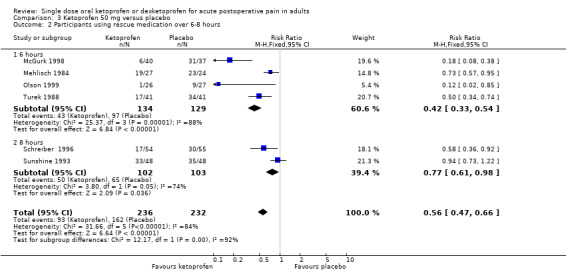
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
4.2. Analysis.
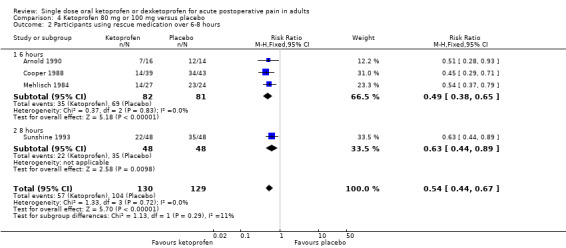
Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
Many more participants needed rescue medication within six hours with the 12.5 mg dose than the higher doses (12.5 mg versus 50 mg: z = 2.37, P = 0.018).
| Summary of results E: participants using rescue medication within 6 hours with ketoprofen | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNTp (95% CI) |
| 12.5 mg | 2 | 198 | 80 | 98 | 0.81 (0.74 to 0.90) | 5.5 (3.8 to 10) |
| 25 mg | 6 | 402 | 46 | 79 | 0.60 (0.52 to 0.69) | 3.0 (2.4 to 4.1) |
| 50 mg | 4 | 349 | 32 | 75 | 0.42 (0.33 to 0.54) | 2.3 (1.8 to 3.4) |
| 80 mg or 100 mg | 3 | 163 | 43 | 85 | 0.54 (0.44 to 0.67) | 2.4 (1.8 to 3.4) |
| CI: confidence interval; NNTp: number needed to treat to prevent an additional harmful event; RR: risk ratio. | ||||||
Use of rescue medication with dexketoprofen
Time to use of rescue medication
Three studies reported the median time to use of rescue medication, all in dental pain (Cooper 1998; Jackson 2004; Moore 2015c). Based on limited data (281 participants in the comparison), the weighted mean of the median time to use of rescue medication was 4.7 hours with dexketoprofen 25 mg, and 1.8 hours with placebo. In one study, the median time to use of rescue medication was 3.6 hours with dexketoprofen 12.5 mg and 1.4 hours with placebo (Moore 2015c). We judged the quality of the evidence as very low due to the small number of studies and participants and a degree of statistical heterogeneity.
Three studies reported the mean time to use of rescue medication, two in dental pain (Gay 1996; McGurk 1998), and one following bunionectomy (Vidal 1999). The times in the bunionectomy study were notably shorter than in the dental studies, with remedication times of 2.3 for dexketoprofen, and 1.7 for placebo. This study used morphine PCA as rescue analgesia, and the data were not combined. Based on very limited data (fewer than 200 participants in each comparison), for dental studies the weighted mean of the mean time to use of rescue medication was 4.9 with dexketoprofen 10 mg or 12.5 mg, 5.2 with dexketoprofen 20 mg or 25 mg, and 3.6 with placebo. We judged the quality of the evidence as very low due to the small number of studies and participants.
Number of participants using rescue medication
Five studies (480 participants) using dexketoprofen 10 mg or 12.5 mg reported proportion of participants using rescue medication, four at six hours (Gay 1996; Harrison 1996; McGurk 1998; Moore 2015c), and one at eight hours (Schreiber 1996). Overall, the mean proportion using rescue medication with dexketoprofen was 44% (107/243) and with placebo was 65% (153/237), giving an NNTp of 4.9 (95% CI 3.4 to 8.5) (Analysis 5.2). For six hours only, the mean proportion using rescue medication with ketoprofen was 49% (93/191) and with placebo was 68% (123/182), giving an NNTp of 5.3 (95% CI 3.5 to 11). We judged the quality of the evidence as high.
Seven studies (635 participants) using dexketoprofen 20 mg or 25 mg reported proportion of participants using rescue medication, five at six hours (Cooper 1998; Gay 1996; Harrison 1996; McGurk 1998; Moore 2015c), one at eight hours (Schreiber 1996), and one at 24 hours (Jackson 2004). Overall, the mean proportion using rescue medication with dexketoprofen was 48% (159/331) and with placebo was 69% (209/304), giving an NNTp of 4.8 (95% CI 3.6 to 7.6) (Analysis 6.2). For six hours only, the mean proportion using rescue medication with ketoprofen was 47% (112/237) and with placebo was 69% (143/208) giving an NNTp of 4.7 (95% CI 3.3 to 8.0). We judged the quality of the evidence as high.
5.2. Analysis.
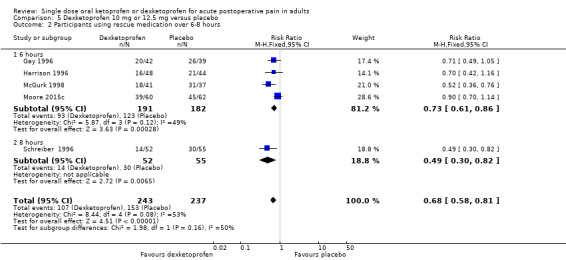
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
6.2. Analysis.
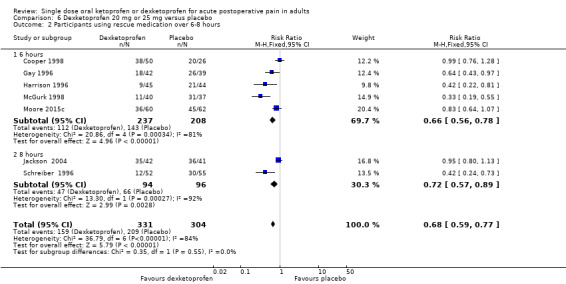
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
There was no difference between these two doses for the number of participants needing rescue medication.
| Summary of results F: participants using rescue medication within 6 hours with dexketoprofen | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNTp (95% CI) |
| 10 mg or 12.5 mg | 4 | 373 | 49 | 68 | 0.73 (0.61 to 0.86) | 5.3 (3.5 to 11) |
| 20 mg or 25 mg | 5 | 445 | 47 | 69 | 0.66 (0.56 to 0.78) | 4.7 (3.3 to 8.0) |
| CI: confidence interval; NNTp: number needed to treat to prevent an additional harmful event; RR: risk ratio. | ||||||
Adverse events with ketoprofen
Any adverse event
Eleven studies reported the numbers of participants experiencing at least one adverse event over a period of six hours post dose (Arnold 1990; Cooper 1984; Cooper 1988; McGurk 1998; Olson 1999; Olson 2001; Seymour 1996; Seymour 2000; Sunshine 1988; Sunshine 1998; Turek 1988). One study did not report on any adverse event (Mehlisch 1984), and three multiple dose studies did not report adverse event data for the single dose phase (Schreiber 1996; Sunshine 1993; Vidal 1999). One study reported the number of participants experiencing nausea within 10 hours post dose, and other specific adverse events at an unspecified time in the evening after surgery (Akural 2009). The authors also reported that there were four to six "unrousable or moderately sedated" participants in each of the four treatment arms (n = 18 to 20), with the maximum number within 1.5 hours from dosing. There was no further comment on this adverse event, and there were no associated withdrawals from the study.
Adverse events were generally described as subjective complaints of mild or moderate intensity, and many could be attributed to the surgical procedure itself, or the anaesthetic.
Three studies using ketoprofen 12.5 mg reported on the number of participants with at least one adverse event (Seymour 1996; Seymour 2000; Sunshine 1998): 6% (8/138) with ketoprofen and 4% (6/136) with placebo (Analysis 1.3).
Seven studies using ketoprofen 25 mg reported on the number of participants with at least one adverse event (Arnold 1990; Cooper 1984; Cooper 1988; Olson 1999; Olson 2001; Seymour 1996; Sunshine 1998): 10% (27/259) with ketoprofen and 10% (22/231) with placebo (Analysis 2.3).
Five studies using ketoprofen 50 mg reported on the number of participants with at least one adverse event (Cooper 1984; McGurk 1998; Olson 1999; Sunshine 1988; Turek 1988): 18% (31/173) with ketoprofen and 11% (18/169) with placebo (Analysis 3.3).
Three studies using ketoprofen 100 mg reported on the number of participants with at least one adverse event (Arnold 1990; Cooper 1984; Cooper 1988): 22% (19/86) with ketoprofen and 18% (16/89) with placebo (Analysis 4.3).
There were too few data for ketoprofen 6.25 mg and 150 mg to carry out any analysis.
1.3. Analysis.
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.
2.3. Analysis.
Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 3 Participants with any adverse event.
3.3. Analysis.
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event.
4.3. Analysis.
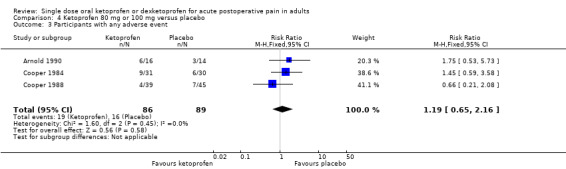
Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 3 Participants with any adverse event.
There was no difference in numbers of participants reporting at least one adverse event between ketoprofen and placebo at any dose tested (Summary of results G). We judged the quality of the evidence as high due to the consistency of results for the different doses.
| Summary of results G: participants with at least one adverse event | ||||||
| Dose | Studies | Participants | Ketoprofen (%) | Placebo (%) | RR (95% CI) | NNH (95% CI) |
| 12.5 mg | 3 | 274 | 5.8 | 4.4 | 1.3 (0.48 to 3.6) | Not calculated |
| 25 mg | 7 | 490 | 10 | 9.1 | 1.2 (0.68 to 2.0) | Not calculated |
| 50 mg | 5 | 342 | 18 | 11 | 1.6 (0.98 to 2.8) | Not calculated |
| 100 mg | 3 | 175 | 22 | 18 | 1.2 (0.65 to 2.2) | Not calculated |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
Serious adverse events
No study reported any serious adverse events. We assessed the quality of evidence as very low quality, based on there being no events, but in single dose studies that are not designed to evaluate serious but rare adverse events.
Adverse events with dexketoprofen
Any adverse event
Four studies reported the numbers of participants experiencing at least one adverse event over a period of six hours post dose (Cooper 1998; Gay 1996; Harrison 1996; McGurk 1998). Two studies reported over 24 hours (Jackson 2004; Moore 2015c), and four multiple dose studies did not report adverse event data for the single dose phase (McQuay 2016; Moore 2016; Schreiber 1996; Vidal 1999). Adverse events were generally described as subjective complaints of mild or moderate intensity, and many could be attributed to the surgical procedure itself, or the anaesthetic.
Four studies (380 participants) using dexketoprofen 10 mg or 12.5 mg reported on the number of participants with at least one adverse event (Gay 1996; Harrison 1996; McGurk 1998; Moore 2015c): 6.8% (13/192) with ketoprofen and 9.6% (18/188) with placebo (Analysis 5.3).
Six studies (536 participants) using dexketoprofen 20 mg or 25 mg reported on the number of participants with at least one adverse event (Cooper 1998; Gay 1996; Harrison 1996; Jackson 2004; McGurk 1998; Moore 2015c): 16% (46/281) with ketoprofen and 10% (26/255) with placebo (Analysis 6.3).
5.3. Analysis.
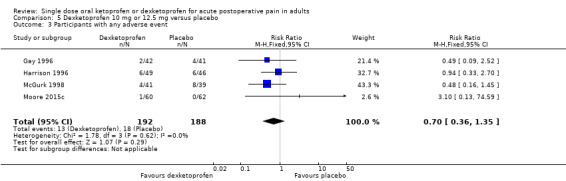
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.
6.3. Analysis.
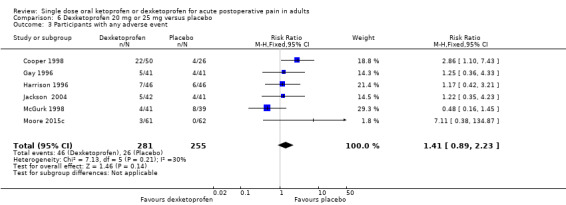
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 3 Participants with any adverse event.
At neither dose was there a significant difference in numbers of participants reporting at least one adverse event between dexketoprofen and placebo (Summary of results H). We judged the quality of the evidence as high due to the consistency of results for the different doses.
| Summary of results H: participants with at least one adverse event | ||||||
| Dose | Studies | Participants | Dexketoprofen (%) | Placebo (%) | RR (95% CI) | NNH (95% CI) |
| 10/12.5 mg | 4 | 380 | 6.8 | 9.8 | 0.70 (0.36 to 1.4) | Not calculated |
| 20/25 mg | 6 | 536 | 16 | 10 | 1.4 (0.89 to 2.2) | Not calculated |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
Serious adverse events
No study reported any serious adverse events. We assessed the quality of evidence as very low quality, based on there being no events, but in single dose studies that are not designed to evaluate serious but rare adverse events.
Withdrawals with ketoprofen and dexketoprofen
Participants who took rescue medication were classified as withdrawals due to lack of efficacy, and details are reported under 'Use of rescue medication' above.
Most studies reported some exclusions from efficacy analyses, and sometimes safety analyses. Exclusions may not be of any particular consequence in single dose acute pain studies, where most result from people having inadequate (less than moderate) pain after surgery to meet study inclusion criteria (McQuay 1982).
Adverse event withdrawals were infrequent. After single doses, Arnold 1990 reported one adverse event withdrawal due to nausea and dizziness with ketoprofen 25 mg, McGurk 1998 reported one with dexketoprofen 50 mg, and one with placebo (no details), and Harrison 1996 reported one with dexketoprofen 25 mg, and one with placebo (no details). In three multiple dose studies, there were two adverse event withdrawals with dexketoprofen 12.5 mg, five with dexketoprofen 25 mg, and four with placebo (Moore 2016; Schreiber 1996; Vidal 1999).
Discussion
Summary of main results
This review included 24 studies, with six additional studies in this update, three of which were large (McQuay 2016; Moore 2015c; Moore 2016). The new studies added 1001 participants involved in placebo comparisons with active drug, and of these, 721 had general surgery, including major orthopaedic surgery or abdominal hysterectomy, and 626 of these were in two multiple dose studies lasting several days but designed to report single dose results separately (McQuay 2016; Moore 2016).
In all 24 included studies, the total number of participants who took medication was 5220, of whom 1084 received ketoprofen alone (dose range 6.25 mg to 150 mg; mostly 25 mg and 50 mg), 1120 received dexketoprofen alone (dose range 5 mg to 100 mg; mostly 12.5 mg and 25 mg), and 1156 received placebo. In the previous version of this review, the numbers were: ketoprofen 968 participants and dexketoprofen 681 participants, making the increase in participants treated with the drugs 12% for ketoprofen and 65% more for dexketoprofen.
For ketoprofen 50 mg, 66% of participants in comparisons with placebo were in dental studies and 34% in other types of surgery. Dental studies gave a distinct dose response relationship, with an NNT of 2.4 at 12.5 mg improving to 1.6 at 100 mg for at least 50% pain relief compared with placebo. There was much less of a dose response relationship when all studies were combined, with NNT values between 2.0 and 2.9. The highest (worst) NNT was with the standard oral dose of ketoprofen 50 mg, where the NNT was 2.9 (95% CI 2.4 to 3.7; 8 studies, 594 participants). There was a distinct and statistically significant (P < 0.00006) difference at ketoprofen 50 mg between dental surgery (NNT 1.8 (95% CI 1.5 to 2.2); 3 studies 190 participants) and other surgery (NNT 4.2 (95% CI 3.0 to 6.7); 5 studies, 404 participants).
For dexketoprofen 25 mg, 38% of participants in comparisons with placebo were in dental studies and 62% in other types of surgery. Dental studies gave a sensible dose‐response relationship with an NNT of 3.6 at 10/12.5 mg improving to 2.7 at 100 mg for at least 50% pain relief compared with placebo. A dose response relationship could not be ascertained for other types of surgery, but NNT values were high, at above 5 for both doses. As a consequence of the larger proportion of participants having had other types of surgery, there was no dose response relationship with all studies together, with NNT values of 3.9 and 4.1 for 10 mg or 12.5 mg and 20 mg or 25 mg, respectively. There was a distinct and statistically significant (P < 0.00006) difference at dexketoprofen 25 mg between dental surgery (NNT 2.7 (95% CI 2.2 to 3.3); 5 studies; 444 participants) and other surgery (NNT 5.7 (95% CI 4.1 to 9.6); 3 studies; 733 participants). Demonstrating a dose response relationship can be difficult except by using a method involving pooling of direct comparison studies (McQuay 2007).
The same problems with small numbers and indirect comparisons affected comparisons of doses of ketoprofen and dexketoprofen, where similar efficacy would be expected for dexketoprofen at half the dose of ketoprofen. The amount of information available was inadequate to exclude that there is a 2:1 dose ratio between ketoprofen and dexketoprofen for the same effect in acute pain. This was not found, though in another review, a direct comparison on very limited numbers across different pain models did find the expected result (Moore 2008c).
Results for different pain models were clearly heterogeneous in this data set, as Figure 4 and Figure 6 show, comparing dental, postsurgical, and bunionectomy studies. There were too few studies to make any sensible cross‐comparisons about effects of different pain models on analgesic efficacy estimates. Where comparison of surgery type has been possible previously, no major effect of pain model has been found, although absolute response rates do differ (Barden 2004; Moore 1998). While third molar extraction studies typically involved participants in their 20s, other types of surgery involved older adults, often in their 40s to 70s. Age might be an issue: data sets in this analysis had many more non‐dental surgery studies than is usual, as third‐molar extraction typically amounts to around 80% of studies and participants in single dose studies (Moore 2015a). In addition, it is not entirely clear whether the effects of the duration of fasting before drug administration might have been responsible for these results, as food has been shown to affect NSAID absorption (Moore 2014).
6.
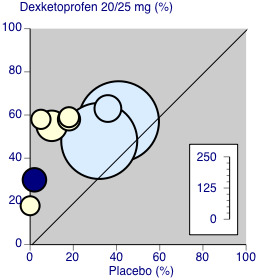
Dexketoprofen 20/25 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.
Overall, the results for ketoprofen and dexketoprofen are those expected for NSAID drugs in acute postoperative pain in participants with established pain of at least moderate intensity. NNTs for at least 50% pain relief for ketoprofen and dexketoprofen were generally between 2 and 3 in dental studies, comparable with other commonly used analgesics at recommended doses (e.g. ibuprofen 400 mg: NNT 2.3, Derry 2009a; diclofenac 50 mg: NNT 2.7, Derry 2009b). Median time to use of rescue medication was also comparable at four to five hours. Efficacy appears to be a little better than with paracetamol 1000 mg (NNT 3.2, Toms 2008), and worse than with etoricoxib 120 mg (NNT 1.6, Clarke 2014).
In these single dose studies, adverse events did not differ from placebo at any dose of ketoprofen and dexketoprofen, and there were no serious adverse events reported. Withdrawals due to adverse events were uncommon and also did not differ from placebo. This is similar to what is usually found in this type of single dose study (Moore 2015b).
Overall completeness and applicability of evidence
The clinical question is not only about short‐term efficacy following single doses, but also about how well analgesics work over several days to address acute pain following surgery. Two of the newer studies included in this review for their single dose results had a design that also looked at longer term results, albeit not for these drugs (McQuay 2016; Moore 2016). They used novel outcomes, including that of having average postoperative pain no worse than mild, an outcome of value to participants (Moore 2013).
Long term multiple dose studies are needed for meaningful analysis of adverse events since analgesics are likely to be used in multiple doses, even in acute pain settings. The difficulty in the postoperative setting is that there are many sequelae of surgery and anaesthesia that manifest as adverse events, such as nausea, vomiting, or abdominal discomfort, while others, such as headache, can be caused by events such as acute caffeine withdrawal over the postoperative period. The main issue is that of rare but serious adverse events, and these are more likely to be found in large observational studies.
Quality of the evidence
Methodological quality was good, with all studies scoring above the minimum required to minimise bias on the Oxford Quality Score; 20 of 24 scored 4/5 or 5/5.
The results of this review are confounded by relatively small numbers of studies and participants, and by clinical heterogeneity in the non‐dental pain models. In particular, a study in bunionectomy was insensitive (Vidal 1999), in keeping with a similar finding in rofecoxib trials (Bulley 2009).
Loss of information from withdrawals or exclusions was small, and was unlikely to have led to an overestimate of efficacy because it is as likely to be related to poor reporting as poor methods. In single dose studies, most exclusions occur for protocol violations such as failing to meet baseline pain requirements, or failing to return for post‐treatment visits after the acute pain results are concluded (McQuay 1982). For missing data, it has been shown that over the four‐ to six‐hour period, there is no difference between baseline observation carried forward and last observation carried forward, but the former gives the more conservative estimate over longer duration observations (Moore 2005).
Potential biases in the review process
We know of no potential biases in the review process. Publication bias was considered unlikely as the susceptibility to publication bias using a limit set at an NNT of 10 would have meant that between 750 and 2100 participants would have been required in unpublished studies of null effect reach that threshold of clinical irrelevance (Moore 2008b). Those numbers are larger than the available data sets.
Agreements and disagreements with other studies or reviews
The results in this updated review are similar to those in the original Cochrane Review (Barden 2009), and a previous meta‐analysis of dexketoprofen (Moore 2008c).
Authors' conclusions
Implications for practice.
For people with acute pain
A single oral dose of ketoprofen 50 mg or dexketoprofen 25 mg provided good levels of pain relief to more people than placebo. Experience has shown that efficacy demonstrated in one acute pain condition is generally applicable in others, although the absolute response rate may vary. Lower doses can also provide good pain relief, but typically to fewer people.
For clinicians
A single oral dose of ketoprofen 50 mg or dexketoprofen 25 mg provided good levels of pain relief to more people than placebo. The magnitude of the effect is similar to other good analgesics, as reported in Cochrane Reviews of individual analgesics and in two overviews. Adverse event rates were low, and similar to placebo.
For policy makers
Ketoprofen 50 mg or dexketoprofen 25 mg is an effective analgesic in acute pain.
For funders
Ketoprofen 50 mg or dexketoprofen 25 mg is an effective analgesic in acute pain.
Implications for research.
General
This review confirms that ketoprofen 50 mg or dexketoprofen 25 mg provide good levels of pain relief in a large proportion of people with acute pain. It is unclear from the available data whether lower doses can provide the same level of efficacy as standard doses of the individual drugs, possibly with reduced adverse events. There is also uncertainty concerning different magnitude of effect of the same dose following third‐molar surgery and other types of surgery. As of 2016, there do not appear to be good reasons for examining that aspect of these results in detail, but the topic of differential magnitude of analgesic effect in different types of surgery might be of value in the future.
Design
The current design of acute pain studies is well understood, and is proven to be robust.
Measurement (endpoints)
Endpoints in these studies have been extensively validated, as have standard pain scoring systems. The main outcome used is one valued by people with pain, and has economic benefits in most circumstances.
Comparison between active treatments
The standardised nature of the study design means that indirect comparisons with placebo are valid, as evidenced by independent research. However, there is a very large body of information amenable to network meta‐analysis. While unlikely to provide much in the way of new insights, it could prove an invaluable tool for testing network meta‐analytical methods.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 30 May 2017 | Amended | Minor typo amended in abstract. |
| 25 May 2017 | Review declared as stable | See Published notes. |
| 28 February 2017 | New citation required but conclusions have not changed | Data from 6 new studies available: 3 studies from new searches, 2 studies previously identified but now with data, and 1 study previously mislabelled (1001 new participants). This update includes updated 'Risk of bias' and GRADE assessments, and Summary of findings tables. No change to conclusions. |
| 23 February 2017 | New search has been performed | New searches carried out in November 2016 and March 2017. |
| 15 September 2011 | Review declared as stable | The authors scanned the literature in August 2011 and are confident that there will be no need to update this search until at least 2015. |
| 8 February 2011 | Amended | Contact details updated. |
| 24 September 2010 | Amended | Contact details updated. |
| 28 September 2009 | Amended | Incorrect date of protocol first published has now been corrected |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in four years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
This review received infrastructure support from the Oxford Pain Relief Trust. Professor Henry McQuay and Jodie Barden were authors on an earlier version of this review.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS), or the Department of Health.
Menarini Group provided copies of published and unpublished studies for dexketoprofen for the earlier review; the company markets products containing both ketoprofen and dexketoprofen.
Appendices
Appendix 1. Glossary
Categorical rating scale: the most common are the four‐category scale for pain intensity (none, mild, moderate, and severe) and the five‐category scale for pain relief (none, slight, moderate, good or lots, and complete). For analysis, numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2, and severe = 3, and for pain relief, none = 0, slight = 1, moderate = 2, good or lots = 3, and complete = 4). Data from different participants are then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. There was good correlation, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
Visual analogue scale (VAS): for pain intensity, lines with left end labelled 'no pain' and right end labelled 'worst pain imaginable', and for pain relief lines with left end labelled 'no relief of pain' and right end labelled 'complete relief of pain', seem to overcome the limitation of forcing participant descriptors into particular categories. Participants mark the line at the point that corresponds to their pain or pain relief. The scores are obtained by measuring the distance between the 'no relief of pain' end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms, and provide many points from which to choose. More concentration and co‐ordination are needed, which can be difficult postoperatively or with neurological disorders.
Total pain relief (TOTPAR): TOTPAR is calculated as the sum of pain relief scores over a period of time. If a participant had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
Summed pain intensity difference (SPID): SPID is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the composite trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See 'Measuring pain' in Bandolier’s Little Book of Pain (Moore 2003).
Appendix 2. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals);
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose‐response gradient.
Appendix 3. CENTRAL search strategy
MESH DESCRIPTOR ketoprofen EXPLODE ALL TREES (430)
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse):TI,AB,KY (144)
(ketoprofen* OR Orudis OR Oruvail):TI,AB,KY (957)
#1 OR #2 OR #3 (1032)
MESH DESCRIPTOR Pain, postoperative EXPLODE ALL TREES (10769)
((postoperative near4 pain*) or (post‐operative near4 pain*) or post‐operative‐pain* or (post* near4 pain*) or (postoperative near4 analgesi*) or (post‐operative near4 analgesi*) or ("post‐operative analgesi*")):TI,AB,KY (20218)
((post‐surgical near4 pain*) or ("post surgical" near4 pain*) or (post‐surgery near4 pain*)):TI,AB,KY (148)
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")):TI,AB,KY (465)
(("post surg*" or post‐surg*) AND (pain* or discomfort)):TI,AB,KY (547)
((pain* near4 "after surg*") or (pain* near4 "after operat*") or (pain* near4 "follow* operat*") or (pain* near4 "follow* surg*")):TI,AB,KY (941)
((analgesi* near4 "after surg*") or (analgesi* near4 "after operat*") or (analgesi* near4 "follow* operat*") or (analgesi* near4 "follow* surg*")):TI,AB,KY (358)
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 (23536)
#4 AND #12 (403)
2009 TO 2017:YR (397652)
#13 AND #14 (150)
Appendix 4. MEDLINE search strategy (via Ovid)
Ketoprofen/ (2532)
(ketoprofen* or alrheum* or profenid or orudis or oruvail).mp. (3624)
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse).mp. (193)
1 or 2 or 3 (3655)
Pain, postoperative/ (32561)
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp. (52819)
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (429)
("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp. (692)
(("post surg*" or post‐surg*) and (pain* or discomfort)).mp. (1526)
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (3131)
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (641)
5 or 6 or 7 or 8 or 9 or 10 or 11 (55263)
randomized controlled trial.pt. (456415)
controlled clinical trial.pt. (93323)
randomized.ab. (348139)
placebo.ab. (171162)
drug therapy.fs. (1966014)
randomly.ab. (239416)
trial.ab. (363498)
groups.ab. (1488935)
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (3755923)
4 and 12 and 21 (373)
limit 22 to yr="2009 ‐Current" (125)
Appendix 5. Embase search strategy (via Ovid)
ketoprofen/ (12022)
(ketoprofen* or alrheum* or profenid or orudis or oruvail).mp. (12452)
(dexketoprofen or Keral or Enantyum or Dolmen or Ketesse).mp. (626)
1 or 2 or 3 (12894)
Pain, postoperative/ (8777)
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp. (91905)
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (1068)
("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp. (1140)
(("post surg*" or post‐surg*) and (pain* or discomfort)).mp. (4190)
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (5086)
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (943)
5 or 6 or 7 or 8 or 9 or 10 or 11 (97035)
(random* or factorial* or crossover or "cross over" or cross‐over).tw. (1252502)
(placebo* or (doubl* adj blind*) or (singl* adj blind*)).tw. (331204)
(assign* or allocat*).tw. (420063)
crossover Procedure/ (55890)
double‐blind procedure/ (142435)
Randomized Controlled Trial/ (487371)
13 or 14 or 15 or 16 or 17 or 18 (1673776)
4 and 12 and 19 (577)
limit 20 to yr="2009 ‐Current" (276)
Appendix 6. Summary of outcomes in individual studies: efficacy
| Study ID | Treatment | Analgesia | Rescue medication | |||
| PI or PR | Number with 50% PR | PGE: very good or excellent | Median time to use (h) | % using | ||
| Akural 2009 | (1) Ketoprofen 100 mg, n = 20 (2) Paracetamol 1000 mg, n = 18 (3) Ketoprofen 100 mg + paracetamol 1000 mg, n = 20 (4) Placebo, n = 20 |
No usable data | No usable data | No data | (1) 5 (3) 9 (4) 1 |
No data |
| Arnold 1990 | (1) ketoprofen 25 mg, n = 14 (2) Ketoprofen 100 mg, n = 16 (3) Ibuprofen 400 mg, n = 15 (4) Placebo, n = 14 |
TOTPAR 6: (1) 6.0 (2) 9.8 (4) 1.5 |
(1) 3/14 (2) 7/16 (4) 0/14 |
At 6 h: (1) 2/14 (2) 7/16 (4) 1/14 |
Mean: (1) 4.8 (2) 4.4 (4) 2.4 |
At 6 h: (1) 46 (2) 45 (4) 83 |
| Balzanelli 1996 | (1) Ketoprofen lysine 80 mg, n = 30 (2) Placebo, n = 30 |
SPID 6: (1) 200 (2) 23.5 |
(1) 18/30 (2) 0/30 |
No data | No data | No data |
| Cooper 1984 | (1) Ketoprofen 25 mg, n = 30 (2) Ketoprofen 50 mg, n = 31 (3) Ketoprofen 100 mg, n = 31 (4) Aspirin 650 mg, n = 31 (5) Placebo, n = 31 |
TOTPAR 6: (1) 13.6 (2) 15.5 (3) 17.1 (5) 4.63 |
(1) 18/30 (2) 23/31 (3) 26/31 (5) 4/31 |
No usable data | Mean: (1) 4.8 (2) 4.8 (3) 4.9 (5) 2.6 |
No data |
| Cooper 1988 | (1) Ketoprofen 25 mg, n = 42 (2) Ketoprofen 100 mg, n = 39 (3) Ibuprofen 400 mg, n = 37 (4) Placebo, n = 43 |
TOTPAR 6: (1) 12.0 (2) 15.2 (4) 4.7 |
(1) 23/42 (2) 28/39 (4) 6/43 |
At 6 h: (1) 17/42 (2) 21/39 (4) 2/43 |
Mean: (1) 5.0 (2) 4.3 (4) 3.0 |
At 6 h: (1) 69 (2) 36 (4) 79 |
| Cooper 1998 | (1) Dexketoprofen 25 mg, n = 50 (2) Dexketoprofen 100 mg, n = 51 (3) Paracetamol 1000 mg, n =50 (4) Placebo, n = 26 |
TOTPAR 6: (1) 5.3 (2) 8.2 (4) 4.5 |
(1) 9/50 (2) 17/51 (4) 0/26 |
No data | (1) 2.1 (2) 3.3 (4) 1.7 |
At 6 h: (1) 76 (2) 57 (4) 78 |
| Gay 1996 | (1) Dexketoprofen 5 mg, n = 41 (2) Dexketoprofen 10 mg, n = 42 (3) Dexketoprofen 20 mg, n = 41 (4) Ibuprofen 400 mg, n = 41 (5) Placebo, n = 41 |
TOTPAR 6: (1) 9.8 (2) 10.5 (3) 11.3 (5) 5.2 |
(1) 18/41 (2) 20/42 (3) 24/41 (5) 7/39 |
No usable data | Mean: (1) 5.0 (2) 4.82 (3) 5.0 (5) 3.65 |
At 6 h: (1) 34 (2) 48 (3) 43 (5) 67 |
| Harrison 1996 | (1) Dexketoprofen 12.5 mg, n = 49 (2) Dexketoprofen 25 mg, n = 46 (3) Placebo, n = 46 |
TOTPAR 6: (1) 10.6 (2) 12.4 (3) 5.2 |
(1) 23/48 (2) 26/45 (3) 8/44 |
No usable data | No data | At 6 h: (1) 33 (2) 20 (3) 48 |
| Jackson 2004 | (1) Dexketoprofen 25 mg, n = 42 (2) Rofecoxib 50 mg, n = 38 (3) Placebo, n = 43 |
No usable data | No usable data | (1) 6.6 (3) 2.5 |
At 24 h: (1) 83 (3) 88 |
|
| McGurk 1998 | (1) Ketoprofen 50 mg, n = 43 (2) Dexketoprofen 12.5 mg, n = 44 (3) Dexketoprofen 25 mg, n = 41 (4) Dexketoprofen 50 mg, n = 43 (5) Placebo, n = 39 |
TOTPAR 6: (1) 10.2 (2) 12.6 (3) 12.3 (4) 12.2 (5) 3.2 |
(1) 22/40 (2) 18/41 (3) 23/40 (4) 24/42 (5) 2/37 |
No usable data | Mean: (1) 5.5 (2) 4.9 (3) 5.3 (4) 5.4 (5) 3.6 |
At 6 h: (1) 15 (2) 41 (3) 27 (4) 24 (5) 71 |
| McQuay 2016 | (1) Dexketoprofen 25 mg, n = 161 (2) Tramadol 100 mg, n = 160 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 159 (4) Placebo, n = 161 |
TOTPAR 6 (SD):
(1) 12 (5.2) (2) 12 (5.2) (3) 13 (5.4) (4) 10 (5.2) |
(1) 92/161 (2) 86/160 (3) 97/159 (4) 66/161 |
At 8 h*:
(1) 44/161 (2) 51/160 (3) 56/159 (4) 18/161 |
No data | At 8 h*: (1) Approx. 10 (2) Approx. 10 (3) Approx. 10 (4) Approx. 25 |
| Mehlisch 1984 | (1) Ketoprofen 25 mg, n = 24 (2) Ketoprofen 50 mg, n = 27 (3) Ketoprofen 100 mg, n = 27 (4) Codeine 90 mg, n = 27 (5) Placebo, n = 24 |
TOTPAR 6: (1) 12.4 (2) 12.7 (3) 12.8 (5) 1.8 |
(1) 14/24 (2) 16/27 (3) 16/27 (5) 0/24 |
No usable data | No data | At 6 h: (1) 54 (2) 72 (3) 51 (5) 96 |
| Moore 2015c | (1) Dexketoprofen 12.5 mg, n = 60 (2) Dexketoprofen 25 mg, n = 60 (3) Tramadol 37.5 mg, n = 59 (4) Tramadol 75 mg, n = 59 (5) Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60 (6) Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62 (7) Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63 (8) Dexketoprofen 25 mg + tramadol 75 mg, n = 61 (9) Ibuprofen 400 mg, n = 60 (10) Placebo, n = 62 |
TOTPAR 6 (SD): (1) 7.9 (5.89) (2) 11.8 (5.60) (3) 4.0 (4.46) (4) 5.4 (6.10) (5) 10.2 (5.52) (6) 13.3 (7.04) (7) 12.6 (6.58) (8) 14.5 (6.14) (9) 10.5 (7.15) (10) 2.9 (4.82) |
(1) 16/60 (2) 33/60 (3) 6/59 (4) 15/59 (5) 22/60 (6) 37/62 (7) 35/63 (8) 44/61 (9) 27/60 (10) 6/62 |
At 24 h: (1) 19/60 (2) 17/60 (3) 5/59 (4) 8/59 (5) 16/60 (6) 29/62 (7) 29/63 (8) 31/61 (9) 20/60 (10) 3/62 |
Median (95% confidence intervals): (1) 3.6 (2.7 to 4.3) (2) 5.6 (4.8 to 7.6) (3) 2.2 (1.3 to 3.0) (4) 2.5 (1.4 to 3.9) (5) 4.9 (4.0 to 5.8) (6) 8.5 (5.9 to 13) (7) 7.3 (6.3 to 9.0) (8) 8.1 (6.3 to 13) (9) 7.1 (4.8 to 8.6) (10) 1.4 (1.2 to 1.8) |
At 6 h: (1) 65 (2) 60 (3) 69 (4) 64 (5) 67 (6) 47 (7) 40 (8) 38 (9) 48 (10) 73 |
| Moore 2016 | (1) Dexketoprofen 25 mg, n = 151 (2) Tramadol 100 mg, n = 150 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 152 (4) Placebo, n = 153 |
TOTPAR 6 (SD):
(1) 11 (5.2) (2) 11 (5.5) (3) 14 (4.6) (4) 8.9 (5.1) |
(1) 72/151 (2) 64/150 (3) 105/152 (4) 49/153 |
At 8 h:
(1) 28/151 (2) 22/150 (3) 42/152 (4) 14/153 |
No data | No data |
| Olson 1999 | (1) Ketoprofen (liquid) 25 mg, n = 28 (2) Ketoprofen (liquid) 50 mg, n = 26 (3) Dipyrone (liquid) 500 mg, n = 27 (4) Placebo, n = 27 |
TOTPAR 6: (1) 14.3 (2) 14.3 (4) 4.8 |
(1) 19/28 (2) 18/26 (4) 5/27 |
No data | Mean: (1) > 6 (2) 5.9 (4) 5.3 |
At 6 h: (1) 0 (2) 4 (4) 33 |
| Olson 2001 | (1) Ketoprofen 25 mg, n = 67 (2) Ibuprofen liquigel 400 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 |
TOTPAR 6: (1) 15.0 (4) 4.3 |
(1) 48/67 (4) 5/39 |
(1) 47/67 (4) 4/39 |
(1) > 6 (4) 1.3 |
At 6 h: (1) 20/67 (4) 31/39 |
| Schreiber 1996 | (1) Ketoprofen 50 mg, n = 54 (2) Dexketoprofen 12.5 mg, n = 52 (3) Dexketoprofen 25 mg, n = 52 (4) Placebo, n = 55 |
TOTPAR 4: (1) 6.8 (2) 8.0 (3) 9.0 (4) 5.8 |
(1) 24/54 (2) 29/52 (3) 33/52 (4) 20/55 |
No usable data | No data | At 8 h: (1) 31 (2) 27 (3) 23 (4) 55 |
| Seymour 1996 | (1) Ketoprofen 12.5 mg, n = 42 (2) Ketoprofen 25 mg, n = 41 (3) Paracetamol 500 mg, n = 41 (4) Paracetamol 1000 mg, n = 41 (5) Placebo, n = 41 |
No usable data | No data | At 6 h: (1) 28/42 (2) 28/41 (5) 8/41 |
(1) 4.0 (2) 4.1 (5) 1.8 |
At 6 h: (1) 75 (2) 76 (5) 97 |
| Seymour 2000 | (1) Buffered ketoprofen 12.5 mg, n = 61 (2) Ibuprofen 200 mg, n = 59 (3) Placebo, n = 60 |
TOTPAR 6: (1) 9.8 (3) 4.1 |
(1) 26/61 (3) 7/60 |
No usable data | (1) 2.7 (3) 1.9 |
At 6 h: (1) 87 (3) 98 |
| Sunshine 1988 | (1) Ketoprofen 50 mg, n = 32 (2) Ketoprofen 150 mg, n = 31 (3) Paracetamol 650 mg + codeine 60 mg, n = 28 (4) Placebo, n = 32 |
TOTPAR 6: (1) 14.6 (2) 14.8 (3) 12.2 (4) 9.5 |
(1) 22/32 (2) 22/31 (3) 16/28 (4) 13/32 |
No usable data | No data | No data |
| Sunshine 1993 | (1) Ketoprofen 50 mg, n = 48 (2) Ketoprofen 100 mg, n = 48 (3) Paracetamol 650 mg, n = 48 (4) Paracetamol 650 mg + oxycodone 10 mg, n = 48 (5) Placebo, n = 48 |
TOTPAR 6: (1) 11.3 (2) 12.9 (5) 8.8 |
(1) 25/48 (2) 29/48 (5) 18/48 |
No usable data | (1) 7.0 (2) 8.8 (5) 6.0 |
At 8 h: (1) 69 (2) 46 (5) 73 |
| Sunshine 1998 | (1) Ketoprofen 6.25 mg, n = 35 (2) Ketoprofen 12.5 mg, n = 35 (3) Ketoprofen 25 mg, n = 35 (4) Ibuprofen 200 mg, n = 35 (5) Placebo, n = 35 |
TOTPAR 6: (1) 7.2 (2) 13.7 (3) 13.0 (5) 3.6 |
(1) 10/35 (2) 23/35 (3) 21/35 (5) 3/35 |
No usable data | No usable data | No usable data |
| Turek 1988 | (1) Ketoprofen 50 mg, n = 41 (2) Ketoprofen 150 mg, n = 39 (3) Paracetamol 650 mg + codeine 60 mg, n = 39 (4) Placebo, n = 42 |
TOTPAR 6: (1) 11.4 (2) 12.2 (4) 4.6 |
(1) 21/41 (2) 22/39 (4) 6/41 |
No usable data | Mean: (1) 2.3 (2) 3.2 (4) 2.2 |
At 6 h: (1) 41 (2) 46 (4) 83 |
| Vidal 1999 | (1) Ketoprofen 50 mg, n = 47 (2) Dexketoprofen 12.5 mg, n = 47 (3) Dexketoprofen 25 mg, n = 47 (4) Placebo, n = 47 |
TOTPAR 6: (1) 2.7 (2) 7.4 (3) 7.4 (4) 2.5 |
(1) 2/47 (2) 14/47 (3) 14/47 (4) 1/47 |
No usable data | Mean: (1) 1.76 (2) 2.31 (3) 2.2 (4) 1.68 |
At 6 h: (1) 98 (2) 91 (3) 93 (4) 100 |
| * Personal communication from study authors. Approx: approximately; h: hour; n: number of participants; PGE: Patient Global Evaluation; PI: pain intensity; PR: pain relief; SD: standard deviation; SPID: summed pain intensity difference; TOTPAR: total pain relief. | ||||||
Appendix 7. Summary of outcomes in individual studies: adverse events and withdrawals
| Study ID | Treatment | Adverse events | Withdrawals | ||
| Any | Serious | Adverse event | Other | ||
| Akural 2009 | (1) Ketoprofen 100 mg, n = 20 (2) Paracetamol 1000 mg, n = 18 (3) Ketoprofen 100 mg + paracetamol 1000 mg, n = 20 (4) Placebo, n = 20 |
No usable data Nausea within 10 h: (1) 4/20 (3) 4/20 (4) 3/20 4‐6 "unrousable or moderately sedated " participants in each of the 4 treatment arms (n = 18‐20), with the maximum number within 1.5 h from dosing |
None | None | 4 exclusions due to protocol violations |
| Arnold 1990 | (1) Ketoprofen 25 mg, n = 14 (2) Ketoprofen 100 mg, n = 16 (3) Ibuprofen 400 mg, n = 15 (4) Placebo, n = 14 |
At 6 h: (1) 3/14 (2) 6/16 (4) 3/14 |
None | (1) 1/14 (nausea and dizziness after 1 h) | None reported |
| Balzanelli 1996 | (1) Ketoprofen lysine 80 mg, n = 30 (2) Placebo, n = 30 |
No usable data | None reported | None | None |
| Cooper 1984 | (1) Ketoprofen 25 mg, n = 30 (2) Ketoprofen 50 mg, n = 31 (3) Ketoprofen 100 mg, n = 31 (4) Aspirin 650 mg, n = 31 (5) Placebo, n = 31 |
At 6 h: (1) 7/30 (2) 10/31 (3) 9/31 (5) 6/30 |
None | None | Exclusions due to not taking medication, protocol violations and loss to follow‐up: (1) 6 (2) 5 (3) 5 (4) 6 (5) 6 |
| Cooper 1988 | (1) Ketoprofen 25 mg, n = 42 (2) Ketoprofen 100 mg, n = 39 (3) Ibuprofen 400 mg, n = 37 (4) Placebo, n = 43 |
At 6 h: (1) 8/44 (2) 4/39 (4) 7/45 |
None reported | None reported | 20 exclusions: 13 lost to follow‐up and 7 protocol violations |
| Cooper 1998 | (1) Dexketoprofen 25 mg, n = 50 (2) Dexketoprofen 100 mg, n = 51 (3) Paracetamol 1000 mg, n = 50 (4) Placebo, n = 26 |
At 6 h: (1) 22/50 (2) 16/51 (4) 4/26 |
None | None | None |
| Gay 1996 | (1) Dexketoprofen 5 mg, n = 41 (2) Dexketoprofen 10 mg, n = 42 (3) Dexketoprofen 20 mg, n = 41 (4) Ibuprofen 400 mg, n = 41 (5) Placebo, n = 41 |
At 6 h: (1) 3/41 (2) 2/42 (3) 5/41 (5) 4/39 |
None | None | 2 exclusions in placebo group due to early remedication |
| Harrison 1996 | (1) Dexketoprofen 12.5 mg, n = 49 (2) Dexketoprofen 25 mg, n = 46 (3) Placebo, n = 46 |
At 6 h: (1) 6/49 (2) 7/46 (3) 6/46 |
None | (1) 0/49 (2) 1/46 (3) 1/46 |
6 exclusions due to protocol violations |
| Jackson 2004 | (1) Dexketoprofen 25 mg, n = 42 (2) Rofecoxib 50 mg, n = 38 (3) Placebo, n = 43 |
At 24 h: (1) 5/42 (3) 4/41 |
None | None | 3 participants excluded from analyses: 2 in placebo group lost to follow‐up, 1 in rofecoxib group did not take medication |
| McGurk 1998 | (1) Ketoprofen 50 mg, n = 43 (2) Dexketoprofen 12.5 mg, n = 44 (3) Dexketoprofen 25 mg, n = 41 (4) Dexketoprofen 50 mg, n = 43 (5) Placebo, n = 39 |
At 6 h: (1) 5/43 (2) 4/41 (3) 4/41 (4) 7/43 (5) 8/39 |
None | (1) 0/42 (2) 0/44 (3) 0/41 (4) 1/43 (5) 1/39 |
10 participants excluded from efficacy analyses due to early remedication or loss to follow‐up: (1) 3 (2) 3 (3) 1 (4) 1 (5) 2 |
| McQuay 2016 | (1) Dexketoprofen 25 mg, n = 161 (2) Tramadol 100 mg, n = 160 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 159 (4) Placebo, n = 161 |
No usable data | None during single dose phase Over 5 days: (1) 1/213 (4 events) (2) 0/212 (3) 1/213 (1 event) |
None | (1) 1/161 (withdrawal by subject) (2) 0/160 (3) 0/159 (4) 2/161 (withdrawal by subject, protocol violation) |
| Mehlisch 1984 | (1) Ketoprofen 25 mg, n = 24 (2) Ketoprofen 50 mg, n = 27 (3) Ketoprofen 100 mg, n = 27 (4) Codeine 90 mg, n = 27 (5) Placebo, n = 24 |
At 6 h: 54 participants in total |
None reported | None reported | 9 participants received medication but were not included in analysis. Reasons and groups not given. |
| Moore 2015c | (1) Dexketoprofen 12.5 mg, n = 60 (2) Dexketoprofen 25 mg, n = 61 (3) Tramadol 37.5 mg, n = 59 (4) Tramadol 75 mg, n = 59 (5) Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60 (6) Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62 (7) Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63 (8) Dexketoprofen 25 mg + tramadol 75 mg, n = 61 (9) Ibuprofen 400 mg, n = 60 (10) Placebo, n = 62 |
Within 24 h: (1) 1/60 (2) 3/61 (10) 0/62 |
(1) 0/60 (2) 0/61 (10) 0/62 |
(1) 0/60 (2) 0/61 (10) 0/62 |
(1) 0/60 (2) 0/61 (10) 0/62 |
| Moore 2016 | (1) Dexketoprofen 25 mg, n = 151 (2) Tramadol 100 mg, n = 150 (3) Dexketoprofen 25 mg + tramadol 75 mg, n = 152 (4) Placebo, n = 153 |
No usable data | No usable data | No usable data | No usable data |
| Olson 1999 | (1) Ketoprofen liquid 25 mg, n = 28 (2) Ketoprofen liquid 50 mg, n = 26 (3) Dipyrone liquid 500 mg, n = 27 (4) Placebo, n = 27 |
No adverse events reported | None | None | None |
| Olson 2001 | (1) Ketoprofen 25 mg, n = 67 (2) Ibuprofen liquigel 400 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 |
At 6 h: (1) 5/67 (4) 2/39 |
None | None | None |
| Schreiber 1996 | (1) Ketoprofen 50 mg, n = 54 (2) Dexketoprofen 12.5 mg, n = 52 (3) Dexketoprofen 25 mg, n = 52 (4) Placebo, n = 55 |
No single dose data | None | Multiple dose: (1) 0/54 (2) 1/52 (3) 2/52 (4) 1/55 |
Multiple dose (includes successful therapy): (1) 35/54 (2) 36/52 (3) 35/52 (4) 39/55 |
| Seymour 1996 | (1) Ketoprofen 12.5 mg, n = 42 (2) Ketoprofen 25 mg, n = 41 (3) Paracetamol 500 mg, n = 41 (4) Paracetamol 1000 mg, n = 41 (5) Placebo, n = 41 |
At 6 h: (1) 0/42 (2) 0/41 (5) 0/41 |
None | None | Exclusions due to early remedication: (1) 2 (3) 1 (4) 1 (5) 2 |
| Seymour 2000 | (1) Buffered ketoprofen 12.5 mg, n = 61 (2) Ibuprofen 200 mg, n = 59 (3) Placebo, n = 60 |
At 6 h: (1) 2/61 (3) 3/60 |
None | None | Exclusions due to protocol violations: (2) 1 (3) 1 |
| Sunshine 1988 | (1) Ketoprofen 50 mg, n = 32 (2) Ketoprofen 150 mg, n = 31 (3) Paracetamol 650 mg + codeine 60 mg, n = 28 (4) Placebo, n = 32 |
At 6 h: (1) 2/32 (3) 1/32 |
None | None | None |
| Sunshine 1993 | (1) Ketoprofen 50 mg, n = 48 (2) Ketoprofen 100 mg, n = 48 (3) Paracetamol 650 mg, n = 48 (4) Paracetamol 650 mg + oxycodone 10 mg, n = 48 (5) Placebo, n = 48 |
No single dose data | "No cases of possible clinical concern" (multiple dose included) | None | None |
| Sunshine 1998 | (1) Ketoprofen 6.25 mg, n = 35 (2) Ketoprofen 12.5 mg, n = 35 (3) Ketoprofen 25 mg, n = 35 (4) Ibuprofen 200 mg, n = 35 (5) Placebo, n = 35 |
At 6 h: (1) 3/35 (2) 6/35 (3) 3/35 (5) 3/35 |
None | None | Exclusions due to early remedication, protocol violation: (2) 1 (3) 1 (5) 2 |
| Turek 1988 | (1) Ketoprofen 50 mg, n = 41 (2) Ketoprofen 150 mg, n = 39 (3) Paracetamol 650 mg + codeine 60 mg, n = 39 (4) Placebo, n = 42 |
At 6 h: (1) 14/41 (2) 8/39 (3) 4/41 |
None | None | 1 exclusion in placebo group due to protocol violation. |
| Vidal 1999 | (1) Ketoprofen 50 mg, n = 47 (2) Dexketoprofen 12.5 mg, n = 47 (3) Dexketoprofen 25 mg, n = 47 (4) Placebo, n = 47 |
No single dose data | None | Multiple dose: (1) 0/43 (2) 1/45 (3) 1/41 (4) 2/43 |
Multiple dose: (1) 0/43 (2) 2/45 (3) 2/41 (4) 3/43 |
| h: hour. | |||||
Data and analyses
Comparison 1. Ketoprofen 12.5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 6 hours | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.68, 6.63] |
| 2 Participants using rescue medication over 6 hours | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3 Participants with any adverse event | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.64] |
Comparison 2. Ketoprofen 25 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 6 hours | 8 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [3.48, 6.85] |
| 1.1 Dental surgery | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.50, 7.36] |
| 1.2 Other surgery | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.77, 8.86] |
| 2 Participants using rescue medication over 6 hours | 6 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| 3 Participants with any adverse event | 7 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.96] |
Comparison 3. Ketoprofen 50 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 4‐6 hours | 8 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.97, 3.14] |
| 1.1 Dental surgery | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.04 [4.23, 19.30] |
| 1.2 Other surgery | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.40, 2.28] |
| 2 Participants using rescue medication over 6‐8 hours | 6 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 2.1 6 hours | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.2 8 hours | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Participants with any adverse event | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.75] |
Comparison 4. Ketoprofen 80 mg or 100 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [3.02, 6.08] |
| 1.1 Dental surgery | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [4.67, 14.86] |
| 1.2 Other surgery | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 3.00] |
| 2 Participants using rescue medication over 6‐8 hours | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.67] |
| 2.1 6 hours | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.65] |
| 2.2 8 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 3 Participants with any adverse event | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
Comparison 5. Dexketoprofen 10 mg or 12.5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 4‐6 hours | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.79, 3.28] |
| 1.1 Dental surgery | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.08, 4.80] |
| 1.2 Other surgery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.35] |
| 2 Participants using rescue medication over 6‐8 hours | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.81] |
| 2.1 6 hours | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.86] |
| 2.2 8 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.82] |
| 3 Participants with any adverse event | 4 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.35] |
Comparison 6. Dexketoprofen 20 mg or 25 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 4‐6 hours | 8 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.68, 2.28] |
| 1.1 Dental surgery | 5 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.12, 6.95] |
| 1.2 Other surgery | 3 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.26, 1.74] |
| 2 Participants using rescue medication over 6‐8 hours | 7 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.77] |
| 2.1 6 hours | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 2.2 8 hours | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.89] |
| 3 Participants with any adverse event | 6 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akural 2009.
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of at least moderate intensity. Pain assessed at baseline and every 15 min to 2 h, then hourly to 8 h. |
|
| Participants | Surgical removal of 1 or 2 impacted third molars. N = 82 (84 cases; 2 participants had 2 operations), 76 (78 cases) assessed (4 protocol violations, 2 inadequate pain). M 31, F 45. Mean age: 23 years. |
|
| Interventions | Ketoprofen 100 mg, n = 20. Paracetamol 1000 mg, n = 18. Ketoprofen 100 mg + paracetamol 1000 mg, n = 20. Placebo, n = 20. |
|
| Outcomes | PI: standard 4‐point scale. Use of rescue medication. Time to use of rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Participants asked to wait ≥ 1 h before using rescue medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned", "computer‐generated allocation schedule". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy design, "patients were given identical sealed containers of study medication". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy design, "patients were given identical sealed containers of study medication". |
| Size | High risk | < 50 participants per treatment arm. |
Arnold 1990.
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. |
|
| Participants | General surgery (including gynaecological and orthopaedic). N = 59. M 35, F 24. Age: 22‐70 years. |
|
| Interventions | Ketoprofen 25 mg, n = 14. Ketoprofen 100 mg, n = 16. Ibuprofen 400 mg, n = 15. Placebo, n = 14. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. |
|
| Notes | Oxford Quality Score: R1, DB2, W0. 4‐h analgesic and anti‐inflammatory washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization chart". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy design. |
| Size | High risk | < 50 participants per treatment arm. |
Balzanelli 1996.
| Methods | RCT, DB, 2 parallel groups, multiple dose study. Medication administered when baseline pain was of moderate to severe intensity, then every 8 h for total of 3 days. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5 ,6, 8 h, then daily. |
|
| Participants | Surgical removal of impacted third molars. N = 60. M 37, F 23. Mean age: approximately 37 years. |
|
| Interventions | Ketoprofen lysine 80 mg, n = 30. Placebo, n = 30. |
|
| Outcomes | PI: 0‐100‐mm VAS. PGE: standard 5‐point scale. AE: any, serious. Withdrawals. Tolerability 4‐point scale at end of study. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "placebo indistinguishable". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "placebo indistinguishable". |
| Size | High risk | < 50 participants per treatment arm. |
Cooper 1984.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 181 (153 analysed). M 48, F 105. Mean age: 23 years. |
|
| Interventions | Ketoprofen 25 mg, n = 30. Ketoprofen 50 mg, n = 31. Ketoprofen 100 mg, n = 31. Aspirin 650 mg, n = 31. Placebo, n = 30. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Time to use of rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 6‐h analgesic, anti‐inflammatory, or sedative washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not reported. |
| Allocation concealment (selection bias) | Low risk | "each study medication bottle was identified only by a sequential code number". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all capsules in each bottle appeared identical". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all capsules in each bottle appeared identical". |
| Size | High risk | < 50 participants per treatment arm. |
Cooper 1988.
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 1, 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 181 (161 analysed). M 59, F 102. Mean age: 23 years. |
|
| Interventions | Ketoprofen 25 mg, n = 42. Ketoprofen 100 mg, n = 39. Ibuprofen 400 mg, n = 37. Placebo, n = 43. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Numbers of participants using rescue medication. Time to use of rescue medication. Numbers with any AE Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "units of medication appeared identical". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "units of medication appeared identical". |
| Size | High risk | < 50 participants per treatment arm. |
Cooper 1998.
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 177. M 75, F 102. Mean age: 23 years. |
|
| Interventions | Dexketoprofen 25 mg, n = 50. Dexketoprofen 100 mg, n = 51. Paracetamol 1000 mg, n = 50. Placebo, n = 26. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Minimum 4‐h analgesic, caffeine, and sedative washout before surgery. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "treatments were randomly allocated", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "study medications all appeared identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "study medications all appeared identical" |
| Size | High risk | < 50 participants in 3 of 4 treatment arms (range 26 to 51). |
Gay 1996.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 206 (204 analysed). M 85, F 119. Mean age: 24 years. |
|
| Interventions | Dexketoprofen tromethamine 5 mg, n = 41. Dexketoprofen tromethamine 10 mg, n = 42. Dexketoprofen tromethamine 20 mg, n = 41. Ibuprofen 400 mg, n = 41. Placebo, n = 41. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "study medication was identical in appearance to maintain blinding". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "study medication was identical in appearance to maintain blinding". |
| Size | High risk | < 50 participants per treatment arm. |
Harrison 1996.
| Methods | RCT, DB, single oral dose, 3 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 141 (137 in efficacy analysis). M 63, F 78. Mean age: 26 years. |
|
| Interventions | Dexketoprofen tromethamine 12.5 mg, n = 49. Dexketoprofen tromethamine 25 mg, n = 46. Placebo, n = 46. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization list was generated by computer program" |
| Allocation concealment (selection bias) | Low risk | Generation of sequence, and preparation of code envelopes and study medication performed by third party; participants assigned consecutively. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind conditions", "tablets of identical size, colour and weight". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind conditions", "tablets of identical size, colour and weight". |
| Size | High risk | < 50 participants per treatment arm. |
Jackson 2004.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6, 7, 8, 24 h. |
|
| Participants | Surgical removal of impacted third molars. N = 123 (120 analysed). M 39, F 81. Mean age: 29 years. |
|
| Interventions | Dexketoprofen trometamol 25 mg, n = 42. Rofecoxib 50 mg, n = 37. Placebo, n = 41. |
|
| Outcomes | PI: standard 4‐point scale and 100‐mm VAS PR: standard 5‐point scale and 100‐mm VAS PGE: standard 5‐point scale. Time use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation carried out by third party, but method of allocation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind", "All study drugs identical [in appearance] with patient numbers only on the packaging". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind", "All study drugs identical [in appearance] with patient numbers only on the packaging". |
| Size | High risk | < 50 participants per treatment arm. |
McGurk 1998.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 210 (200 in efficacy analysis). M 88, F 122. Mean age: 28 years. |
|
| Interventions | Dexketoprofen trometamol 12.5 mg, n = 44. Dexketoprofen trometamol 25 mg, n = 41. Dexketoprofen trometamol 50 mg, n = 43. Ketoprofen 50 mg (racemic), n = 43. Placebo, n = 39. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization list generated by a computer program in blocks of five patients". |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "tablets of identical appearance to ensure double‐blind conditions". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "tablets of identical appearance to ensure double‐blind conditions". |
| Size | High risk | < 50 participants per treatment arm. |
McQuay 2016.
| Methods | Multicentre, RCT, DB (double‐dummy), multiple dose, placebo‐controlled (first dose), and active comparator, 4 parallel groups. Medication administered orally every 8 h over 5‐day period. First dose administered after cessation of postoperative analgesia once participants able to take oral medication and PI ≥ 40/100. Pain assessed at 30 min, and 1, 1.5, 2, 3, 4, 6, 8 h following first dose. |
|
| Participants | Standard unilateral total hip arthroplasty due to osteoarthritis. Age 18 to 80 years; moderate to severe pain at rest on day after surgery. N = 641. M 295, F 346. Mean age: 62 years (range 29 to 80). Baseline PI: moderate in 324, severe in 315. |
|
| Interventions | Single dose phase. Dexketoprofen 25 mg, n = 161. Tramadol 100 mg, n = 160. Dexketoprofen 25 mg + tramadol 75 mg, n = 159. Placebo, n = 161. |
|
| Outcomes | PI: 100‐mm VAS. PR: standard 5‐point VRS (0 = none, 4 = complete). PGE: standard 5‐point VRS (1 = poor, 5 = excellent) at 24 h or use of rescue medication/withdrawal. Use of rescue medication. Time to use of rescue medication. AEs. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: metamizole 500 mg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence stratified by baseline PI‐VAS categories [moderate pain (40 to 60) and severe pain (> 60) with an imbalanced 1:3:1:3:1:3 ratio, using block size of 12]". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 159 to 161). |
Mehlisch 1984.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 138 (129 analysed). M/F not given. Mean age: 26 years. |
|
| Interventions | Ketoprofen 25 mg, n = 24. Ketoprofen 50 mg, n = 27. Ketoprofen 100 mg, n = 27. Codeine 90 mg, n = 27. Placebo, n = 24. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: 5‐point scale (1 to 5 and reverse order). Number using rescue medication. AEs: any. |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Minimum 3‐h analgesic, anti‐inflammatory, and psychotropic washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random code", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind", "identical capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double blind", "identical capsules". |
| Size | High risk | < 50 participants per treatment arm. |
Moore 2015c.
| Methods | Multicentre, RCT, DB (double‐dummy), placebo‐controlled and active comparator, 10 parallel groups. Medication administered within 4 h of surgery when PI ≥ 40/100 and 4‐point VRS ≥ 2. Pain assessed at 0, 15, 30, 45 min, and 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 24 h. |
|
| Participants | Outpatient surgical removal, under local anaesthesia, of ≥ 1 third molar (≥ fully or partially impacted in mandibular bone). N = 606 for efficacy, 611 for safety. M 247, F 359. Mean age: 27 years (range 18 to 64). Baseline PI: 64% moderate, 35% severe (3 mild, 2 missing data). |
|
| Interventions | Dexketoprofen 12.5 mg, n = 60. Dexketoprofen 25 mg, n = 60. Tramadol 37.5 mg, n = 59. Tramadol 75 mg, n = 59. Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60. Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62. Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63. Dexketoprofen 25 mg + tramadol 75 mg, n = 61. Ibuprofen 400 mg, n = 60. Placebo, n = 62. |
|
| Outcomes | PI: standard 4‐point VRS (0 = none, 3 = severe). PR: standard 5‐point VRS (0 = none, 4 = complete). PGE: standard 5‐point VRS (1 = poor, 5 = excellent) at 24 h or use of rescue medication/withdrawal. Use of rescue medication. Time to use of rescue medication. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: paracetamol 1000 mg (maximum 4 doses in 24 h) after ≥ 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated" "blocked randomisation procedure, with block size of 10". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 59 to 63). |
Moore 2016.
| Methods | Multicentre, RCT, DB, placebo‐controlled and active control, 4 parallel groups. Single and multiple dose phases. Medication administered orally every 8 h over 3‐day period. First dose administered after cessation of postoperative analgesia once participants able to take oral medication and PI ≥ 40/100. Pain assessed at 30 min, and 1, 1.5, 2, 3, 4, 6, 8 h. |
|
| Participants | Abdominal hysterectomy for benign conditions. N = 606. All F. Mean age: 48 years (range 25 to 73). Baseline PI: moderate 38%, severe 62%. |
|
| Interventions | Dexketoprofen 25 mg, n = 151. Tramadol 100 mg, n = 150. Dexketoprofen 25 mg + tramadol 75 mg, n = 152. Placebo, n = 153. |
|
| Outcomes | PI: 100‐mm VAS. PR: standard 5‐point VRS (0 = none, 4 = complete). PGE: standard 5‐point VRS (1 = poor, 5 = excellent) at 8 h or use of rescue medication/withdrawal. Use of rescue medication. Time to use of rescue medication. AEs. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: metamizole 500 mg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence stratified by baseline PI‐VAS categories [moderate pain (40 to 60) and severe pain (> 60)] with an imbalanced 3:3:3:1:1:1 ratio, using block size of 12]". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 150 to 153). |
Olson 1999.
| Methods | RCT, DB, single dose oral liquid formulation of ketoprofen, 4 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Episiotomy. N = 108 (terminated early, recruitment target N = 276). All F. Mean age: 24 years. |
|
| Interventions | Ketoprofen 25 mg liquid formulation, n = 28. Ketoprofen 50 mg liquid formulation, n = 26. Dipyrone 500 mg liquid formulation, n = 27. Placebo, n = 27. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs; any, severe. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB1, W1. 2 women entered with 2nd degree vaginal tears. Minimum 6‐h washout before surgery for any medication that could confound results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported, medication assignment in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study medications not identical in appearance. Nurse preparing study medication also administered it. A second nurse, blinded to the medication given, observed the woman. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study medications not identical in appearance. Nurse preparing study medication also administered it. A second nurse, blinded to the medication given, observed the woman. |
| Size | High risk | < 50 participants per treatment arm. |
Olson 2001.
| Methods | RCT, DB, triple dummy, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 239. M 76, F 163. Mean age: 23 years. |
|
| Interventions | Ketoprofen 25 mg, n = 67. Ibuprofen liquigel 400 mg, n = 67. Paracetamol 1000 mg, n = 66. Placebo, n = 39. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Analgesic and anti‐inflammatory washout before surgery (5 × half‐life). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomization schedule generated by the sponsor", method not described. |
| Allocation concealment (selection bias) | Low risk | "Numbers were assigned to subjects in sequential order within the appropriate strata". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules and caplets matched the active treatments; "all unit doses were identical in appearance". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo capsules and caplets matched the active treatments; "all unit doses were identical in appearance". |
| Size | High risk | < 50 participants in 1 of 4 treatment arms (range 39 to 67). |
Schreiber 1996.
| Methods | RCT, DB, single and multiple oral dose phases, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 4 h after the 1st dose. |
|
| Participants | Knee (meniscus or ligament reconstruction) or ankle surgery. N = 230. M 110, F 103. Mean age: 40 years. |
|
| Interventions | Dexketoprofen tromethamine 12.5 mg, n = 52. Dexketoprofen tromethamine 25 mg, n = 52. Ketoprofen 50 mg, n = 54. Placebo, n = 55. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation list", in blocks of 8. Judged low risk as computer randomisation described in related studies carried out by same sponsor. |
| Allocation concealment (selection bias) | Low risk | Generation of sequence and preparation of code envelopes and study medication performed by third party; participants assigned consecutively. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "tablets of identical size, colour and weight", packaging indistinguishable except for randomisation number. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "tablets of identical size, colour and weight", packaging indistinguishable except for randomisation number. |
| Size | Unclear risk | 50 to 199 participants per treatment arm (range 52 to 55) |
Seymour 1996.
| Methods | R, DB, double dummy, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 206. M 66, F 140. Mean age: 25 years. |
|
| Interventions | Ketoprofen 12.5 mg, n = 42. Ketoprofen 25 mg, n = 41. Paracetamol 500 mg, n = 41. Paracetamol 1000 mg, n = 41. Placebo, n = 41. |
|
| Outcomes | PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized trial", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy technique", "ketoprofen dosages identical in appearance and standard paracetamol tablets were used. Matched placebos were prepared for both medications". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy technique", "ketoprofen dosages identical in appearance and standard paracetamol tablets were used. Matched placebos were prepared for both medications". |
| Size | High risk | < 50 participants per treatment arm. |
Seymour 2000.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. |
|
| Participants | Surgical removal of impacted third molars. N = 180. M 58, F 122. Mean age: 27 years. |
|
| Interventions | Buffered ketoprofen 12.5 mg, n = 61. Ibuprofen 200 mg, n = 59. Placebo, n = 60. |
|
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy technique", "both tablets and dragees were of identical appearance, irrespective of their contents". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy technique", "both tablets and dragees were of identical appearance, irrespective of their contents". |
| Size | Unclear risk | 50‐199 participants per treatment arm. |
Sunshine 1988.
| Methods | RCT, DB, single dose, parallel groups. Medication administered when baseline pain was of at least moderate intensity. Pain assessed at 0, 30, 60 min then hourly to 6 h. |
|
| Participants | Study 3. 'General surgery' procedures (details not reported). N = 123. All M (Veterans Administration hospital). Age: not reported. |
|
| Interventions | Ketoprofen 50 mg, n = 32. Ketoprofen 150 mg, n = 31. Paracetamol 650 mg + codeine 60 mg, n = 28. Placebo, n = 32. |
|
| Outcomes | PI: 4‐point VRS. PR: 5‐point VRS. PGE: 4‐point medication rating (0 = no help, 3 = excellent) and 7‐point VRS overall rating (1 = very much worse, 7 = very much better). |
|
| Notes | Oxford Quality Score: R1, DB1, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not specifically mentioned, but used the same methods as other studies described as randomised. Method of sequence generation not described. "The same general methods were used in all of the studies under discussion. All studies met current standards of well‐controlled trials" |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "double‐blind", method not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "double‐blind", method not reported. |
| Size | High risk | < 50 participants per treatment arm. |
Sunshine 1993.
| Methods | RCT, DB, single and multiple oral dose, parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 30, 60 min then hourly to 8 h. |
|
| Participants | Caesarean section. N = 250. All F. Mean age: 26 years. |
|
| Interventions | Ketoprofen 50 mg, n = 48. Ketoprofen 100 mg, n = 48. Paracetamol 650 mg, n = 48. Paracetamol 650 mg + oxycodone 10 mg, n = 48. Placebo, n = 48. |
|
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study medication was identical in appearance". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study medication was identical in appearance". |
| Size | High risk | < 50 participants per treatment arm. |
Sunshine 1998.
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30 min, and 1, 1.5, 2, 3, 3.5, 4, 5, 6 h. |
|
| Participants | Surgical removal of ≥ 1 impacted third molars. N = 179 (175 analysed for efficacy). M 58, F 117. Mean age: 22 years. |
|
| Interventions | Ketoprofen 6.25 mg, n = 35. Ketoprofen 12.5 mg, n = 35. Ketoprofen 25 mg, n = 35. Ibuprofen 200 mg, n = 35. Placebo, n = 35. |
|
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 24‐h analgesic washout before surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", randomisation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all study medication identical in appearance". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all study medication identical in appearance". |
| Size | Unclear risk | < 50 participants per treatment arm. |
Turek 1988.
| Methods | RCT, DB, single oral dose, 3 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h |
|
| Participants | Elective surgery (113 orthopaedic, 23 abdominal, 11 gynaecology, 8 urology, and 6 miscellaneous procedures). N = 161 (160 analysed). M 81, F 81. Mean age: 47 years. |
|
| Interventions | Ketoprofen 50 mg, n = 41. Ketoprofen 150 mg, n = 39. Paracetamol 650 mg + codeine 60 mg, n = 39. Placebo, n = 42. |
|
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. |
|
| Notes | Oxford Quality Score: R1, DB2, W1. 3‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "double‐blind", method not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "double‐blind", method not described. |
| Size | High risk | < 50 participants per treatment arm. |
Vidal 1999.
| Methods | RCT, DB, single and multiple oral dose phases, 4 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6 h for single dose phase. |
|
| Participants | Hallux vagus (bunion) surgery. N = 188 (172 analysed). M 25, F 163. Mean age: 54 years. |
|
| Interventions | Dexketoprofen trometamol 12.5 mg, n = 47. Dexketoprofen trometamol 25 mg, n = 47. Ketoprofen 50 mg, n = 47. Placebo, n = 47. |
|
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. Withdrawals. |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication via PCA morphine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation "by computer program" for each centre. |
| Allocation concealment (selection bias) | Low risk | Generation of sequence, and preparation of code envelopes and study medication performed by third party; allocation "in chronological order of inclusion in each centre". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All the treatments .... were tablets of identical size, colour and weight". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All the treatments .... were tablets of identical size, colour and weight". |
| Size | High risk | < 50 participants per treatment arm. |
AE: adverse event; DB: double blind; F: female; h: hour; M: male; min: minute; N: number of participants in study; n: number of participants in treatment arm; PCA: patient‐controlled analgesia; PGE: Patient Global Evaluation of efficacy; PI: pain intensity; PR: pain relief; R: randomised (Oxford Quality Score); RCT: randomised controlled trial; VAS: visual analogue scale (see 'Glossary'; Appendix 4); VRS: verbal rating scale; W: withdrawal (Oxford Quality Score).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avila 1991 | No placebo, no baseline pain. |
| Bagan 1998 | No placebo. |
| Berti 2000 | No placebo, preoperative administration. |
| Esparza‐Villalpando 2016 | No relevant control for postoperative administration, intervention given irrespective of baseline pain intensity. |
| Gallardo 1982 | 3‐hour study period, no 4‐hour data. |
| Giudice 1987 | No placebo. |
| Jimenez‐Martinez 2004 | No placebo. |
| Kantor 1984 | Included women with uterine cramps. |
| Letarget 1998 | No placebo. |
| Lobo 1983 | 3‐hour study period, no 4‐hour data. |
| Olmedo 2001 | No 4‐ to 6‐hour data reported. |
| Perez 2002 | No placebo. |
| Schreiber 1998 | No placebo. |
| Sunshine 1986 | Included women with uterine cramps. |
| Tufano 1981 | Study not randomised or double blind. Intravenous route. |
| Zapata 2000 | No placebo. |
Characteristics of studies awaiting assessment [ordered by study ID]
Yatomi 1979.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Japanese ‐ unable to obtain copy. |
Differences between protocol and review
We changed the title to 'ketoprofen or dexketoprofen' to clarify that the interventions were not taken together.
This review included updated 'Risk of bias' and GRADE assessments, and 'Summary of findings' tables. We have not included sensitivity analyses by size and Oxford Quality Score.
Contributions of authors
For the original review: JB, SD, and RAM carried out searching, data extraction, and analysis, including assessment of study quality. HJM helped with analysis and acted as arbitrator. All review authors contributed to the writing of the protocol and review.
For this update: HG and SD carried out searching, data extraction, and analysis, including assessment of risk of bias and quality of the evidence. RAM acted as arbitrator; he did not participate in searching, or data extraction, analysis, assessment of risk of bias and quality of the evidence for the included studies in which he was an author. All authors contributed to the writing of the review.
Sources of support
Internal sources
Oxford Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
NIHR Biomedical Research Centre Programme, UK.
Declarations of interest
HG: none known; HG is a retired geriatrician and has treated patients with acute pain.
SD: none known.
PW: none known.
RAM is an author of three of the included trials. He has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Akural 2009 {published data only}
- Akural E. A combination of ketoprofen and paracetamol analgesia after oral surgery: a prospective, randomized, double‐blinded, placebo‐controlled single‐dose study. Pain Practice 2009;9 (Suppl 1):139‐40. [DOI: 10.1111/j.1533-2500.2009.00267.x] [DOI] [Google Scholar]
- Akural EI, Järvimäki V, Länsineva A, Niinimaa A, Alahuhta S. Effects of combination treatment with ketoprofen 100 mg + acetaminophen 1000 mg on postoperative dental pain: a single‐dose, 10‐hour, randomized, double‐blind, active‐ and placebo‐controlled clinical trial. Clinical Therapeutics 2009;31(3):560‐8. [DOI: 10.1016/j.clinthera.2009.03.017] [DOI] [PubMed] [Google Scholar]
Arnold 1990 {published data only}
- Arnold JD. Ketoprofen, ibuprofen, and placebo in the relief of postoperative pain. Advances in Therapy 1990;7:264‐75. [Google Scholar]
Balzanelli 1996 {published data only}
- Balzanelli B, Lorenzi C. Efficacy and tolerability 80 mg granulated ketoprofen lysine salt in posttraumatic orodental pain: double blind vs placebo study. Minerva Stomatologica 1996;45(1‐2):53‐9. [PubMed] [Google Scholar]
Cooper 1984 {published data only}
- Cooper SA, Gelb SB, Cavaliere MB, Crohn P, Dyer C. An analgesic relative potency assay comparing ketoprofen and aspirin in postoperative dental pain. Advances in Therapy 1984;1(6):410‐8. [Google Scholar]
Cooper 1988 {published data only}
- Cooper SA, Berrie R, Cohn P. Comparison of ketoprofen, ibuprofen, and placebo in a dental surgery pain model. Advances in Therapy 1988;5(3):43‐53. [Google Scholar]
Cooper 1998 {published data only}
- Cooper SA, Reynolds DC, Reynolds B, Hersh EV. Analgesic efficacy and safety of (R)‐ketoprofen in postoperative dental pain. Journal of Clinical Pharmacology 1998;38(2 Suppl):11S‐18S. [DOI: 10.1002/j.1552-4604.1998.tb04412.x] [DOI] [PubMed] [Google Scholar]
Gay 1996 {published data only}
- Gay C, Planas E, Donado M, Martinez J, Artigas R, Torres F, et al. Analgesic efficacy of low doses of dexketoprofen in the dental pain model. Clinical Drug Investigation 1996;11:320‐30. [DOI: 10.2165/00044011-199611060-00002] [DOI] [Google Scholar]
Harrison 1996 {unpublished data only}
- Harrison F. Double‐blind randomised, parallel‐group comparison of the safety and efficacy of single oral doses of LM‐1158.tris (dexketoprofen tromethamine salt, 12.5 mg or 25 mg) to placebo in patients with moderate to severe dental pain due to removal of impacted third molar teeth. Clinical trial report 1996.
Jackson 2004 {published data only}
- Jackson ID, Heidemann BH, Wilson J, Power I, Brown RD. Double‐blind, randomised, placebo‐controlled trial comparing rofecoxib with dexketoprofen trometamol in surgical dentistry. British Journal of Anaesthesia 2004;92:675‐80. [DOI] [PubMed] [Google Scholar]
McGurk 1998 {published data only}
- McGurk M, Robinson P, Rajayogeswaran V, Luca M, Casini A, Artigas R, et al. Clinical comparison of dexketoprofen trometamol, ketoprofen, and placebo in postoperative dental pain. Journal of Clinical Pharmacology 1998;38(12 Suppl):46S‐54S. [PubMed] [Google Scholar]
McQuay 2016 {published data only}
- McQuay HJ, Moore RA, Berta A, Gainutdinovs O, Fulesdi B, Porvaneckas N, et al. Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate‐to‐severe pain after total hip arthroplasty. Revista de la Sociedad Espanola del Dolor 2015;22(5):186‐99. [Google Scholar]
- McQuay HJ, Moore RA, Berta A, Gainutdinovs O, Fülesdi B, Porvaneckas N, et al. Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate‐to‐severe pain after total hip arthroplasty. British Journal of Anaesthesia 2016;116(2):269‐76. [CTG: NCT01902134; DOI: 10.1093/bja/aev457] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehlisch 1984 {published data only}
- Mehlisch D, Frakes L, Cavaliere MB, Gelman M. Double‐blind parallel comparison of single oral doses of ketoprofen, codeine, and placebo in patients with moderate to severe dental pain. Journal of Clinical Pharmacology 1984;24(11‐12):486‐92. [DOI] [PubMed] [Google Scholar]
Moore 2015c {published data only}
- Moore RA, Gay‐Escoda C, Figueiredo R, Tóth‐Bagi Z, Dietrich T, Milleri S, et al. Dexketoprofen/tramadol: randomised double‐blind trial and confirmation of empirical theory of combination analgesics in acute pain. Journal of Headache and Pain 2015;16:541. [CTG: NCT01307020; DOI: 10.1186/s10194-015-0541-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2016 {published data only}
- Moore RA, McQuay HJ, Tomaszewski J, Raba G, Tutunaru D, Lietuviete N, et al. Dexketoprofen/tramadol 25 mg/75 mg: randomised double‐blind trial in moderate‐to‐severe acute pain after abdominal hysterectomy. BMC Anesthesiology 2016;16:9. [CTG: NCT01904149; DOI: 10.1186/s12871-016-0174-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 1999 {published data only}
- Olson NZ, Sunshine A, Zighelboim I, Lange R. Analgesic efficacy of liquid ketoprofen compared to liquid dipyrone and placebo administered orally as drops in postepisiotomy pain. International Journal of Clinical Pharmacology and Therapeutics 1999;37(4):168‐74. [PubMed] [Google Scholar]
Olson 2001 {published data only}
- Olson NZ, Otero AM, Marrero I, Tirado S, Cooper S, Doyle G, et al. Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. Journal of Clinical Pharmacology 2001;41(11):1238‐47. [DOI: 10.1177/00912700122012797] [DOI] [PubMed] [Google Scholar]
Schreiber 1996 {unpublished data only}
- Schreiber M. Double‐blind, randomized, parallel‐group comparison of the safety and efficacy of oral doses of dexketoprofen tromethaine salt (LM‐1158.TRIS, 12.5 mg or 25 mg) with racemic ketoprofen (50 mg) and placebo in patients with moderate or severe pain following orthopaedic surgery. Clinical trial report 1996.
Seymour 1996 {published data only}
- Seymour RA, Kelly PJ, Hawkesford JE. The efficacy of ketoprofen and paracetamol (acetaminophen) in postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1996;41(6):581‐5. [DOI: 10.1046/j.1365-2125.1996.34015.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2000 {published data only}
- Seymour RA, Watkinson H, Hawkesford JE, Moore U. The efficacy of buffered ketoprofen in postoperative pain after third molar surgery. European Journal of Clinical Pharmacology 2000;55(11‐12):801‐6. [DOI: 10.1007/s002280050700] [DOI] [PubMed] [Google Scholar]
Sunshine 1988 {published data only}
- Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. Journal of Clinical Pharmacology 1988;28(S1):S47‐54. [DOI: 10.1002/j.1552-4604.1988.tb05977.x] [DOI] [PubMed] [Google Scholar]
Sunshine 1993 {published data only}
- Sunshine A, Olson NZ, Zighelboim I, Castro A. Ketoprofen, acetaminophen plus oxycodone, and acetaminophen in the relief of postoperative pain. Clinical Pharmacology and Therapeutics 1993;54(5):546‐55. [DOI: 10.1038/clpt.1993.187] [DOI] [PubMed] [Google Scholar]
Sunshine 1998 {published data only}
- Sunshine A, Olson NZ, Marrero I, Tirado S. Onset and duration of analgesia for low‐dose ketoprofen in the treatment of postoperative dental pain. Journal of Clinical Pharmacology 1998;38(12):1155‐64. [DOI: 10.1177/009127009803801211] [DOI] [PubMed] [Google Scholar]
Turek 1988 {published data only}
- Turek MD, Baird WM. Double‐blind parallel comparison of ketoprofen (Orudis), acetaminophen plus codeine, and placebo in postoperative pain. Journal of Clinical Pharmacology 1988;28(12 Suppl):S23‐8. [DOI: 10.1002/j.1552-4604.1988.tb05973.x] [DOI] [PubMed] [Google Scholar]
Vidal 1999 {unpublished data only}
- Vidal F, Marinez, P, Montero, A, Puig M, Aliag L, Planell J, et al. Clinical trial to assess the analgesic efficacy and safety of LM‐1158.TRIS (12.5 and 25 mg tid) versus ketoprofen (50 mg tid) and placebo after oral administration in patients with acute post‐surgery pain. Clinical trial report 1999.
References to studies excluded from this review
Avila 1991 {published data only}
- Avila G, Balbo G, Biasia R, Brighenti FM, Conte R, Donini I, et al. Ketoprofen in the prevention of postoperative pain in abdominal surgery. A multicenter study. Il Giornale di chirurgia 1991;12(8‐9):456‐8. [PubMed] [Google Scholar]
Bagan 1998 {published data only}
- Bagán JV, Lopez Arranz S, Valencia E, SantamarÍa J, Eguidazu I, Horas M, et al. Clinical comparison of dexketoprofen trometamol and dipyrone in postoperative dental pain. Journal of Clinical Pharmacology 1998;38 (Suppl):55S‐64S. [PubMed] [Google Scholar]
Berti 2000 {published data only}
- Berti M, Albertin A, Casati A, Palmisano S, Municino G, Gama Malcher M, et al. A prospective, randomized comparison of dexketoprofen, ketoprofen or paracetamol for postoperative analgesia after outpatient knee arthroscopy. Minerva Anestesiologica 2000;66(7‐8):549‐54. [PubMed] [Google Scholar]
Esparza‐Villalpando 2016 {published data only}
- Esparza‐Villalpando V, Chavarria‐Bolanos D, Gordillo‐Moscoso A, Masuoka‐Ito D, Martinez‐Rider R, Isiordia‐Espinoza M, et al. Comparison of the analgesic efficacy of preoperative/postoperative oral dexketoprofen trometamol in third molar surgery: a randomized clinical trial. Journal of Cranio‐Maxillofacial Surgery 2016;44(9):1350‐5. [DOI: 10.1016/j.jcms.2016.06.002] [DOI] [PubMed] [Google Scholar]
Gallardo 1982 {published data only}
- Gallardo F, Rossi E, Ciscutti V. Analgesic efficacy of ketoprofen on postoperative pain following periodontal surgery. IRCS Medical Science 1982;10(12):1036‐7. [Google Scholar]
Giudice 1987 {published data only}
- Giudice G, Rizzi F. Ketoprofen, tromethamine tiaprofenate, versus lysine acetyl salicylate in the control of postoperative pain. Minerva Anestesiologica 1987;53(10):587‐94. [PubMed] [Google Scholar]
Jimenez‐Martinez 2004 {published data only}
- Jimenez‐Martinez E, Gasco‐Garcia C, Arrieta‐Blanco JJ, Gomez del Torno J, Bartolome Villar B. Study of the analgesic efficacy of dexketoprofen trometamol 25mg. vs. ibuprofen 600mg. after their administration in patients subjected to oral surgery. Medicina Oral : Organo Oficial de la Sociedad Espanola de Medicina Oral y de la Academia Iberoamericana de Patologia y Medicina Bucal 2004;9(2):143‐48(English), 138‐43(Spanish). [PubMed] [Google Scholar]
Kantor 1984 {published data only}
- Kantor T, Cavaliere MB, Hopper M, Roepke S. A double‐blind parallel comparison of ketoprofen, codeine, and placebo in patients with moderate to severe postpartum pain. Journal of Clinical Pharmacology 1984;24(5‐5):228‐34. [DOI] [PubMed] [Google Scholar]
Letarget 1998 {unpublished data only}
- Letarget J (Principal Investigator). A comparative study on safety and efficacy of dexketoprofen trometamol versus paracetamol codeine (Dafalgan Codeine) in the treatment of moderate to severe pain in the post‐operative follow‐up of hip‐replacement surgery. Clinical trial report 1998.
Lobo 1983 {published data only}
- Lobo R, Gallardo F, Henriquez E, Iriarte E. Analgesic activity of ketoprofen in post‐operative oral surgery pain. IRCS Medical Science 1983;11:639‐40. [Google Scholar]
Olmedo 2001 {published data only}
- Olmedo MV, Galvez R, Vallecillo M. Double‐blind parallel comparison of multiple doses of ketorolac, ketoprofen and placebo administered orally to patients with postoperative dental pain. Pain 2001;90(1‐2):135‐41. [DOI] [PubMed] [Google Scholar]
Perez 2002 {unpublished data only}
- Perez A. A multicentre clinical trial evaluating the analgesic efficacy and safety of dexketoprofen trometamol (25 mg tid) versus diclofenac (50 mg tid) for the treatment of pain subsequent to ambulatory surgery. Clinical trial report 2002.
Schreiber 1998 {unpublished data only}
- Schreiber M. Comparison of efficacy and tolerability of oral administration of 25 mg dexketoprofen (trometamol) vs 50 mg tramadol in patients with post‐operative pain. Clinical trial report 1998.
Sunshine 1986 {published data only}
- Sunshine A, Zighelboim I, Laska E, Siegel C, Olson NZ, Castro A. A double‐blind, parallel comparison of ketoprofen, aspirin, and placebo in patients with postpartum pain. Journal of Clinical Pharmacology 1986;26(8):706‐11. [DOI] [PubMed] [Google Scholar]
Tufano 1981 {published data only}
- Tufano R, Santangelo E, Esposito O, Brancadoro V. Ketoprofen and postoperative pain [Ketoprofene e dolore post‐operatorio]. Acta Anaesthesiologica Italica 1981;32(2):333‐41. [Google Scholar]
Zapata 2000 {unpublished data only}
- Zapata A, Cegarra F, Artigas R, Keller F. Dexketoprofen vs tramadol: randomised double‐blind trial in patients with postoperative pain. Clinical trial report 2000.
References to studies awaiting assessment
Yatomi 1979 {published data only}
- Yatomi H, Hamada G, Ozaki T, Ogawa T. Analgesic effects of ketoprofen (Orudis) on pains following tooth extraction. Shikai Tenbo 1979;54(2):355‐8. [PubMed] [Google Scholar]
Additional references
Aldington 2011
- Aldington DJ, McQuay HJ, Moore RA. End‐to‐end military pain management. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 2011;366(1562):268‐75. [DOI: 10.1098/rstb.2010.0214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbanoj 2001
- Barbanoj MJ, Antonijoan RM, Gich I. Clinical pharmacokinetics of dexketoprofen. Clinical Pharmacokinetics 2001;40(4):245‐62. [DOI: 10.2165/00003088-200140040-00002] [DOI] [PubMed] [Google Scholar]
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107(1‐2):86‐90. [DOI: 10.1016/j.pain.2003.09.021] [DOI] [PubMed] [Google Scholar]
BNF 2016
- British National Formulary. Peri‐operative analgesia. Non‐opioid analgesics, 2016. www.medicinescomplete.com/mc/bnf/current/PHP78461‐peri‐operative‐analgesia.htm (accessed 8 November 2016).
Bulley 2009
- Bulley SJ, Derry S, Moore RA, McQuay RA. Single dose oral rofecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004604.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2014
- Clarke R, Derry S, Moore RA. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD004309.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI: 10.1016/S0304-3959(97)00005-5] [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91(1‐2):189‐94. [DOI: 10.1016/S0304-3959(00)00435-8] [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI: 10.1136/bmj.310.6977.452] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The Design of Analgesic Clinical Trials. Advances in Pain Research Therapy. Vol. 18, New York (NY): Raven Press, 1991:117‐24. [Google Scholar]
Dechartes 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta‐analyses. JAMA 2014;312:623‐30. [DOI: 10.1001/jama.2014.8166] [DOI] [PubMed] [Google Scholar]
Derry 2009a
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2009b
- Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2015
- Derry S, Wiffen PJ, Moore RA. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2016
- Derry S, Cooper TE, Phillips T. Single fixed‐dose oral dexketoprofen plus tramadol for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012232.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. Resources for review authors, 2015. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 13 February 2017).
FitzGerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [DOI: 10.1056/NEJM200108093450607] [DOI] [PubMed] [Google Scholar]
Grahame‐Smith 2002
- Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy. 3rd Edition. Oxford (UK): Oxford University Press, 2002. [Google Scholar]
Gregory 2016
- Gregory J, McGowan L. An examination of the prevalence of acute pain for hospitalised adult patients: a systematic review. Journal of Clinical Nursing 2016;25(5‐6):583‐98. [DOI: 10.1111/jocn.13094] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66:151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66:158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Hawkey 1999
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. [DOI: 10.1016/S0140-6736(98)12154-2] [DOI] [PubMed] [Google Scholar]
Hernández‐Diaz 2000
- Hernández‐Díaz S, García Rodríguez LA. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Archives of Internal Medicine 2000;160:2093‐9. [DOI: 10.1001/archinte.160.14.2093] [DOI] [PubMed] [Google Scholar]
Herrero 2003
- Herrero JF, Romero‐Sandoval EA, Gaitan G, Mazario J. Antinociception and the new COX inhibitors: research approaches and clinical perspectives. CNS Drug Reviews 2003;9:227‐52. [DOI: 10.1111/j.1527-3458.2003.tb00251.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI: 10.1016/0304-3959(96)03033-3] [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI: 10.1016/0197-2456(95)00134-4] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI: 10.7326/0003-4819-107-2-224] [DOI] [PubMed] [Google Scholar]
Laporte 2004
- Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Safety 2004;27(6):411‐20. [DOI: 10.2165/00002018-200427060-00005] [DOI] [PubMed] [Google Scholar]
McQuay 1982
- McQuay HJ, Bullingham RE, Moore RA, Evans PJ, Lloyd JW. Some patients don't need analgesics after surgery. Journal of the Royal Society of Medicine 1982;75(9):705‐8. [PMC free article] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI: 10.1136/pgmj.2004.024737] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2012
- McQuay HJ, Derry S, Eccleston C, Wiffen PJ, Moore RA. Evidence for analgesic effect in acute pain ‐ 50 years on. Pain 2012;153(7):1364‐7. [DOI: 10.1016/j.pain.2012.01.024] [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI: 10.1016/0304-3959(96)03032-1] [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI: 10.1016/S0304-3959(96)03251-4] [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI: 10.1016/S0304-3959(96)03306-4] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Measuring pain. Bandolier's Little Book of Pain. Oxford (UK): Oxford University Press, 2003:7‐13. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI: 10.1016/j.pain.2005.05.001] [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford (UK): Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Moore 2008a
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2008b
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2008c
- Moore RA, Barden J. Systematic review of dexketoprofen in acute and chronic pain. BMC Clinical Pharmacology 2008;8:11. [DOI: 10.1186/1472-6904-8-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore RA, Derry S, Straube S, Ireson‐Paine J, Wiffen PJ. Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain 2014;155(1):14‐21. [DOI: 10.1016/j.pain.2013.08.013] [DOI] [PubMed] [Google Scholar]
Moore 2015a
- Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD008659.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015b
- Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011407.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
PACT 2016
- Prescribing and Medicines Team Health and Social Care Information Centre. Prescription Cost Analysis, England 2015. Leeds (UK): Health and Social Care Information Centre, 2016. [ISBN: 978‐1‐78386‐680‐9] [Google Scholar]
PaPaS 2012
- Cochrane Pain, Palliative and Supportive Care Group (PaPaS) author and referee guidance. papas.cochrane.org/papas‐documents (accessed 23 February 2017).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PloS One 2011;6(10):e25491. [DOI: 10.1371/journal.pone.0025491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI: 10.1136/bmj.315.7109.635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visentin 2005
- Visentin M, Zanolin E, Trentin L, Sartori S, Marco R. Prevalence and treatment of pain in adults admitted to Italian hospitals. European Journal of Pain 2005;9(1):61‐7. [DOI: 10.1016/j.ejpain.2004.04.004] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Barden 2009
- Barden J, Derry S, McQuay HJ, Moore RA. Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007355.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


