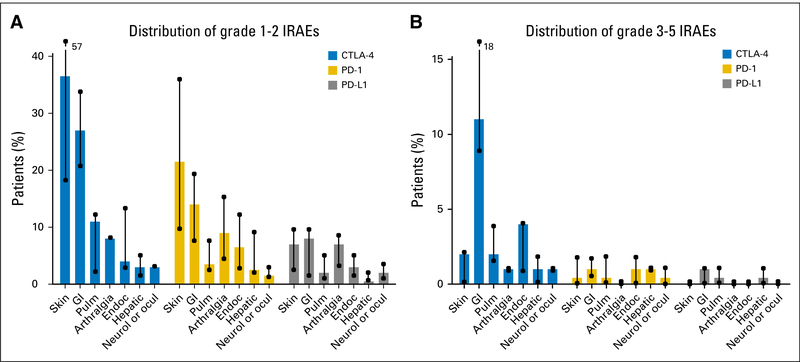
Fig A1.
Distribution of (A) grade 1 to 2 and (B) grade 3 to 5 immune-related adverse events (irAEs) for all tumor types in the main clinical trials with anti- cytotoxic T-cell lymphocyte-4 (anti–CTLA-4), anti-programmed death 1 (PD-1), or anti–PD ligand 1 (PD-L1) antibodies as single therapies. The values quoted are the median (range) irAE rates for the set of clinical trials as a whole. Adapted from European Journal of Cancer, Vol 54, J.M. Michot et al, Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review, 139–149, Copyright 2016, with permission from Elsevier. Endoc, endocrinology; Neurol, neurology; ocul, ocular; Pulm, pulmonary.