Abstract
Autoantigen-specific immunotherapy promises effective treatment for devastating tissue specific autoimmune diseases like multiple sclerosis (MS) and type 1 diabetes (T1D). Because activated dendritic cells (DCs) stimulate the differentiation of autoreactive T cells involved in the initiation of autoimmunity, blocking the activation of DCs may be an effective strategy for inhibiting tissue specific autoimmunity. Following this approach, immature DCs were shown to remain inactive after treatment with chimeric fusion proteins composed of the cholera toxin B subunit adjuvant linked to autoantigens like proinsulin (CTB-INS). Mass spectrometer analysis of human DCs treated with CTB-INS suggest that upregulation of the tryptophan catabolic enzyme indoleamine 2, 3-dioxygenase (IDO1) is responsible for inhibiting DC activation thereby resulting in a state of immunological tolerance within the DC. Here we show that the fusion protein CTB-INS inhibits human monocyte derived DC (moDC) activation through stimulation of IDO1 biosynthesis and that the resultant state of DC tolerance can be further enhanced by the presence of residual E. coli lipopolysaccharide (LPS) present in partially purified CTB-INS preparations. Additional experiments showed that LPS enhancement of DC tolerance was dependent upon stimulation of IDO1 biosynthesis. LPS stimulation of increased levels of IDO1 in the DC resulted in increased secretion of kynurenines, tryptophan degradation products known to suppress DC mediated pro-inflammatory T cell differentiation and to stimulate the proliferation of regulatory T cells (Tregs). Further, the presence of LPS in CTB-INS treated DCs stimulated the biosynthesis of costimulatory factors CD80 and CD86 but failed to upregulate maturation factor CD83, suggesting CTBINS treated DCs may be maintained in a state of semi-activation. While treatment of moDCs with increasing amounts of LPS free CTB-INS was shown to increase DC secretion of the anti-inflammatory cytokine IL-10, the presence of residual LPS in partially purified CTB-INS preparations dramatically increased IL-10 secretion, suggesting that CTB-INS may enhance DC mediated immunological tolerance by stimulating the proliferation of anti-inflammatory T cells. While the extraction of LPS from bacterial generated CTB-INS may remove additional unknown factors that may contribute to the regulation of IDO1 levels, together, our experimental data suggest that LPS stimulates the ability of CTB-INS to induce IDO1 and IL-10 important factors required for establishment of a state of functional immunological tolerance in human DCs. Regulation of the ratio of LPS to CTB-INS may prove to be an effective method for optimization of readily available “off the shelf” CTB-INS mediated immune-therapy for tissue specific autoimmune diseases including type 1 diabetes.
Keywords: LPS, CTB, IDO1, immunotherapy, dendritic cells, T1D autoimmunity
INTRODUCTION:
Tissue specific autoimmune diseases of dysregulated metabolism such as Type 1 diabetes (T1D) predispose to serious medical conditions leading to an overall reduction in life span [1, 2]. A treatment that could prevent or reverse the course of autoimmunity would exert a major impact over the life span of a rapidly increasing number of patients [3]. A critical immune cell component considered to be the key to the pathogenesis of autoimmunity is the dendritic cell (DC). Activation of DCs and their presentation of autoantigens such as insulin or glutamate decarboxylase (GAD) to naïve autoreactive T cells results in their differentiation into effector T cells capable of initiating T1D autoimmunity. Insulin specific effector T cells were shown to destroy the pancreatic insulin-producing β-cells, resulting in early mortality [4–8]. Current immunotherapeutic strategies can reduce autoimmune disease induced inflammation by functionally tolerizing the DCs, thereby inhibiting DC induction of pro-inflammatory autoreactive effector T cell differentiation. However, current immunosuppressive strategies have significant disadvantages [9, 10]. A major drawback is their establishment of a state of broad immune tolerization that extends beyond specific autoimmune responses and can compromise normal immunity to infection [11–17]. Thus, no effective intervention is available to slow or arrest the processes responsible for development of tissue specific autoimmune diseases like T1D. An immune suppression strategy shown to prevent and partially reverse autoimmunity in animal models of tissue specific autoimmunity, involves conjugation of tissue specific autoantigens to an immune stimulating adjuvant [18–23]. Fusions of the pancreatic islet antigen, proinsulin, with the cholera toxin B subunit adjuvant (CTB-INS) were shown to prevent insulitis and hyperglycemia in pre-diabetic mice [24–29], while cell culture experiments showed DCs stimulate autoimmunity in the mice [30, 31]. In contrast, the induction of tolerance in murine DCs was shown to prevent T1D autoimmunity. Together, these experiments suggest CTB-INS prevention of T1D is correlated with treatments that generate DC tolerance. Further studies suggest CTB-autoantigen fusion proteins induce tolerance by expanding Foxp3 (+) T regulatory cell populations [32–36], while other studies suggest Foxp3 (−) T cells induce tolerance by upregulating anti-inflammatory cytokines TGF-β and IL-10. [32–36]. Stimulating human DCs with CTB-INS was shown to upregulate the biosynthesis of IDO1, a key regulatory enzyme in the tryptophan degradation pathway known to induce a state of functional tolerance in DCs [37]. Further, upregulation of IDO1 in the DCs occurs via activation of the non-canonical NF-kB signaling pathway, although receptors involved in CTB-INS signaling remain unknown [37]. When stimulated with CTB-INS, DCs isolated from the blood of healthy subjects were shown to upregulate anti-inflammatory cytokines TGF-β and IL-10, previously known to antagonize pro-inflammatory T cells and stimulate immune tolerance [37–40]. The outcome of DC -T cell co-culture experiments showed that CTB-INS treated DCs inhibit the proliferation of pro-inflammatory T cells [41]. Further, the upregulation of IDO1 and its tryptophan degradation products (kynurenines) were shown to stimulate DC tolerance and may also recruit Tregs that further inhibit DC activation resulting in a state of functional DC tolerance [37, 42–44]. In this study, we show that residual LPS present in CTB-INS fusion protein used for treatment of healthy human DCs enhances CTB-INS mediated upregulation of IDO1.
MATERIALS AND METHODS:
Amplification of recombinant CTB-INS fusion protein in E. coli BL21 cells.
The recombinant gram negative E. coli expression vector pRSETA containing the CTB proinsulin fusion gene (pRSET-CTB-INS), was transformed into competent cells of the E. coli host strain BL-21 (DE3). The synthesis of CTB-INS fusion protein in the recombinant E. coli strain was confirmed by immunoblotting of the crude homogenate. The purification of CTB-INS from the recombinant E. coli was accomplished as previously described [37].
Partial purification and removal of LPS endotoxin from CTB-INS made in E. coli.
E. coli BL-21 cells producing recombinant CTB-INS were lysed in 20 ml of Buffer Z (8.0 M urea, 100 mM NaCl, 20 mM HEPES, pH 8.0) by sonication on ice with a Sonic 60 Dismembrator (Fisher Sci. Sunnyvale, CA) using 5 × 10 second bursts at a sonicator setting of 12 W. Cell debris was removed from the homogenate by centrifugation in a 50 ml polypropylene conical tube for 20 min at 13,000 rpm and 4 °C in a Sorvall SA-600 rotor (Sorvall, Porton Down, UK), imidazole was added to the bacterial lysate supernatant to a final concentration of 10 mM. A suspension of 1ml Ni-NTA agarose (QIAGEN, Germany) was poured into 2 glass chromatography columns and pre-equilibrated with 1XPBS buffer. The cleared lysate was loaded onto the columns and unbound and weakly bound proteins were eluted from the columns with 4 column volumes of (1X) PBS wash buffer containing 20 mM Imidazole to remove weakly bound proteins. One histidine-affinity column was washed with PBS wash buffer containing 0.1% TritonX-114 (Sigma, St. Louis, MO) to remove LPS contamination. Both columns were washed with PBS until the Triton X-114 content of the aqueous phase were equivalent as determined by measuring the absorbance at 280 nm with a Nano Drop spectrophotometer (Thermo Fisher Scientific Inc, Waltham, MA). The His-tagged recombinant proteins were eluted from the columns with 1XPBS buffer containing 500 mM imidazole. To remove imidazole from the samples, the eluted protein fractions were dialyzed overnight at 4 °C against 1.0 li of HyClone PBS buffer (GE Healthcare Life Sciences, Logan, Utah). Residual endotoxins were removed from the two fractions with EndoTrap HD resin (Hyglos GmbH, Bernried, Germany) according to the manufacturer’s instructions.
Determination of LPS endotoxin levels in purified CTB-INS protein.
To determine the level of recombinant CTB-INS freedom from LPS contamination following partial and complete purification methods, LPS endotoxin was identified in CTB-INS isolated from recombinant E.coli via the Limulus amebocyte Lysate (LAL) assay procedure. Detection of LPS levels were performed using the Toxin Sensor Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ), according to the manufacturer’s instructions. Triplicate samples of each CTB-INS extraction were analyzed in a 96-well format and read in a Bio-Rad microplate reader (Bio-Rad, Hercules, CA, USA). One EU of LPS/ml is equal to 0.1–0.2 ng endotoxin/ml. The CTBINS protein concentration in each preparation was determined using the Bradford protein assay reagent (Sigma) with bovine serum albumin used as the protein standard.
Ethics.
Ex vivo experiments with monocyte-derived dendritic cells (DCs), were performed using aphaeresis isolated leukocytes from the peripheral blood of healthy human subjects provided by the Life Stream Blood Bank (384 West Orange Show Road, San Bernardino CA, 92408). The experiments presented in this manuscript were approved by the Loma Linda University Adventist Health Science Center, Institutional Review Board (IRB), and with written consent of the blood donors. Blood donor information was anonymized and de-identified prior to initiation of the study.
Isolation and culture of human peripheral blood monocyte - derived dendritic cells.
Before the method of CTB-INS induction of DC tolerance can be used for protection against autoimmune disease development in patients, the mechanism underlying CTB-INS induction of DC tolerance in healthy subjects must be elucidated. To accomplish this goal, monocyte-derived dendritic cells (moDCs) were differentiated from freshly collected human peripheral blood monocytes. Peripheral blood monocytes were isolated from 12 anonymized 18 – 40 yr old blood donors from the San Bernardino Blood Bank. Equal numbers (n=6) of randomly selected healthy male and female blood donors were chosen for this study. After collection of peripheral blood from the subjects, erythrocytes were removed from the blood samples by lysis with NH4Cl and monocytes were isolated from the lymphocyte fraction by CD14+ selection on antibody coated magnetic beads (Miltenyi Biotec, Auburn CA). The monocytes were counted and cultured for 6 days in complete RPMI culture medium (ThermoFisher), containing 10% bovine serum, GMCSF and IL-4 to differentiate monocytes into immature DCs as previously described Kim et al., [42].
Determination of IDO1 protein synthesis in LPS and CTB-INS treated dendritic cells.
Approximately 1–2 X 106 monocyte-derived DCs from each subject were inoculated with: 1) LPS at the following concentrations (0, 0.001, 0.1, 1, 10, 100 and 1,000 ng/ml), 2) 10 μg/ml of CTB-INS isolated from E.coli BL-21 cells (containing 66,700 EU LPS/ml) and 3) 10 μg/ml of CTB-INS treated with both Triton X-114 and Endotrap to remove residual LPS (final concentration 0.01 EU LPS/ml). After treatment, the DCs were incubated at 37°C for an additional 0, 2, 4, 8, 16 or 24 hours followed by cell lysis in Buffer C (20 mM HEPES, 0.42 M KCl, 26% Glycerol, 0.1 mM EDTA, 5 mM MgCl2, 0.2% NP40, 37°C), containing a tablet of Complete Protease Inhibitor (Roche, Basel, Switzerland). A minimum of 50 μg of protein isolated from the DC lysate was separated by electrophoresis on 12% polyacrylamide separating gels (SDS-PAGE). After transfer of the electrophoretically separated proteins to polyvinylidene difluoride (PVDF) membranes (Millipore, Temecula, CA), the presence of IDO1 protein (NP- 002155.1) was identified by incubation of the blot for 12 hours at 4° C with rabbit anti-IDO1 monoclonal primary antibody (Cat. 04–1056, clone EPR1230Y), (Millipore, Temecula, CA). For detection of the IDO1 signal, the blot was washed 3 times with PBST (1X PBS, 0.02% tween 20, pH 7.4) and incubated for 2 hours at room temperature in the presence of a monoclonal anti-rabbit IgG γ-chain specific secondary antibody conjugated to alkaline phosphatase (Cat. A-2556, clone RG-96) (Sigma-Aldrich). The immunoblots were washed 3 times in PBST and incubated in 200 μL of Novex® AP chemiluminescent substrate (Invitrogen, Carlsbad, CA) for 5 minutes prior to exposure to X -ray film (Fuji Photo Film Co. HR-G30, Tokyo, Japan) for 3 minutes. The IDO1 signal intensity was quantified via Image J software v. 1.48h. (Image J, NIH).
Measurement of IDO1 activity in LPS +/− CTB-INS treated DCs.
IDO1 activity was quantified by measuring the DC culture supernatant for the production of kynurenines in CTB-INS treated DCs by colorimetry as previously described Mbongue et al., 2015 - [37]. Briefly, in a 1.5 ml polypropylene Eppendorf tube, 100 μL of 30% trichloroacetic acid (TCA) was added to 200 μL of culture supernatant and the contents mixed. Next, in a flat bottom microtiter plate, 125 μL of the TCA soluble phase was added to 125 μl of Ehrlich’s reagent (100 mg of p-dimethyl benzaldehyde dissolved in 5 mL glacial acetic acid), (Sigma). The optical density (OD) was measured at 492 nm and the kynurenine concentration calculated by referral to the kynurenine (Sigma) standard curve. One unit of IDO1 enzyme activity was defined as the amount of IDO1 enzyme that produced 1 nmol of kynurenine/h.
Detection of IDO1 RNA in CTB-INS treated DCs by (RT-qPCR).
Total RNA from 1 Х 106 DCs was prepared using Trizol reagent (Invitrogen) as previously described (Kim et al., 2016), [42]. Complementary DNA was synthesized from 2 μg total RNA with oligo (dT) primer in a 20 μl reaction volume according to the manufacturer’s recommendations (Thermo Fisher Scientific Inc). Dendritic cell IDO1 (NM_002164.5) mRNA was quantified by RT-qPCR relative to β-actin using the following primers: IDO1 forward, 5’-TCT GGC CAG CTT CGA GAA AG-3’; IDO1 reverse, 5’-AGA ACT AGA CGT GCA AGG CG-3’; β-actin forward, 5’-GCA TTG CTT TCG TGT AAA TTA TGT-3’; β-actin reverse, 5’-ACC AAA AGC CTT CAT ACA TCT CA-3’. The quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR) was initiated by SYBR Green JumpStart Taq Ready Mix (BioRad), according to the manufacturer’s instructions and run in triplicate on 96-wells plates with the CFX 96™ Real-Time PCR Detection System (Bio-Rad). The reactions were prepared in a total volume of 25 μl containing: 5 μl of template DNA (Chip or input), 2 μl of each amplification primer (final concentration 50 nM), 12.5 μl of 2Х iQ SYBR Green Supermix (Bio-Rad). The cycling conditions were set as follows: initial denaturation step of 95°C for 10 min to activate the iTaq DNA polymerase, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 1 min. The amplification process was followed by a melting curve analysis, ranging from 65°C to 95°C, with temperature increasing steps of 2°C every 1 min. Baseline and threshold cycles (Ct) were automatically determined using the Bio-Rad CFX Manager 2.1. All the PCR measurements were performed in triplicate and validated when the difference in threshold cycle (Ct) between the 3 measurements was <0.3. The ratio of gene of interest/housekeeping gene was calculated according to the formula: ratio=2−dCt (dCt= mean Ct gene – mean Ct housekeeping gene). Dendritic cell synthesized β-actin was used to normalize for the presence of IDO1 mRNA. To establish statistical significance, the experiment was performed five times per subject DC collection. The samples were electrophoresed on a 1.5% (w/v) agarose gel, and observed under UV light. Three biological replicates for each sample were used for real-time PCR analysis and three technical replicates were analyzed for each biological replicate.
Detection of CTB-INS-induced IDO1 expression in DCs with small interfering RNA (siRNA).
To define CTB-INS-induced IDO1 expression in DCs, human IDO1-small interfering RNA (IDO1 siRNA, sc-45939) and non-targeting siRNA (Control siRNA Fluorescein conjugate, sc36869) were purchased from Santa Cruz Biotechnology (Santa Cruz, Delaware, CA). Human monocytes were cultured in six-well plates for 4 days with 50 ng/ml of hGM-CSF and 10 ng/ml of IL-4 to differentiate the monocytes into immature DCs. The siRNAs were transfected into DCs using Lipofectamine® RNAiMAX reagent (Invitrogen) according to the manufacturer’s protocol. Three microliters of 10 μM siRNA was mixed with 150 μl of Opti-MEN (Gibco-Life Technologies, Paisley, UK), while 9 μl of Lipofectamine® RNAiMAX reagent was incubated in 150 μl of Opti-MEN at room temperature for 5 min. The diluted siRNA and Lipofectamine® RNAiMAX reagent were incubated for an additional 20 min at room temperature for complex formation. The complexes were added to the wells. The final siRNA concentration was 25 pmol. Incubation of the DCs was continued at 37°C in 5% humidified CO2 for 48 h which was sufficient time to significantly knock down the target protein levels. To evaluate transfection efficiency, FITC labeled control RNA was used instead of siRNA. After 24 hours incubation, the transfected DCs were analyzed by fluorescence microscopy to assess intracellular FITC content. The DCs were treated for 6hrs after transfection and lysed in buffer C containing phosphatase inhibitors (50 mM Sodium-beta-glycerophosphate, 1mM Sodium fluoride, and 1.0 mM Sodium-ortho-vanadate). Western blot analysis with anti-IDO1 rabbit monoclonal primary antibody (Millipore) and monoclonal anti-rabbit IgG γ-chain specific alkaline phosphatase conjugated secondary antibody (Sigma-Aldrich) was performed to detect the level of IDO1 expression.
Flow cytometric identification of co-stimulatory factor synthesis in CTB-INS treated DCs.
Peripheral blood monocyte samples were plated in 6 well plates (5×105 cells/well), in RPMI 1640 culture medium containing 10% FBS and human GM-CSF (50 ng/ml) and human IL-4 (10 ng/ml). After 6 days culture at 37°C, the CTB-INS treated DCs were stained with Annexin V to assess viability and for identification of DC co-stimulatory factors with fluorescent antibodies against CD86, (antiCD80 -FITC, Biolegend, San Diego, CA), CD80, (anti-CD86-PE, Biolegend), and maturation factor CD83 (anti CD83-APC, Biolegend), and anti-CD11c - PE-Cy7, Biolegend). One aliquot of cells was labelled with isotype-specific control antibodies in excess concentration as a control for nonspecific Ab binding. UltraComp eBeads (Affymetrix, Santa Clara, CA) were used for compensation. For flow cytometric analysis of CD80, CD83 and CD86 surface expression, the median fluorescence intensity (MFI) of the DCs were recorded for each sample. The MFI fold-changes were calculated in comparison with the control cells (PBS/BSA-induced cells) for each cell type, to allow a direct comparison of the different cell types measured with different voltages and/or compensation settings. For analysis of CD11c surface expression, cells were gated according to the individual isotype controls and the percentages of CD11c positive cells were used as readout. The differentiating cells were identified by flow cytometry (MacsQuant, Miltenyi Biotec, Auburn, CA). The level of DC activation was determined by flow cytometry and the data generated was analyzed with FlowJo software, Version 10 (Treestar), (FLOWJo, LLC).
Quantification of DC secreted cytokines by ELISA.
The determination of DC secreted cytokines was carried out by ELISA assessment of DC culture media previously aliquoted and stored at −80 C. Repeated freeze-thaw cycles were avoided. Pro- and anti-inflammatory cytokines secreted by DCs into the culture medium were quantified by ELISA according to the manufacturer’s instructions, using the human IL-10/IL-12 ELISA ReadySet-Go kit (eBioscience, San Diego, CA).
Statistical Analysis.
The data generated by LPS, CTB-INS and IDO1 effects on human DC activation were expressed as the mean +/−SD and statistical analysis of the data were generated using Graph Pad Prism software v.6.01 (La Jolla, CA). Each experiment was repeated twice with at least 3 replicates of each sample to determine the level of reproducibility. Both LPS(+) and LPS(−) treatment groups were evaluated separately and together. A paired Student’s t-test was used to compare treatment effects with and without CTB-INS. Treatment effects of CTB-INS on DCs and accounting for (LPS) effects on the DCs were evaluated by ANOVA, (P<0.05).
RESULTS:
LPS stimulates IDO1 mRNA synthesis in CTB-INS treated DCs.
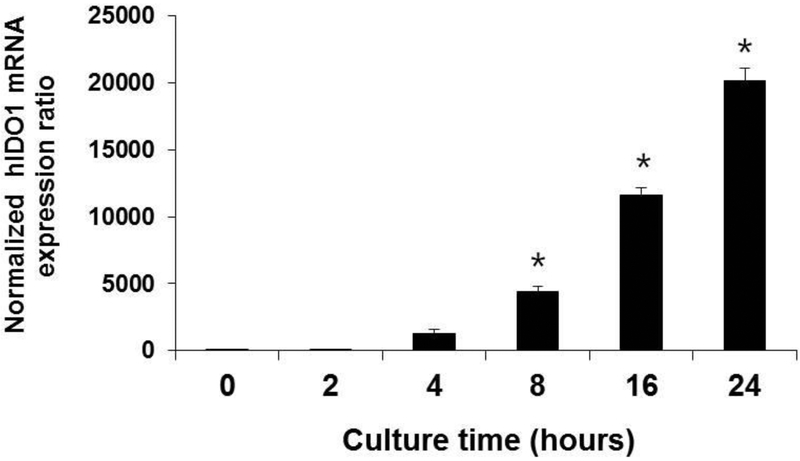
Monocytes isolated from healthy donor peripheral blood were differentiated into immature DCs (iDCs) and the DCs treated with LPS as shown in (Figure 1). IDO1 mRNA levels quantified by rtPCR in DC cell samples harvested at increasing time intervals showed that IDO1 mRNA was visible as early as 2 hours following LPS addition and IDO mRNA increased in a linear fashion up to at least 24 hr after LPS addition to the sample.
Figure 1. LPS stimulation of IDO1 mRNA biosynthesis in human monocyte-derived DCs (moDCs).
Monocytes (CD14+) were isolated by magnetic affinity columns extraction from peripheral blood PBMCs of healthy volunteers. Immature DCs were differentiated from the monocytes by 6 days incubation in RPMI culture medium supplemented with GMCSF and IL-4. The immature DCs were treated with LPS (1μg/mL) synthesized in recombinant E. coli (Sigma Chem Co.) for 0, 2, 4, 8, 16, and 24 h. The LPS treated DCs were harvested and total RNA extracted. The expression of IDO1 mRNA was quantified by reverse transcriptase real-time PCR (rtPCR) as described in the section on Materials and Methods. In three separate experiments, the DC samples were assayed in triplicate and the experimental results averaged and presented graphically as the mean ± the standard deviation (SD) of three independent time course experiments. *p < 0.05, compared with the negative control group (NC), (0hr).
LPS upregulates IDO1 protein synthesis in CTB-INS treated DCs.
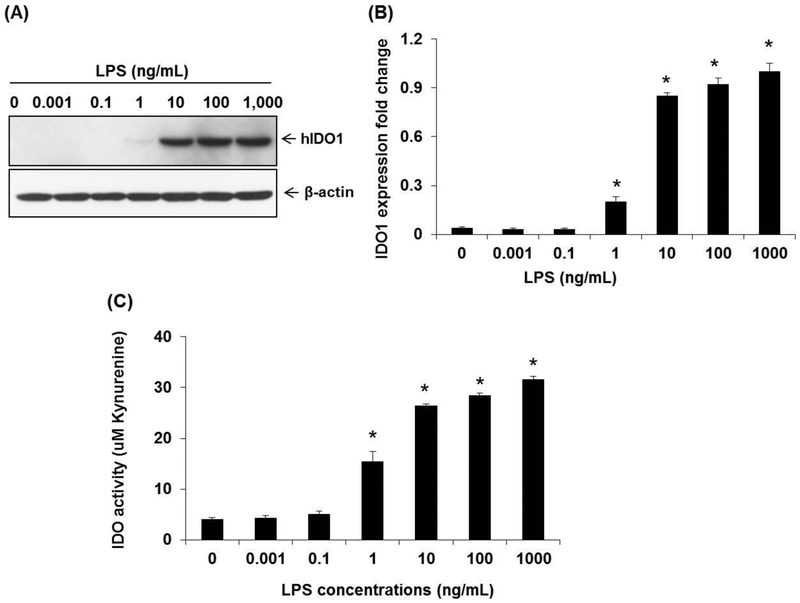
As little as 10 ng of LPS was shown to stimulate detectable amounts of IDO1 protein biosynthesis in western blots of LPS treated human DCs (Figure 2. A). A 10 fold increase in LPS concentration (1 – 10 ng/ml LPS), generated a threefold increase in IDO1 levels in CTB-INS treated DCs (Figure 2, B). A 10 fold increase in LPS concentration (0.1 to 1.0 ng/ml) tripled kynurenine levels produced in the DCs. However, a 100 fold increase in LPS concentration (1.0 – 10 ng/ml LPS) only increased kynurenine levels in the DCs by 30%, indicating a plateau in LPS stimulation of IDO1 activity was achieved. (Figure 2, C).
Figure 2. LPS stimulation of IDO1 protein synthesis in human dendritic cells.
Human moDCs were differentiated from peripheral blood monocytes and treated with increasing amounts of LPS (0.001 – 1,000 ng/mL) for 24 h at 37°C. Panel (A) is a western blot indicating the amount of IDO1 protein produced in monocyte derived DCs treated with increasing amounts of LPS. Panel (B), is a graphic representation of the fold change in IDO1 produced in the DCs after incubation with increasing amounts of LPS for 24 hr at 37°C. The IDO levels presented are representative of three normal donors and are expressed as the standard error of the mean. Each experiment was performed in triplicate. Panel (C) is a graph of IDO1 activity as a function of tryptophan degradation product (kynurenine) levels detected in the DC culture medium. *p < 0.05, in comparison with the NC group, (0hr).
LPS enhances the production of IDO1 in CTB-INS treated DCs.
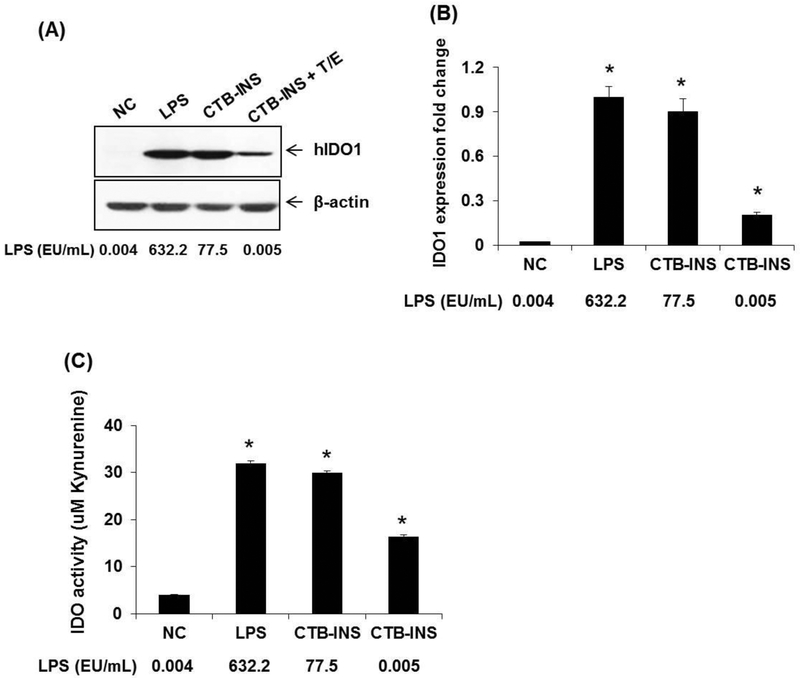
Human DCs incubated with CTB-INS in which trace levels of LPS were removed by Triton X-114 and (or) Endotrap resin treatment (Table 1), produced significant levels of IDO1 in comparison with untreated DCs, as detected by western blotting (Figure 3, A, B). The activity of IDO1 (amount of kynurenines) detected in LPS-free CTB-INS treated DCs was approximately 5 fold greater than kynurenine levels detected in untreated DCs (Figure 3, C). In contrast, CTB-INS purified from E. coli but retaining detectable levels of LPS (77.5 EU/ml), generated almost 4 times the amount of IDO1 detected in LPS-free CTB-INS treated DCs (Figure 3, A, B). DCs treated with CTB-INS isolated from E. coli and containing LPS generated almost 10 fold more kynurenine activity than DCs not treated with CTB-INS and produced almost 2 fold more kynurenines in comparison with LPS-free CTB-INS treated DCs.
Table 1.
Endotoxin Levels in Purified CTB-INS Protein Preparations
| Vaccine Samples | Triton X-114 | Endotrap Resin | Purification conditions | CTB-INS protein recovery (%) | Endotoxin levels (EU/mL) |
|---|---|---|---|---|---|
| CTB-INS | − | − | D | 100 | 66,700 |
| CTB-INS | − | + | D | 77.9 | 1.3 |
| CTB-INS | + | − | D | 86.1 | 1.1 |
| CTB-INS | + | + | D | 55.6 | 0.01 |
Removal of residual LPS endotoxin from CTB-INS protein isolated from E. coli. Partially purified recombinant CTB-INS fusion protein was isolated from sonicated E. coli BL-21 cells by nickel – agarose (Ni-NTA) affinity column chromatography under denaturing conditions of 8.0 M urea (D). Residual LPS was removed from the partially purified CTB-INS protein by treatment with 0.1% Triton X-114 or by passage of the protein over an Endotrap resin column. For maximum endotoxin removal, CTB-INS protein was purified by treatment with Triton X-114 followed by treatment with Endotrap resin.
Figure 3. IDO1 protein synthesis and kynurenine activity in DCs treated with CTB-INS protein partially or completely purified from LPS.
Panel (A), Human moDCs suspended in 2.0 mL of RPMI culture medium were treated with: 1) PBS as a negative control (NC), 2) LPS (1 μg/mL), 3) partially purified CTB-INS (10 μg/mL) isolated by nickel affinity column from E. coli BL-21 cells, containing residual levels of LPS, and 4) completely purified CTB-INS (10 μg/mL) treated with both Triton X-114 and Endotrap resin (T/E) to remove residual LPS. The levels of LPS were determined by Limulus amebocyte assay of all DC samples. Panel B, the DCs were cultured at 37°C for 24h and the amount of IDO1 protein synthesized in the DCs was identified by western blotting. Panel C, The activity of IDO1 synthesized in response to the different treatments was determined in all DC samples by kynurenine assay of the cell culture medium. The experimental data presented was from an individual donor representative of a total of three DC donors. The data for each measurement is expressed as the standard error of the mean of triplicate DC samples obtained from each donor. *p < 0.05, in comparison with the NC group.
LPS stimulates CTB-INS activation of human dendritic cells.
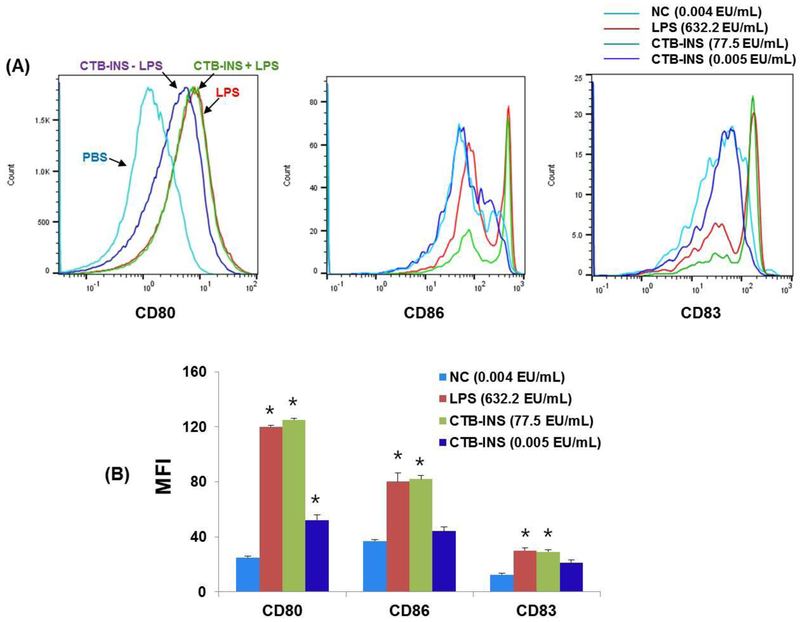
The production of costimulatory factors CD86, CD80 and maturation factor CD83 are considered to be signals required for antigen mediated DC activation (maturation). Flow cytometry of iDCs incubated with CTB-INS containing LPS produced CD86, CD80 and CD83 costimulatory factors at levels determined to be intermediate between the high levels of costimulatory factors generated by LPS alone and the lowest levels detected in LPS-free CTB-INS treated DCs (Figure 4, A). In the absence of LPS, CTB-INS stimulated lower levels of costimulatory factors than did DCs treated with CTB-INS containing LPS. (Figure 4, B), as represented by the primary flow cytometry data in Figure 3, A.
Figure 4. Activation of human moDCs treated with CTB-INS in the presence or absence of residual LPS endotoxin.
Panel (A), are histograms generated by flow cytometric identification of DC costimulatory factors CD80, CD86 and maturation factor CD83 induced by CTB-INS fusion protein in the presence of residual LPS or absence of LPS endotoxin. The DCs were stained for surface expression of CD80, CD86 and CD83 and the costimulatory factor markers were determined by flow cytometric measurement (x-axis = MFI; y-axis = cell #). DC activation is expressed as mean fluorescence intensity (MFI). Panel (B), is a bar graph representing the data in panel A. Immature moDC activation is expressed as mean fluorescence intensity (MFI) after 24 h incubation of the DCs in RPMI culture medium containing 1μg/mL of LPS (red bar); 10 μg/mL of CTB-INS with residual LPS (green bar), 10 μg/mL of pure CTB-INS treated with both triton X-114 and Endotrap resin to remove residual LPS (blue bar) or PBS as a no treatment negative control (NC), (sky blue bar). The amount of LPS in each sample was determined. The experimental treatments are represented by the same colors used in panel (A). The data presented was taken from a representative donor from a total of three donors and is expressed as mean standard error for triplicate samples from the donor. *p < 0.05, in comparison with the NC group.
LPS enhances anti-inflammatory cytokine production in CTB-INS treated DCs.
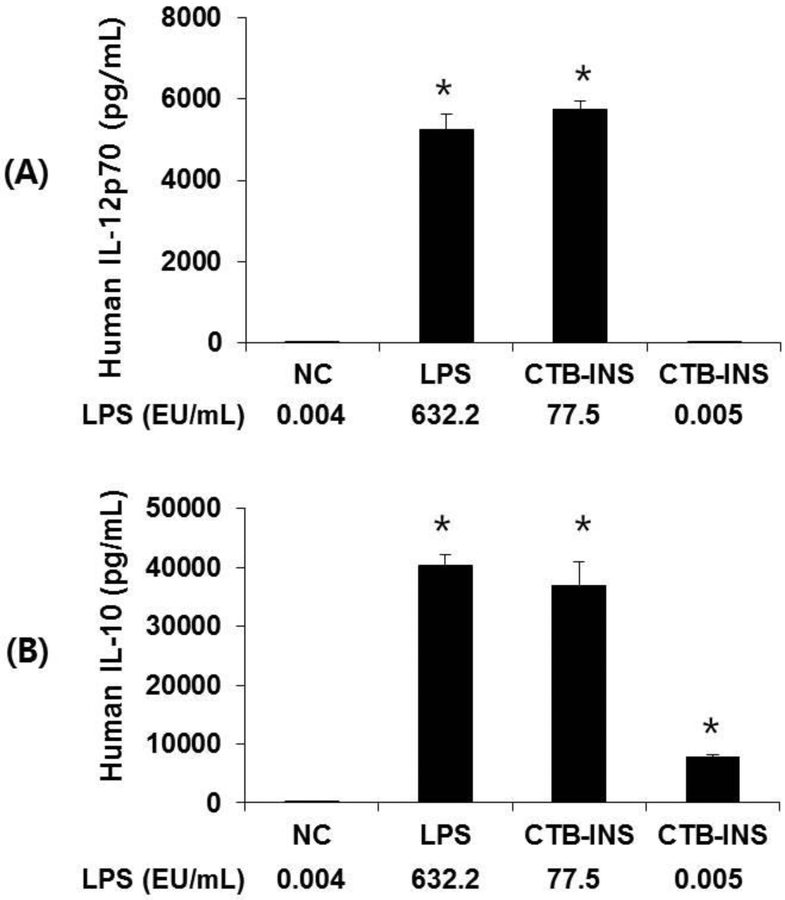
Enzyme linked immunosorbent assay (ELISA) determination of pro-inflammatory cytokine IL-12p70 and the anti-inflammatory cytokine IL-10 levels in the culture medium of CTB-INS treated DCs is shown in Figure 5. Dendritic cells treated with LPS-free CTB-INS increased IL-10 levels to 10,000 pg/ml in comparison with untreated DCs which did not synthesize detectable levels of IL-10. In addition, LPS-free CTB-INS treated DCs did not synthesize detectable IL-12 levels. In contrast, DCs treated with CTB-INS containing LPS (77.5 EU/ml), increased IL-10 levels 4 fold to about 40,000 pg/ml (Figure 5), while the levels of IL-12 p70 in LPS containing CTB-INS treated DCs increased from zero to only about 6,000 pg/ml, far less than the increase in IL-10 levels generated by DCs treated with CTB-INS containing LPS.
Figure 5. Detection of cytokines secreted by dendritic cells treated with CTB-INS.
Panel A, Graphic representation of ELISA assay of DC secretion of the pro-inflammatory cytokine IL-12p70 detected in RPMI culture medium after 24 h treatment of moDCs with PBS (NC), 1μg/mL of LPS, 10 μg/mL of CTB-INS containing residual LPS, 10 μg/mL of CTB-INS treated with Triton X-114 and Endotrap resin to remove residual LPS. Panel B is a graphic representation of DC secretion of the anti-inflammatory cytokine IL-10. The error bars represent the standard deviation from the mean of three independent ELISA experiments. *p < 0.05, in comparison with the NC.
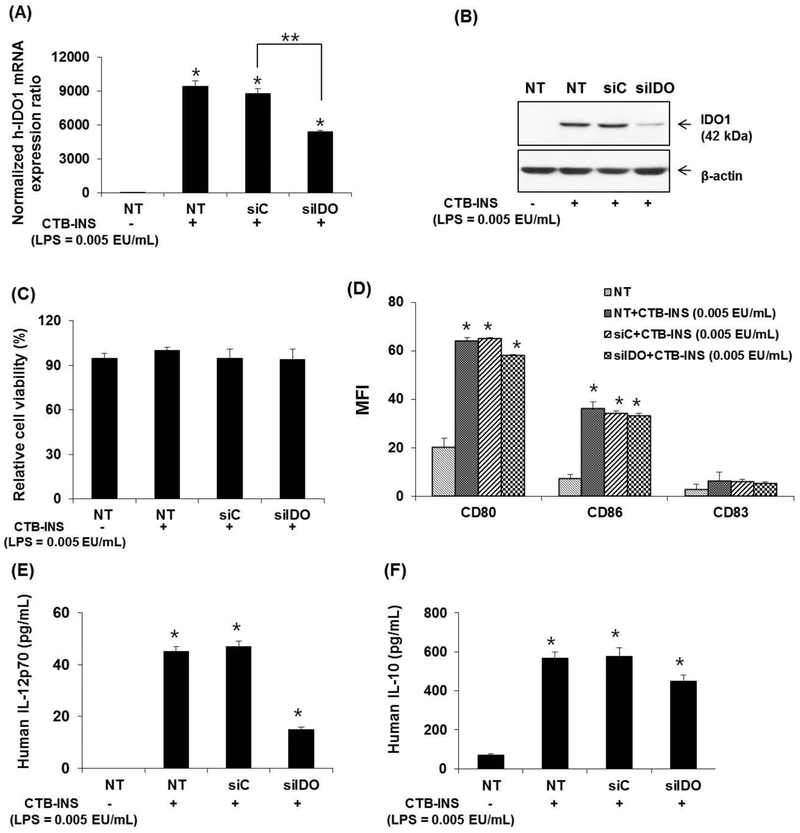
Knock down of IDO1 in LPS free CTB-INS treated human DCs inhibits pro-inflammatory cytokine secretion.
The addition of IDO1 siRNA (siIDO1) to LPS-free CTB-INS treated DCs reduced IDO1 mRNA and protein levels with no significant reduction in DC viability, (Figure 6. A, B, C). However, the secretion of IL-12 was significantly reduced but IL-10 secretion remained relatively unaffected (Figure 6, E, F). Treatment of DCs with LPS-free CTB-INS resulted in a significant reduction of costimulatory factor CD80 while CD86 and CD83 levels were unaltered (Figure 5, D).
Figure 6. Effects of the inhibition of IDO1 on CTB-INS treated DCs.
Panel (A) is a graphic representation of quantitative real time-PCR (RT-qPCR) analysis of human IDO1 mRNA levels synthesized in untreated (NT) or LPS free CTB-INS (LPS = 0.005EU/mL) treated moDCs transfected with human IDO1-specific small interfering RNA (siIDO1), or a negative control siRNA (siC). IDO1 gene expression was normalized in comparison with β-actin gene expression. Panel (B) Western blot analysis of IDO1 protein synthesis in moDCs treated with CTB-INS and transfected with IDO1-specific siRNA (siIDO) or negative control (siC) using human anti-IDO1 as the primary antibody. Panel (C) Viability of CTB-INS treated human moDCs after transfection with IDO1-specific siRNA (siIDO). DC viability was measured as the percentage of CTB-INS treated DCs negative for uptake of viability dye FVD450. Panel (D) Graphic representation of siRNA inhibition of IDO1 effects on LPS free CTB-INS activation of DC costimulatory factor biosynthesis measured by flow cytometry (MFI). Panels (E and F) graphic representation of ELISA quantification of secreted IL-12p70 and IL-10 cytokines from LPS free CTB-INS treated moDCs. The graph bars represent the mean of three independent experiments. NT− = siIDO1 RNA transfected DCs in the absence of LPS free CTB-INS fusion protein treatment. NT+ = siIDO1 RNA transfected DCs treated with CTB-INS (LPS = 0.005 EU/mL). The DC samples were assayed in triplicate and the data represent the mean ± SD of three independent experiments. *p < 0.05, compared with the NT and **p < 0.05 in comparison with siC.
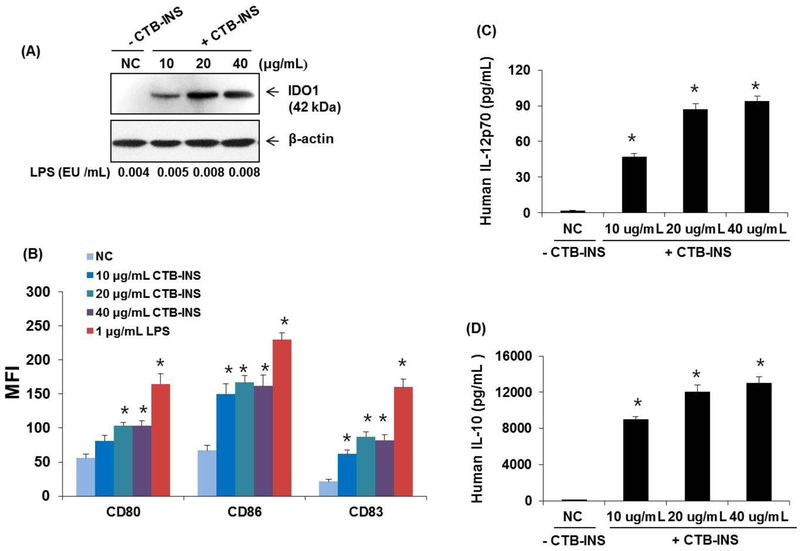
The effect of CTB-INS levels on DC activation.
Increasing the amount of LPS-free CTB-INS treatment of normal monocyte derived DCs above 20 μg CTB-INS/ml did not significantly increase the upregulation of IDO1, costimulatory or maturation factor levels, or the level of secretion of IL-10 or IL-12 cytokines (Figure 7, A, B).
Figure 7. Increased amounts of CTB-INS stimulate DC activation and cytokine secretion.
Panel (A) Immunoblot detection of IDO1 levels in human moDCs treated with increasing amounts of LPS free CTB-INS. PBS treated DCs are a negative control (NC) for addition of CTB-INS. The amount of LPS in each CTB-INS treated DC sample was determined by the Limulus amebocyte lysate assay. Actin protein bands provide a loading control for DC protein in each well. Panel (B) graphic representation of costimulatory factors CD80, CD86 and CD83 synthesized on LPS free CTB-INS treated DCs determined by flow cytometric detection of fluorescent Ab binding to DC co-stimulatory factors (MFI). Panels (C and D) graphic representation of ELISA determination of pro-inflammatory (IL-12p70) and anti-inflammatory (IL-10) cytokines secreted by DCs into the culture medium following treatment of the DCs with increasing amounts of LPS free CTB-INS. Individual bars represent the mean and standard deviation (SD) of three independent ELISA cytokine determinations. *p < 0.05, in comparison with NC.
DISCUSSION:
While the precise mechanism of CTB-INS induced IDO1 mediated suppression of DC activation in vivo remains unclear, interferons, cytokines and specifically IFN-γ, is required for the induction of IDO1 [45]. Through selective blocking of the canonical and the non-canonical NF-κB pathway pathways with small interfering RNAs, IDO1 expression in human DCs was shown to require non-canonical NF-κB signaling for downregulation of pro-inflammatory cytokine production [46]. Further, activation of the non-canonical NF-κB pathway resulted in the differentiation of DCs that suppressed T cell activation and promoted the development of regulatory T cells [46]. Reinforcing these observations, activation of IDO by LPS was demonstrated experimentally in pigs both in vivo and ex vivo [47]. Here we show that LPS stimulation of IDO1 biosynthesis in human immature monocyte derived DCs occurs as early as 2 hr after addition of endotoxin to the DCs and continues to stimulate tolerance in the DCs for at least 24 hr (Figure 1), suggesting that the addition of LPS rapidly induces DC tolerance. While the ultimate length of LPS induced DC tolerance in the presence of CTB-INS remains unknown, longer exposure to LPS may be required to generate a durable state of functional DC tolerance. The addition of LPS to immature DCs substantially increased IDO1 biosynthesis resulting in increased levels of IDO1 enzyme activity that was reflected as an increase in kynurenine production (Figure 2,C). Increased IDO1 enzyme activity may stimulate functional tolerance in CTB-INS treated DCs by exhausting the essential amino acid tryptophan required for DC metabolism in addition to the secretion of immune-inhibitory kynurenines. While LPS upregulation of IDO1 in moDCs was shown to stimulate DC maturation, IDO1 was also shown to expand CD4+CD25 high regulatory T cell populations that could play an additional role in LPS mediated inhibition of DC maturation [48]. Kynurenine release from LPS and CTB-INS treated DCs is predicted to inhibit pro-inflammatory Th1, Th17 and Th2 effector cell populations, while stimulating Treg activation adding to the suppression of DC mediated pro-inflammatory T cell development. In comparison with phosphate buffered saline, preparations of CTB-INS free of LPS upregulated IDO1 expression (Figure 3 B).
The presence of residual LPS in CTB-INS treated DCs activated both CD80 and CD86 costimulatory factors, but did not stimulate upregulation of maturation factor CD83, suggesting CTB-INS treated DCs may remain in a semi-active state (Figure 4, A, B). The increase in IL-10 secretion detected following DC treatment with LPS-free CTB-INS and ELISA (Figure 5), supports our hypothesis that CTB-INS without enhancement may inhibit DC activation by inducing a state of functional tolerance in the immature DCs. The increased levels of IL-10 detected in DCs treated with CTB-INS containing LPS suggest that small increases in LPS may enhance CTB-INS induced DC tolerance. The presence of increased IL12 levels in LPS containing CTB-INS treated DCs suggests LPS maintains its ability to stimulate DC activation in the presence of CTB-INS which could down regulate immuno-suppressive functions of the fusion protein. The duration of IL-12 upregulation in CTB-INS treated DCs remains unknown. The knock down of IDO1 levels in LPS-free CTB-INS treated DCs mediated by siRNA (Figure 6, A, B), is accompanied with a significant reduction in IL-12 levels while IL-10 levels remain unaffected. This result suggests that IL-10 may have a significant effect on the maintenance of DC mediated tolerance. The lack of DC maturation factor CD83 upregulation in comparison with costimulatory factors CD86 and CD80 following DC treatment with LPS-free CTB-INS reinforces the hypothesis that DC immaturity is maintained during IDO1 regulation of DC mediated tolerogenesis (Figure 6, D). Because IDO1 siRNA knock down does not significantly reduce CD86 or CD80 levels in CTB-INS treated DCs this result further suggests that the DCs may exist in a state of partial or semi-maturation during CDB-INS mediated tolerogenesis. Interestingly, the addition of LPS free CTB-INS to immature DCs increased IL-10 biosynthesis without significantly upregulating costimulatory factor synthesis, (Figure 7, B, D). it was shown by Salazar et al., that in comparison with the unprimed controls, TLR4 ligation in LPS-primed DCs induced higher levels of IDO isoforms as well as the aryl-hydrocarbon receptor (AhR) transcription factor. Further, LPS was shown to induce an anti-inflammatory phenotype in the DCs - with an increase in IL-10 and higher expression of programmed death ligands PD-L1 and PD-L2 partially contingent on IDO1. In addition, the authors demonstrated that an aryl hydrocarbon (AhR-IDO) pathway could be responsible for preferential activation of the non-canonical NF-κB pathway in LPS-conditioned DCs [49]. The possibility exists that Triton detergent or EndoTrap removal of LPS from CTB-INS could also remove additional factors responsible for or contributing to DC stimulation of IDO1 or IL-10 synthesis. Experiments in which highly purified CTB-INS is reconstituted with LPS will help to confirm or dismiss this possibility.
Taken together, our experimental data suggest that LPS stimulates CTB-INS induced DC synthesis of the immune-inhibitory enzyme IDO1, which upregulates the release of kynurenines, tryptophan degradation products that initiate a state of functional tolerance in human moDCs. Application of this experimental strategy could lead to the arrest of DC mediated pro-inflammatory T cell responses important in the development of T1D autoimmunity. Further, our data suggest the presence of residual LPS in the CTB-INS fusion protein could greatly enhance DC mediated tolerance by: 1) inhibiting iDC activation (maturation), 2) by increasing the levels of enzymatically active IDO1, 3) by increasing the production of functional kynurenines and 4) by increasing DC secretion of the anti-inflammatory cytokine IL-10.
These DC functions may act in a coordinated fashion to induce a state of functional DC tolerance capable of inhibiting T1D autoimmunity. Thus, LPS augmentation of DC inhibited T1D autoimmunity could alter the existing paradigm for T1D therapy in an unexpected way. Because the presence of bacterial LPS in therapeutics is thought to be pro-inflammatory it is considered to be detrimental for human health. Shifting the paradigm of LPS applications for therapeutic downregulation of immune responses provides a novel application to use LPS for down regulation of chronic inflammation thought by many to be responsible for the development of tissue specific autoimmunity. Our experimental data provides an initial step in understanding how bacterial toxins may serve to enhance immunosuppressive therapy for protection against tissue specific autoimmune diseases. Translating these findings in vivo in humanized mice and ultimately in T1D patients will establish a strong foundation for addition of LPS as an effective contributor to immuno-therapeutic strategies for “off the shelf” treatment for tissue specific autoimmune diseases including T1D.
Highlights of our manuscript:
Explores the mechanism of how the bacterial endotoxin LPS amplifies CTB-INS induction of immunological tolerance in immature human dendritic cells (DCs) for effective protection against organ specific type 1 diabetes autoimmunity.
Pre-treatment of CTB-INS with the E. coli lipopolysaccharide (LPS) stimulates IDO1 biosynthesis in CTB-INS treated immature DCs (iDCs) to increase the secretion of immunoregulatory products (kynurenines), that suppress DC mediated pro-inflammatory T cell differentiation.
LPS supplementation of CTB-INS treated iDCs stimulates the biosynthesis of costimulatory factors CD80 and CD86 but does not upregulate maturation factor CD83, suggesting CTB-INS treated DCs may remain in a state of semi-activation.
CTB-INS alone stimulates DC secretion of IL-10. However, when CTB-INS is supplemented with LPS the secretion of IL-10 is greatly enhanced.
Increasing the dose of CTB-INS dramatically increases the secretion of IL-10 suggesting CTB-INS levels are critical for regulating DC mediated pro-inflammatory T cell differentiation.
Acknowledgements:
The authors would like to acknowledge financial support from NIH award DK-99–013 to W. Langridge and financial contributions from the Korean government NRF-20151A1A3A04001542 to Dr. Nansun Kim that provided funding for many of the experiments presented in this manuscript. We would also like to acknowledge financial support from the John Backer Philanthropic Fund that without his support many of the experiments presented in this manuscript would not have been possible
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Livingstone Shona J. MSc; Levin Daniel MSc; Helen C. Looker MBBS; Robert S. Lindsay FRCP; Sarah H. Wild FRCP; Nicola Joss MD; Graham Leese MD; Peter Leslie MD; Rory J. McCrimmon FRCP; Wendy Metcalfe MD; John A. McKnight FRCP; Andrew D. Morris FRCP; Donald W. M. Pearson FRCP; John R. Petrie MD; Philip Sam MD; Naveed A. Sattar FRCP; Jamie P. Traynor MD; Helen M. Colhoun MD; (2015). Estimated Life Expectancy in a Scottish Cohort With Type 1 Diabetes, 2008–2010 JAMA. 2015; 313(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scottish Diabetes Research Network epidemiology group and the Scottish Renal Registry.
- 2.de Ferranti Sarah D. MD, MPH; Ian H. de Boer MD, MS; Vivian Fonseca MD; Caroline S. Fox MD, MPH*; Sherita Hill Golden MD, MHS; Carl J. Lavie MD; Sheela N. Magge MD, MSCE; Nikolaus Marx MD; Darren K. McGuire MD; Trevor J. Orchard MD, MMedSci; Bernard Zinman MD; and Robert H. Eckel MD, FAHA, (2014).Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association Diabetes Care 2014;37:2843–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Type 1 Diabetes, 2011; JDRF FACTS, www.jdrf.org, (> 15,000 children and 15,000 adults annually, approximately 80 people per day, are diagnosed with this tissue specific autoimmune disease in the U.S and there is a 3.0% increase in disease incidence annually).
- 4.Roep Bart O 1 and Mark Peakman, (2012) Antigen Targets of Type 1 Diabetes Autoimmunity Cold Spring Harbor Perspect Med. April; 2(4): a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham Kate L 1 Robyn M. Sutherland 2,3 Stuart I. Mannering 1,4 Yuxing Zhao 1 Jonathan Chee 1,4 Balasubramanian Krishnamurthy 1,4 Helen E. Thomas 1,4 Andrew M. Lew 2,3 and Thomas W.H. Kay 1,4 (2012). Pathogenic Mechanisms in Type 1 Diabetes: The Islet is Both Target and Driver of Disease, Rev Diabet Stud., Winter; 9(4): 148–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen Annemarie, Martin Van Hagen and Hemmo A Drexhaga, (1995). Defective maturation and function of antigen-presenting cells in type 1 diabetes, The Lancet, Vol 345, February 25, 491–492. [DOI] [PubMed] [Google Scholar]
- 7.Lee Li-Fen *, Baohui Xu †, Sara A. Michie †, Georg F. Beilhack ‡, Tibor Warganich *, Shannon Turley §, and Hugh O. McDevitt *¶ (2005), The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: Analysis of dendritic cell maturation. PNAS, November 1, vol. 102: no. 44, 15995–16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morel PA, (2013). Dendritic Cell Subsets in Type 1 Diabetes: Friend or Foe? Front Immunol.; 4: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatenoud Lucienne 1 Katharina Warncke 2, 3 and Anette -G. Ziegler 3,4 Clinical Immunologic Interventions for the Treatment of Type 1 Diabetes (2012). Cold Spring Harb Perspect Med. August; 2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluestone Jeffrey A. and Hélène Bour-Jordan (2012). Current and Future Immunomodulation Strategies to Restore Tolerance in Autoimmune Diseases Cold Spring Harb Perspect Biol. November; 4(11): a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannoukakis N,C Andred Bonham, Siguang Quian, Zhongyou Zhou, Lansha Peng, Jo Harana, Wei Li, Angus W. Thomson, John J. Fung, Paul D.. Robbins and Lina Lu. (2000). Prolongation of Cardiac Allograft Survival Using Dendritic Cells treated with NF-kB Decoy Oligodeoxyribonucleotides. Molecular Therapy, Vol 1, No 5, May 200, Part 1 of 2 parts. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins MK, Schwartz RH, and Pardoll DM, (1988). Effects of cyclosporine A on T cell development and clonal deletion, Science, 241:1655–1658. [DOI] [PubMed] [Google Scholar]
- 13.Lu L 1, Li W, Fu F, Chambers FG, Qian S, Fung JJ, Thomson AW, (1997). Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. December 27; 64(12):1808–15. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Lee W-C, Gambotto A, Zhong C, Robbins PD, Qian S, Fung JJ, and Thomson AW. (1999). Transduction of Dendritic Cells With Adenoviral Vectors Encoding CTLA4-Ig Markedly Reduces Their Allostimulatory Activity. Transplantation Proceedings, 31, 797. [DOI] [PubMed] [Google Scholar]
- 15.Lee Wei-Cheng 3; Zhong Cuiping 3; Qian Shiguang 3; Wan Yonghong 5; Gauldie Jack 5; Mi Zhibao 6; Robbins Paul D. 6; Thomson Angus W. 3,4,6; Lu Lina 3,4. (1998). Phenotype, Function, And In Vivo Migration And Survival Of Allogeneic Dendritic Cell Progenitors Genetically Engineered To Express TGF-β1, 2 Transplantation: 27 December - Volume 66 - Issue 12 - pp 1810–1817. [DOI] [PubMed] [Google Scholar]
- 16.Stepkowski SM 1, Wang ME, Condon TP, Cheng-Flournoy S, Stecker K, Graham M, Qu X, Tian L, Chen W, Kahan BD, Bennett CF (1998). Protection against allograft rejection with intercellular adhesion molecule-1 antisense oligodeoxynucleotides. Transplantation. Sep 27; 66(6):699–707. [DOI] [PubMed] [Google Scholar]
- 17.Stepkowski SM, Tu Y, Condon TP and Bennett CF. (1995). Blocking of heart allograft rejection by intercellular adhesion molecule-1 antisense oligonucleotides alone or in combination with other immunosuppressive modalities. J Immunol. February 1, 154 (3) 1521. [PubMed] [Google Scholar]
- 18.Phipps PA, Stanford MR, Sun JB et al. (2003). Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit, Eur J Immunol, 33, pp. 224–232. [DOI] [PubMed] [Google Scholar]
- 19.Petersen JS, Bregenholt S, Apostolopolous V, Homann D, Wolfe T, Hughes A, De Jongh K, Wang M, Dyrberg T, Von Herrath MG, (2003). Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes Clin Exp Immuno l. October; 134(1): 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George Chandy Annie, Susanne Hultkrantz, Raghavan Sukanya, Czerkinsky Cecil, Lebens Michael, Telemo Esbjörn, Holmgren Jan. (2006). Oral tolerance induction by mucosal administration of cholera toxin B-coupled antigen involves T-cell proliferation in vivo and is not affected by depletion of CD25+ T cells, Immunology. July; 118(3): 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dénes Béla, István Fodor, Langridge William H. R., (2013) Persistent Suppression of Type 1 Diabetes by a Multicomponent Vaccine Containing a Cholera Toxin B Subunit-Autoantigen Fusion Protein and Complete Freund’s Adjuvant. Clin Dev Immunol.. Volume 2013, Article ID 578786, 16 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, Mizushima Y, Holmgren J, Lehner T. (2004). Oral tolerization with peptide 336–351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease, Clin Exp Immunol. July; 137(1):201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JB 1, Czerkinsky C, Holmgren J. (2012). B lymphocytes treated in vitro with antigen coupled to cholera toxin B subunit induce antigen-specific Foxp3(+) regulatory T cells and protect against experimental autoimmune encephalomyelitis J Immunol. February 15;188(4):1686–97. [DOI] [PubMed] [Google Scholar]
- 24.Aspord C and Thivolet C(2002). Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. November; 130(2): 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploix Corinne, Bergerot Isabelle, Durand Annie, Czerkinsky Cecil, Holmgren Jan, and Thivolet Charles (1999). Oral Administration of Cholera Toxin B–Insulin Conjugates Protects NOD Mice From Autoimmune Diabetes by Inducing CD4+ Regulatory T-Cells, Diabetes, 4 8 :2150–2156. [DOI] [PubMed] [Google Scholar]
- 26.Arakawa T, Chong DKX, Yu J, Hough J, Engen PC, Elliott JF and Langridge WHR, (1998). A Plant-based Cholera Toxin B Subunit-insulin Fusion Protein Protects Against Development of Autoimmune Diabetes, Nature Biotechnology, Vol.16:934–938. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa T, Chong DKX, Yu J, Hough J, Engen PC, Elliott JF and Langridge WHR, (1999), Suppression of Autoimmune Diabetes by a Plant-delivered Cholera Toxin B Subunit-Human Glutamate Decarboxylase Fusion Protein, Transgenics, Vol.3:1,1–10. [Google Scholar]
- 28.Denes B, Krausova V, Fodor N, Timiryasova T, Henderson D, Hough J, Yu J, Fodor I, Langridge WH (2005). Protection of NOD mice from type 1 diabetes after oral inoculation with vaccinia viruses expressing adjuvanted islet autoantigens. J Immunother. Sep-Oct; 28(5):438–48. [DOI] [PubMed] [Google Scholar]
- 29.Denes B, Fodor I and Langridge WHR, (2010). Autoantigens plus Interleukin-10 Suppress Diabetes Autoimmunity, Diabetes Technology and Therapeutics 12:8649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. (2014). A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 41:657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chilton PM, Rezzoug F, Fugier-Vivier I, Weeter LA, Xu H, Huang Y, et al. (2004). Flt3ligand treatment prevents diabetes in NOD mice. Diabetes 53:1995–2002. [DOI] [PubMed] [Google Scholar]
- 32.Sun J-B *, Czerkinsky C & Holmgren J * (2010). Mucosally induced Immunological Tolerance, Regulatory T Cells and the Adjuvant Effect by Cholera Toxin B Subunit Scandinavian Journal of Immunology 71, 1–11. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez Joaquín and Jan Holmgren (2011). Cholera toxin- A foe & a friend, Indian J Med Res. February; 133(2): 153–163. [PMC free article] [PubMed] [Google Scholar]
- 34.Hajishengallis G, Hollingshead SK, Koga T and Russell MW (1995). Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol; 154:4322–4332. [PubMed] [Google Scholar]
- 35.Roland Tisch * and Hugh McDevitt †, (1996). Insulin-Dependent Diabetes Mellitus, Cell, Vol. 85, 291–297. [DOI] [PubMed] [Google Scholar]
- 36.Mizuho. Shinomiya, Seijin Nadano, *Hiroto Shinomiya, and Morikazu Onji (2000). In Situ Characterization of Dendritic Cells Occurring in the Islets of Nonobese Diabetic Mice During the Development of Insulitis. Pancreas, Vol. 20, No. 3, pp. 290–296. [DOI] [PubMed] [Google Scholar]
- 37.Mbongue Jacques C 1, 2, Dequina Nicholas 1,3, Kangling Zhang 3,4, Brittany N. Hamilton 1,5, Marco Larios 1, Guangyu Zhang 3, Kazuo Umezawa 6, Anthony Firek 7, and William H.R. Langridge 1,3 (2015), Induction of Indoleamine 2, 3 Dioxygenase in Human Dendritic Cells by a Cholera Toxin B Subunit - Proinsulin Vaccine PLOS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esebanmen Grace and William H.R. Langridge, (2017). Mechanism of chimeric vaccine stimulation of indoleamine 2, 3-dioxygenase biosynthesis in human dendritic cells is independent of TGF-β signaling (2017). Cellular Immunology.8/21 (available on line). [DOI] [PubMed] [Google Scholar]
- 39.Odumosu Oludare, Payne Kimberly, Baez Mavely, Jutzy Jessica, Wall Nathan, Langridge William (2010). Suppression of Dendritic Cell Activation by Diabetes Autoantigens Linked to the Cholera Toxin B Subunit. Immunobiology. April; 216(4):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odumosu O, Nicholas D, Payne K, Langridge W. (2011). Cholera toxin B subunit linked to glutamic acid decarboxylase suppresses dendritic cell maturation and function. Vaccine. October 26; 29(46):8451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odumosu Oludare, (2011). Cholera Toxin B subunit-Diabetes Autoantigen Fusion Proteins Modulate Dendritic Cell Function and T Cell Morphogenesis. Doctoral Dissertation, Loma Linda University, School of Medicine, 1–211 [Google Scholar]
- 42.Kim Nan-Sun 1, 2, Jacques C. Mbongue 1, 3, Dequina A. Nicholas 1,4, Juli J. Unternaehrer 4, Anthony F. Firek 5 and William H. R. Langridge 1,4* (2016) Chimeric Vaccine Stimulation of Human Dendritic Cell Indoleamine 2, 3-Dioxygenase Occurs via the Noncanonical NF-κB pathway PLoS ONE 11(2) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R 1, Wei F, Yu J, Li H, Ren X, Hao X. IDO1 inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther. 2009. July; 8(14):1402–8. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado Roberto A 1 and Ulrich H. von Andrian 1, 2 How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010; 108: 111–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duk Chang-Min Leea Young-Il Jeongb Jun SikLeec Won Sun Parkd Jin Hand Yeong-Min Parka (2007). Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells Volume 581, Issue 7, 3 April, Pages 1449–1456. [DOI] [PubMed] [Google Scholar]
- 46.Tas Sander W. 1, Margriet J. Vervoordeldonk 1, Najat Hajji 1, Joost H. N. Schuitemaker 2, Koen F. van der Sluijs 3, Michael J. May 4, Sankar Ghosh 5, Martien L. Kapsenberg 2, Paul P. Tak 1 and Esther C. de Jong 2 (2007). Non-canonical NF-κB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation Blood prepublished online May 4, 2007; DOI 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 47.Wirthgen E 1, Tuchscherer M, Otten W, Domanska G, Wollenhaupt K, Tuchscherer A, Kanitz E. (2014). Activation of indoleamine 2,3-dioxygenase by LPS in a porcine model. Innate Immun. January;20(1):30–9. doi: 10.1177/1753425913481252. [DOI] [PubMed] [Google Scholar]
- 48.Marcelo Hill *1,2,3, Sverine Tanguy-Royer *4, Pierre Royer 4, Christine Chauveau 1,2,3, Kashif Asghar 1,2,3, Laurent Tesson 1,2,3, Frederic Lavainne *1,2,3, Severine Rmy 1,2,3, Regis Brion 1,2,3, Franois-Xavier Hubert 1,2,3, Michle Heslan 1,2,3, Marie Rimbert 1,2,3, Laureline Berthelot 1,2,3, John R. Moffett 5, Regis Josien 1,2,3, Marc Gregoire **4 and Ignacio Anegon **1,2,3 (2007). IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur. J. Immunol 37: 3054–3062. [DOI] [PubMed] [Google Scholar]
- 49.Salazar Fabián Dennis Awuah, Negm Ola H., Shakib Farouk & Amir M Ghaemmaghami Published: 03 March (2017). The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs Scientific Reports volume7, Article number: 43337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]