Figure 1. Trial schema.
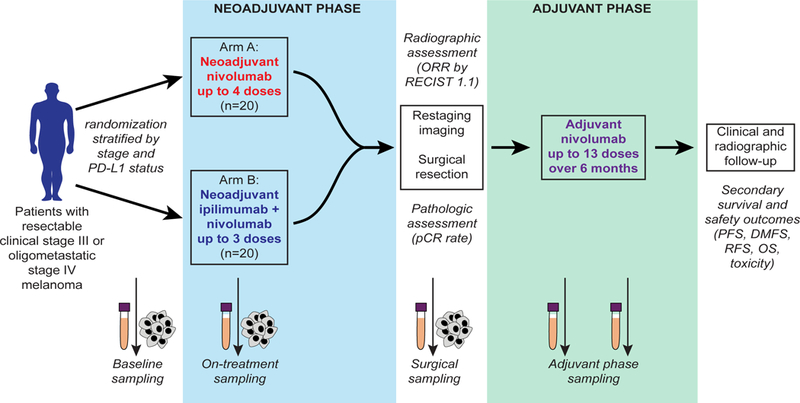
Patients with resectable clinical stage III or oligometastatic stage IV melanoma were stratified by stage and PD-L1 status and randomized in 1:1 ratio to neoadjuvant nivolumab 3 mg/kg for up to 4 doses (Arm A) or ipilimumab 3 mg/kg with nivolumab 1 mg/kg for up to 3 doses (Arm B), followed by surgical resection and then adjuvant nivolumab for 6 months. Primary endpoint of the trial was pathologic complete response (pCR) rate, defined as complete eradiation of tumor. Additional secondary endpoints included overall response rate by RECIST 1.1, survival outcomes, and immune correlates. Longitudinal tumor and blood samples were collected at baseline, prior to cycle 2, prior to cycle 3, and at the time of surgery followed by adjuvant blood collection every 3 months at the time of restaging.
