Abstract
Background
Alvimopan is used in abdominal surgery to reduce postoperative ileus in patients undergoing small bowel resections with primary anastomosis. The role and efficacy of alvimopan in patients undergoing radical cystectomy with urinary diversion is not well understood.
Objectives
To assess the effects of alvimopan in the context of enhanced recovery pathways compared to enhanced recovery pathways alone for perioperative bowel dysfunction in patients undergoing radical cystectomy.
Search methods
The terms alvimopan and cystectomy were used to search the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase. We also reviewed abstracts from the past four years (2013 to 2016) of the American Urologic Association, Society of Urologic Oncology, and American Society of Clinical Oncology Genitourinary Cancers.
Selection criteria
We searched for randomized controlled trials that compared alvimopan to placebo.
Data collection and analysis
This study was based on a published protocol. We performed a comprehensive search of multiple databases including CENTRAL in the Cochrane Library, MEDLINE, Embase, LILACS, Web of Science, Scopus and Biosis, which we last updated on 6 February 2017. We also searched abstract proceedings for major relevant meetings (2013 to 2016), databases of the grey literature, trial registries, citations of relevant reviews and contacted clinical experts and the drug manufacturer.
Two independent reviewers screened the literature in two stages (title and abstract, full‐text) using Covidence software. Two independent reviewers assessed the risk of bias on a 'per outcome' basis using the Cochrane 'Risk of bias; tool and rated the quality of evidence according to GRADE. Results of the single eligible trial were reported in a 'Summary of findings' table based on an intention‐to‐treat analysis.
Main results
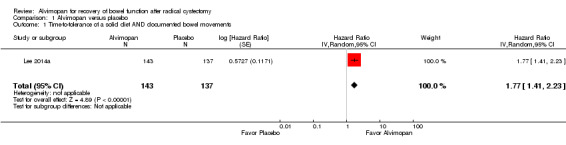
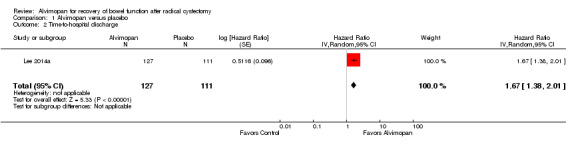
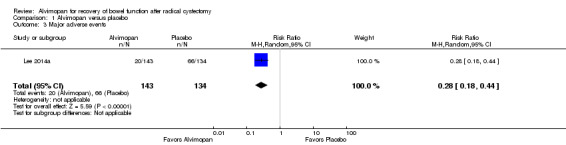
Based on a single trial and moderate‐quality evidence, alvimopan reduced the time to reach a composite endpoint of tolerance of solid food and documented bowel movements (hazard ratio (HR) 1.77, 95% confidence interval (CI) 1.41 to 2.23). This represents 165 more patients (109 more to 207 more) per 1000 meeting this endpoint within 10 days of surgery. Based on moderate‐quality evidence, alvimopan reduced the time to hospital discharge (HR 1.67, 95% CI 1.38 to 2.01). This represents 138 more patients (82 more to 198 more) per 1000 being discharged within 10 days of surgery. Also based on moderate‐quality evidence, alvimopan was associated with a reduced risk of major adverse events (risk ratio (RR) 0.28, 95% CI 0.18 to 0.44) representing 355 fewer patients (404 fewer to 276 fewer) with major adverse events per 1000. We downgraded this outcome for indirectness as it included adverse events that we did not consider major.
In terms of secondary outcomes, alvimopan did not appear to alter the rate of readmission (RR 0.89, 95% CI 0.59 to 1.33), change the rate of any cardiovascular event (RR 0.54, 95% CI 0.27 to 1.05) or alter the mean narcotic pain medication use (mean difference 0, 95% CI 14.08 fewer to 14.08 more morphine equivalents). The quality of evidence was moderate for all three outcomes. Based on high‐quality evidence, alvimopan reduced the rate of nasogastric tube replacement (RR 0.31, 95% CI 0.16 to 0.59). We did not find evidence for the drug's impact on rates of parenteral nutrition. All outcomes were short term and limited to a 30‐day time horizon.
Based on the existence of only one trial, we were unable to perform any subgroup or sensitivity analyses.
Authors' conclusions
In patients undergoing radical cystectomy and urinary diversion, the use of alvimopan administered as part of an enhanced recovery pathway for a limited duration (up to 15 doses for up to seven days) probably reduces the time to tolerance of solid food, time to hospital discharge and rates of major adverse events. Readmission rates, rates of cardiovascular events and narcotic pain requirements are probably similar. The need for reinsertion of nasogastric tubes is reduced. We found no evidence for the impact on rates of parenteral nutrition within 30 postoperative days.
Plain language summary
Alvimopan for recovery of bowel function after radical cystectomy
Review question
In patients who have their bladder removed, does the drug alvimopan compared to placebo help them recover their bowel function more quickly?
Background
Surgical removal of the bladder is a major operation that requires a stay of several days in hospital. One of the issues that keeps patients in hospital is not being able to eat normal food and not having bowel movements, an issue that is referred to as ileus. Alvimopan is a drug that is being used to treat this problem but it is uncertain how well it works and what its side effects are when used in this setting.
Study characteristics
We performed a comprehensive literature search for randomized controlled trials and found one study that addressed our question. This study was a randomized trial of adults undergoing surgery to remove their bladder. They received either 12 mg alvimopan of up to 15 doses over seven days (143 patients) or placebo (137 patients). This study was conducted at centres that did many of these operations (at least 50 per year), had experienced surgeons and also used other measures such as asking patients to get out of bed soon after surgery to hasten bowel recovery.
Key results
We found that patients who receive alvimopan short‐term probably tolerate solid food faster, are discharged from the hospital more quickly and have fewer major adverse events. We did not find any differences with regards to these patients' need to be readmitted to hospital, their risk of heart problems or their need for narcotic pain medications. Patients taking alvimopan were less likely to have a tube placed back into their stomach.
Quality of the evidence
The quality of evidence was rated as at least moderate as per GRADE for all primary outcomes. This means that our estimates of how well alvimopan works is likely close to how well it really works although there is a possibility that it may be different.
Summary of findings
Summary of findings for the main comparison. Alvimopan compared to placebo for recovery of bowel function after radical cystectomy.
| Alvimopan compared to placebo for recovery of bowel function after radical cystectomy | |||||
| Bibliography: Sultan S, Coles B, Dahm P. Alvimopan for recovery of bowel function after radical cystectomy. Cochrane Database of Systematic Reviews [Year], Issue [Issue]. | |||||
| Outcomes | № of participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with Alvimopan | ||||
| Time‐to‐tolerance of a solid diet and documented bowel movements | 277 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.77 (1.41 to 2.23) | Study population | |
| 746 per 1000 | 165 more per 1000 (109 more to 207 more) | ||||
| Time‐to‐hospital discharge | 277 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.67 (1.38 to 2.01) | Study population | |
| 269 per 1000 | 138 more per 1000 (82 more to 198 more) | ||||
| Major adverse events | 277 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | RR 0.28 (0.18 to 0.44) | Study population | |
| 493 per 1000 | 355 fewer per 1000 (404 fewer to 276 fewer) | ||||
| Readmission | 277 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | RR 0.89 (0.59 to 1.33) | Study population | |
| 269 per 1000 | 30 fewer per 1000 (110 fewer to 89 more) | ||||
| Any cardiovascular event | 277 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | RR 0.54 (0.27 to 1.05) | Study population | |
| 157 per 1000 | 72 fewer per 1000 (114 fewer to 8 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Crosses threshold of clinically important difference of 1 day; downgraded for imprecision.
2 Serious adverse event category included postoperative ileus and dehydration, which we did not judge as severe; downgraded for indirectness.
3 Confidence interval consistent with both a clinically important reduction or increase in incidence of outcome; downgraded for imprecision.
Background
Description of the condition
Postoperative ileus (POI) is a common complication that affects patients after surgical removal of the bladder for bladder cancer or benign disease (haemorrhagic cystitis, bilharziosis), and urinary diversion. It is found in both developed and developing countries, with a reported average incidence of approximately 10% (Ramirez 2013). The associated delayed return of bowel function is the leading cause of prolonged hospitalisation and hospital readmission in these patients. It is also associated with an increase in treatment‐related costs (Pruthi 2010).
The term POI refers to the transient cessation of coordinated bowel motility that is common after many types of abdominal surgery. POI may be accentuated if concomitant urinary diversion is performed using the bowel, as is the case in radical cystectomy patients. Patients suffering from POI may suffer from variable clinical symptoms, ranging from minor complaints (i.e. mild distension, burping) to significant discomfort (i.e. painful abdominal distention, abdominal cramps, nausea and vomiting), which commonly prolong hospitalisation (Traut 2008). Morbidity and costs associated with POI have stimulated the development of enhanced recovery protocols, for example through the use of epidural analgesia to reduce the use of systemic opioids (Arumainayagam 2008; Melnyk 2011; Pruthi 2010; Toren 2009). While POI after cystectomy is thought to be multifactorial, one key aetiology appears to be opioid‐induced, which is the target of the drug class of μ‐opioid (or mu‐opioid) antagonists (McNicol 2008).
Description of the intervention
Alvimopan (trade name Entereg®) is an oral medication that works as a μ‐opioid antagonist, and has been used in surgical patients to reduce the incidence and severity of POI. The US Food and Drug Administration (FDA) initially approved its use in patients undergoing small bowel resections with primary anastomosis in 2008 (Erowele 2008). However, it may also have a positive effect on large bowel motility (Kraft 2010).
Alvimopan is administered as a single preoperative dose, and then re‐administered every 12 hours postoperatively for up to seven days (up to a total of 15 doses; FDA 2013b).
Randomized controlled trials in general surgery literature have shown the benefit of decreasing POI and length of stay, as summarized in a Cochrane Review (McNicol 2008). Since its approval in colorectal patients, urologists have become increasingly interested in its potential role in patients with radical cystectomies (Tobis 2014). The urinary diversion portion of a radical cystectomy requires a small bowel resection and primary anastomosis for the formation of an ileal conduit or continent diversion. In 2013, the FDA expanded the indications for use of alvimopan to include any surgery that involves a small bowel resection and primary anastomosis, including radical cystectomy (FDA 2013b; FDA 2013c). However, due to concerns over increased rates of ischaemic cardiovascular events, its current use in the United States is limited to inpatients, and a total of 15 doses (12 mg per dose) per patient (FDA 2013b; FDA 2013c). Other reported side‐effects include gastrointestinal complaints (constipation, flatulence), anaemia, hypokalaemia, back pain and urinary retention (Kraft 2010).
To date, there is no other single pharmaceutical agent that has been shown to decrease POI; instead surgeons rely on a series of measures bundled together in a so‐called 'enhanced recovery pathway' (ERAS pathway) to minimize ileus duration (Raynor 2014). Measures incorporated in these pathways include: omission or early removal of a nasogastric tube with or without concurrent use of metoclopramide; omission of a standard bowel preparation; and chewing gum. In the radical cystectomy setting, a study using matched controls suggested that a protocol focusing on avoiding bowel preparation and nasogastric tube, early feeding, non‐narcotic pain medication and the use of cholinergic and μ‐opioid receptor antagonists expedites bowel function recovery and shortens hospital stay without increasing hospital readmission rates (Daneshmand 2014). A recent editorial found a paucity of direct evidence for ERAS in cystectomy patients (Patel 2014). Based on a systematic review of the literature, no high‐quality evidence derived from randomized controlled trials supports any of these individual interventions in cystectomy patients (Ramirez 2013). Lastly, a recent guideline concluded that ERAS has not been widely implemented in urology yet and also concluded that the evidence for individual interventions remains limited (Cerantola 2013).
How the intervention might work
Mu‐opioid receptors in the gastrointestinal tract are essential for normal motility; however, opioids used postoperatively lead to decreased gastrointestinal motility. This in turn leads to patient discomfort, decreases the ability to tolerate oral nutrition, and may lead to nasogastric tube placement, and occasionally to parenteral nutrition (Bauer 2004).
Perioperative blockade of gastrointestinal μ‐opioid receptors by μ‐opioid receptor antagonists, such as alvimopan, is thought to decrease opioid‐induced bowel dysfunction while still allowing patients to achieve adequate pain control (Taguchi 2001). Alvimopan is a μ‐opioid receptor‐preferring antagonist with a peripherally restricted site of action; given its polar structure, alvimopan has low systemic absorption and a limited ability to enter the brain. Alvimopan is formulated for oral intake to block μ‐opioid receptors in the gut with a prolonged duration of action (FDA 2013a; FDA 2013b; FDA 2013c).
Why it is important to do this review
Radical cystectomy is a morbid procedure with numerous potential complications. POI is seen in approximately 10% of patients (range 2% to 24%) who undergo radical cystectomy (Ramirez 2013). POI contributes significant cost, prolongs hospitalisation and causes patient discomfort. The use of alvimopan may have a positive impact on these outcomes, but may also have important adverse events. To date, there is no high‐quality published systematic review on the use of alvimopan that summarizes the benefits and side‐effects in patients undergoing cystectomy and addresses the quality of evidence by outcome.
Objectives
To assess the effects of alvimopan in the context of enhanced recovery pathways compared to enhanced recovery pathways alone for perioperative bowel dysfunction in patients undergoing radical cystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials and quasi‐randomized controlled trials. We excluded non‐randomized cohort studies and case series. We excluded cluster‐randomized or cross‐over studies. We included studies regardless of their publication status or language of publication.
Types of participants
We included studies of male and female participants undergoing radical cystectomy for primary bladder cancer, including urothelial carcinoma, squamous carcinoma, or adenocarcinoma (or other rare histological types), and participants undergoing cystectomy for benign disease such as haemorrhagic cystitis. We excluded studies of participants who did not undergo a concomitant urinary diversion. We included people who had undergone prior radiation therapy for prostate cancer, but excluded those who had had radiation therapy for bladder cancer. We considered studies irrespective of participant age, clinical stage, or surgical approach (i.e. open, laparoscopic, or robotic‐assisted).
Types of interventions
The experimental intervention studied was the use of the μ‐opioid antagonist, alvimopan. We included studies irrespective of the administered individual dose, cumulative dose, or dose schedule. We considered all studies using concomitant interventions (i.e. use of gastrointestinal prokinetics, chewing gum) as long as they were equally applied to participants in both the experimental and comparator arms.
Eligible comparators were usual care or usual care and placebo. Concomitant interventions (i.e. enhanced recovery pathway) had to be the same in the intervention and comparator groups to establish a fair comparison.
Types of outcome measures
Measurement of particular outcomes were not used to determine eligibility of studies for the review.
Primary outcomes
Time‐to‐tolerance of a solid diet AND documented bowel movements (composite endpoint; time‐to‐event outcome measured from the time of surgery).
Time‐to‐hospital discharge (time‐to‐event outcome measured from the time of surgery).
Major adverse events (dichotomous outcome) of surgical nature (Dindo‐Clavien grades III to V; Dindo 2004); or medical nature (Common Terminology Criteria for Adverse Events grades III to V; CTCAE 2010).
If we were unable to retrieve the necessary information to analyse time‐to‐event outcomes, we planned to assess the number of events per total for dichotomised outcomes at 7, 14, and 21 days after surgery; however, this was not necessary.
Secondary outcomes
Nasogastric tube (re‐)placement within 30 postoperative days (dichotomous outcome).
Initiation of total parenteral nutrition within 30 postoperative days (dichotomous outcome).
Readmission (number of participants readmitted) within 30 postoperative days.
Any cardiovascular event (dichotomous outcome).
Narcotic pain medication use measured in morphine equivalents (continuous outcome).
Main outcomes for 'Summary of findings' table
All three pre‐identified primary outcomes as well as two of the five secondary outcomes — namely readmissions and cardiovascular events, which we deemed most important for participants — were included in the Table 1.
Search methods for identification of studies
We searched for studies without placing restrictions on language of publication or publication status. We re‐ran the search within three months prior to publication of this review.
Electronic searches
We searched the following databases through 24 June 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library (Wiley).
MEDLINE OvidSP (from 1946).
PreMEDLINE.
PubMed (from 1946).
Embase OvidSP (from 1979).
Web of Science databases Science Citation Index Expanded (from 1900) and Conference Proceedings Citation Index‐Science (from 1990) (Thomson Reuters).
LILACS (Latin American and the Caribbean Health Sciences Literature; www.bireme.br/; from 1982).
BioMed Central (from 2000).
BIOSIS (1926 to 14 February 2014) and BIOSIS Citation Index (from 2014).
Scopus (from 1966).
Appendix 1 contains the search strategy for MEDLINE, which we adapted for use in other databases.
We also searched the following trials registers and grey literature sources.
ClinicalTrials.gov (clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en).
Grey Literature Report (www.greylit.org).
We searched abstracts from the past four years (2013 to 2016) of the American Urologic Association (www.auanet.org), Society of Urologic Oncology (suonet.org/meetings/past‐meetings.aspx), the European Association of Urology (www.uroweb.org/urosource) and American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium and ASCO annual meeting (both: meetinglibrary.asco.org/).
Searching other resources
We handsearched reference lists of the included studies and any identified relevant reviews, and checked for errata related to included studies. We contacted the manufacturer of alvimopan (initially Cubist Pharmaceuticals LLC; now Merck and Co, Inc.) and experts in the field, for the results of unpublished or ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (SS, PD) independently screened the titles and abstracts of records retrieved from each search to determine eligibility through Covidence (www.covidence.org), which also provided a method for the detection of duplicates.
We obtained full‐length reports of records of potentially eligible studies. Ultimate study eligibility was determined by the two review authors independently using Covidence. We recorded disagreements, which we resolved by discussion and consensus.
We mapped all included records (e.g. abstracts or publications providing extended follow‐up or secondary analyses) to unique studies in Covidence.
We summarized the results of our comprehensive search in a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (SS, PD) independently extracted all study data onto a standardised data abstraction form in Covidence, which we pilot tested in advance. We recorded disagreements, which we resolved by discussion and consensus. We did not need to engage a third review author as tie‐breaker.
We extracted the following study characteristics.
Study design.
Study dates.
Study setting.
Study inclusion and exclusion criteria.
Participant baseline characteristics (age, gender, clinical stage, performance status, use of bowel preparation, receipt of neoadjuvant chemotherapy).
Number of participants randomized to study and to each intervention group.
Characteristics of the interventions (dose, dose schedule, duration of administration, route).
Characteristics of co‐interventions (e.g. chewing gum, gastrointestinal prokinetics, use of epidural analgesia).
Perioperative factors (i.e. duration of surgery, open versus laparoscopic versus robotic‐assisted approach).
Definitions of outcomes relevant to this review, and how and when they were measured.
Relevant subgroups (as defined below) analysed for each relevant outcome.
Study funding sources.
Declarations of interest by the study investigators.
We also extracted outcomes data relevant to this review. For dichotomous outcomes, we attempted to obtain numbers of events and totals to populate a two‐by‐two table, as well as summary statistics with corresponding measures of variance. For time‐to‐event outcomes, we obtained hazard ratios (HRs) with corresponding measures of variance or data necessary to calculate this information. For continuous outcomes, we obtained means and standard deviations (or sought to retrieve data necessary to calculate this information).
For missing data, we attempted to contact one or more of the study authors to obtain key missing information.
Assessment of risk of bias in included studies
Two review authors (SS, PD) assessed the risk of bias of each included study independently. We resolved disagreements by consensus, or by consultation with a third review author.
We assessed the risk of bias using the up‐to‐date 'Risk of bias' tool, provided in the online version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Risk of bias was determined as low, high, or unclear for the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
With regards to the risk for detection bias, we grouped outcomes as 'subjective' or 'objective'. We considered all outcomes to be 'objective' and therefore at low risk for detection bias. This categorization did not affect the determination of risk for performance bias. We also assessed the risk of bias in the incomplete outcome data domain on an outcome‐specific basis, and grouped outcomes with like judgements when reporting our findings in the 'Risk of bias' tables.
We further summarized the risk of bias for each outcome (across domains) for each study.
Measures of treatment effect
For the pre‐identified time‐to‐event outcomes (time‐to‐tolerance of a solid diet and documented bowel movements; time‐to‐hospital discharge), we reported effect sizes as HRs with corresponding 95% confidence intervals (CIs). For dichotomous outcomes (major adverse events; cardiovascular events; nasogastric tube (re‐)placement; initiation of total parenteral nutrition; readmission), we reported effect sizes as risk ratios (RRs) with corresponding 95% CIs. We expressed continuous data (narcotic pain medication use measured in morphine equivalents) as mean differences (MDs) with corresponding 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. We did not encounter trials with multiple intervention groups (Higgins 2011b).
Dealing with missing data
We attempted to conduct all efficacy analyses on an intention‐to‐treat basis. In the case of missing data, we planned to contact the study authors to potentially obtain additional data but this was not necessary. Had we been unable to conduct the analysis as intention‐to‐treat, we would have reported an available case analysis and identified that as such. We did not use statistical imputation methods.
We conducted the analyses of adverse events on an 'as‐treated' basis.
Assessment of heterogeneity
We planned to calculate the I² statistic to assess heterogeneity. According to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), we would interpret the I² value as follows.
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² would have depended on magnitude and direction of effects, as well as strength of evidence for heterogeneity (e.g. P value from a Chi² test) (Deeks 2011). In this review, we only identified one eligible trial; therefore we did not assess heterogeneity.
Assessment of reporting biases
We planned to obtain study protocols to assess for outcome reporting bias. Since we only identified a single eligible study, we were unable to formally assess for publication bias.
Data synthesis
We planned to perform statistical meta‐analysis of all available studies, using a random‐effects model but only identified a single study and therefore did not perform meta‐analysis. We used Review Manager 5 (RevMan 5) software to report the data for the single trial (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following five clinical subgroup analyses to explore potential heterogeneity (I² ≥ 30%) given that observational data and expert opinion by reviewers of this protocol suggest they may impact ileus duration, and cause interaction.
Use of epidural analgesia (epidural analgesia used versus no epidural analgesia used).
Type of urinary diversion using small bowel (e.g. ileal conduit) versus those that include large bowel (e.g. colon pouch).
Surgical approach (open versus robotic‐assisted or laparoscopic).
Cancer indication (radical cystectomy) versus non‐cancer indication ('simple' cystectomy).
Receipt of neoadjuvant chemotherapy versus no chemotherapy.
In addition, we sought to explore potential heterogeneity (I² ≥ 30%) by comparing studies at low versus unclear or high risk of bias. We would have used the test for subgroup differences in Review Manager 5 to compare subgroup analyses if there had been sufficient studies (Review Manager 2014). However, given that we only found one trial, no subgroup analyses were conducted.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess the impact of:
excluding studies at high risk of bias;
excluding studies at high or unclear risk of bias.
Since we only found a single eligible trial, we did not conduct a sensitivity analysis.
'Summary of findings' table
We present the quality of the evidence for each outcome according to the GRADE approach (Guyatt 2008). We judged the quality of the evidence for each outcome by considering the following five dimensions that affect our confidence in the estimates of effect.
Study limitations.
Inconsistency.
Imprecision.
Indirectness.
Publication bias.
For each comparison, two review authors (SS, PD) independently rated the quality of the evidence for each outcome as high, moderate, low, or very low according to GRADE (Guyatt 2008). We resolved discrepancies by consensus; arbitration by a third review author was not required. We used the online Guideline Development Tool to generate a 'Summary of findings' table for the single comparison (GRADEpro GDT; Guyatt 2011).
Results
Description of studies
Results of the search
A literature search identified 150 references, which included 57 duplicates. We ultimately screened 93 references at the title and abstract phase of which five studies were assessed for full‐text eligibility (Figure 1).
1.
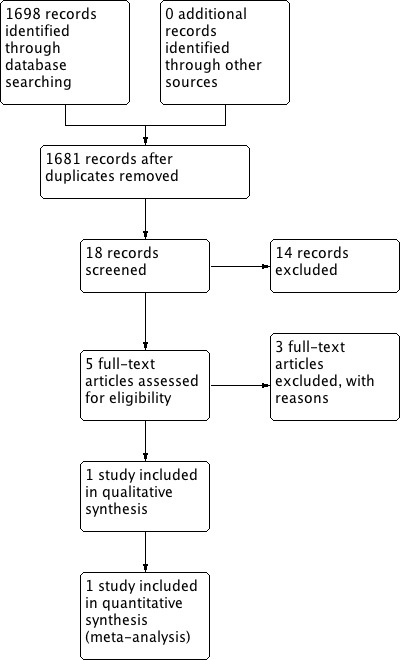
Study flow diagram.
Included studies
One study met inclusion criteria for this Cochrane Review (Lee 2014a). This study was a randomized multicentre placebo‐controlled trial of adults undergoing radical cystectomy at 21 high‐volume (greater than 50 cases per year) clinical venues across the United States.
The trial was sponsored by Cubist Pharmaceuticals LLC and prospectively registered on 27 June 2008 (NCT00708201). The study randomized participants to alvimopan 12 mg with a maximum of 15 inpatient doses (n = 143) versus placebo (n = 137). The primary endpoint of the study was a composite endpoint of time to upper (first tolerance of solid food) and lower (first bowel movement) gastrointestinal recovery. Secondary outcomes were time to discharge order written; actual postoperative length of stay; hospital readmission; nasogastric tube reinsertion; readmission; incidence of nausea, abdominal bloating and antiemetic use; and total postoperative pain medication use measured in morphine equivalents. All reported outcome data were limited to 30 days.
Excluded studies
We initially identified 126 references which included 53 duplicates which were removed. We screened the 73 remaining studies, excluding 70 based on the title and abstract alone. Three studies were excluded in full‐text review stage: Delaney 2005 for the wrong patient population (not cystectomy patients); Vora 2012 for the wrong study design (not RCT); and Kauf 2014 as being a secondary economic analysis that was outside the scope of this review.
Risk of bias in included studies
The risk of bias is summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The patients were randomized using a sponsor‐generated, random‐allocation sequence stratified by study site and presence or absence of cardiovascular disease. The risk of bias was rated as low.
Allocation concealment
There is no explicit description of allocation concealment; the risk of bias was rated as unclear.
Blinding
Blinding of participants and personnel
The study was described as double‐blind placebo‐controlled and made explicit mention of the use of an identical placebo as well as masking of participants and personnel; the risk of bias for performance bias was rated as low for all outcomes.
Blinding of outcome assessment
Outcome assessors for all outcomes were masked; this included adjudicators of the adverse events. We judged the risk of bias for detection bias as low.
Incomplete outcome data
For the two primary efficacy outcomes of this review (time‐to‐tolerance of a solid diet and documented bowel movements; and time to discharge from the hospital), all except three participants who did not undergo surgery were included in the analysis; the risk of bias was judged as low for both outcomes.
For the primary harm‐related outcome, all except three participants who did not undergo surgery were included in the analysis; the risk of bias was judged as low.
Similarly, the risk of bias for all five secondary outcomes was rated as low.
Selective reporting
No selective reporting bias was identified with reported outcomes being consistent with the information provided at the time of trial registration.
Other potential sources of bias
We did not identify other potential sources of bias.
Effects of interventions
See: Table 1
Alvimopan versus placebo
Primary Outcomes
1. Time to tolerance of solid food and documented bowel movements
Based on a single trial providing moderate‐quality evidence, alvimopan reduced the time to reach a composite endpoint of tolerance of solid food and documented bowel movements (HR 1.77, 95% CI 1.41 to 2.23). This corresponded to a reduction of the mean time to reaching this outcome by 1.3 (95% CI 1.9 to 0.7) days. In absolute terms, this corresponded to 165 more patients per 1000 (95% CI 109 to 207 more) meeting this endpoint by the time of discharge (or within 10 days of surgery). We downgraded the quality of evidence for imprecision assuming that one day represented a clinically meaningful threshold for this composite outcome (Table 1).
2. Time to hospital discharge
Based on moderate‐quality evidence, alvimopan reduced the time to hospital discharge (HR 1.67, 95% CI 1.38 to 2.01). This corresponded to a reduction of the mean time to reaching this outcome by 0.9 (95% CI 0.4 to 1.5) days. In absolute terms, this corresponded to 138 more patients per 1000 (95% CI 82 to 198) meeting this endpoint within 10 days of surgery. We downgraded the quality of evidence for imprecision assuming that one day represented a clinically meaningful threshold for hospital stay (Table 1).
3. Major adverse events
Based on moderate‐quality evidence, alvimopan was associated with a reduced risk of major adverse events (RR 0.28, 95% CI 0.18 to 0.44) within 30 days of surgery. In absolute terms, this corresponded to 355 fewer patients per 1000 (404 to 276 fewer). We downgraded the quality of evidence for indirectness as Lee 2014a included postoperative ileus and dehydration in this outcome. We were unable to analyze the data separately (Table 1).
Secondary Outcomes
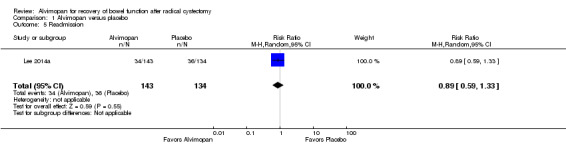
1. Readmission
Based on moderate‐quality evidence, alvimopan did not change the rate of readmission (RR 0.89, 95% CI 0.59 to 1.33) within 30 days of surgery. In absolute terms, this corresponded to 30 fewer patients per 1000 (110 fewer to 89 more). We downgraded for imprecision as the confidence interval was consistent with both a clinically important reduction or increase in readmission rates (Table 1).
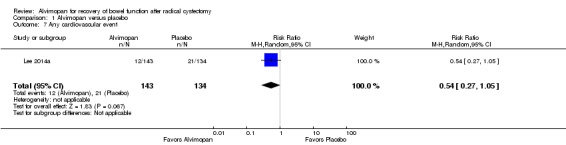
2. Any cardiovascular events
Based on moderate‐quality evidence, alvimopan did not change the rate of any cardiovascular event (RR 0.54, 95% CI 0.27 to 1.05) for the 30‐day postoperative time period. In absolute terms, this corresponded to 72 fewer patients per 1000 (114 fewer to 8 more). We downgraded for imprecision as the confidence interval was consistent with both a clinically important reduction or increase in cardiovascular event rates (Table 1).
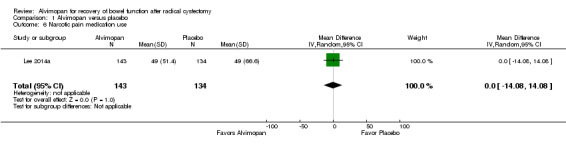
3. Narcotic pain medication use
Based on moderate‐quality evidence, alvimopan did not change the mean narcotic pain medication use for the postoperative hospital stay (mean difference 0, 95% CI 14.08 fewer to 14.08 more morphine equivalents). We downgraded for imprecision as the confidence interval was consistent with both a clinically important reduction or increase in pain medication use (Table 1).
4. Nasogastric tube (re‐)placement within 30 postoperative days
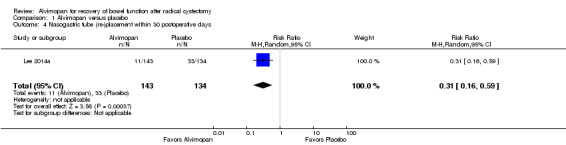
Based on high‐quality evidence, alvimopan reduced the rate of nasogastric tube replacement (RR 0.31, 95% CI 0.16 to 0.59). In absolute terms, this corresponded to 170 fewer patients per 1000 (207 fewer to 101 fewer). We saw no reasons to downgrade the quality of evidence.
5. Initiation of total parenteral nutrition within 30 postoperative days
We did not find any evidence for this outcome.
Discussion
Summary of main results
The findings of this systematic review indicate that 1) time to tolerance of solid food and documented bowel movements, and 2) time to hospital discharge were clinically significantly reduced (all moderate‐quality evidence). At the same time, 3) major adverse events were reduced.
In terms of secondary outcomes, alvimopan also reduced the rates for replacement of an NG tube (high‐quality evidence). Rates of readmission, cardiovascular events and the amount of narcotic pain medication used appeared similar in the groups that received or did not receive alvimopan (all moderate‐quality evidence).
Overall completeness and applicability of evidence
The results of this systematic review are based on a single, albeit methodologically rigorous, randomized controlled trial conducted primarily at high volume, tertiary care centres all located in the United States (Lee 2014a). These centres typically have well‐established clinical care pathways that are designed to optimize patient recovery and in particular speed up bowel recovery and reduce prolonged hospital stays due to postoperative ileus. It is in this context — as an adjunct to other measures to improve bowel recovery — that the results of this study should be interpreted.
The vast majority of patients in this trial (approximately 85%) underwent radical cystectomy using an open surgical approach (Lee 2014a). At least in the United States, there is a trend towards performing radical cystectomy using a robotic‐assisted laparoscopic approach (Monn 2014), although the relative merits of this approach are not well defined (Tan 2016). There is some uncertainty to what extent the results of this trial may generalize to robotic‐assisted cystectomy. We were also unable to explore the impact of other potentially important baseline characteristics such as use of epidural analgesia, type of urinary diversion, indication (cancer versus non‐cancer) and use of neoadjuvant chemotherapy.
We limited this review to evidence derived from randomized controlled trials as being the study design most likely to yield high‐quality evidence, which also follows the standard Cochrane approach for questions of therapeutic effectiveness. Given that all predefined outcomes were rated at least as moderate according to GRADE, it appears unlikely that the inclusion of observational studies would have provided evidence in which we would have placed similar confidence. Had we found only low‐ or moderate‐quality evidence an expanded inclusion of comparative observational studies might have added meaningful additional evidence (Schünemann 2013); however, we did not encounter that situation. One exception may have been long‐term treatment‐related harms such as increased cardiovascular event rates in patients receiving alvimopan, for which longer‐term observational studies might provide additional evidence. However, a scoping search would suggest that no such long‐term observational studies exist.
The reported evidence of this review relates to the short‐term use of alvimopan in a clearly defined postoperative setting with a maximum of 15 doses, which was the basis for FDA approval under a Risk Evaluation and Mitigation Strategy (REMS) called the Entereg Access Support and Education (E.A.S.E.) Program (Kraft 2010; FDA 2013a). The specific concern was that of a "potentially greater incidence of myocardial infarction in alvimopan‐treated patients compared to placebo‐treated patients ... although a causal relationship has not been established"; this was based on a 12‐month (unpublished) study of patients treated with opioids for chronic non‐cancer pain (alvimopan 0.5 mg, n = 538; placebo, n = 267). No further details about the event rates and relative risks were available. The E.A.S.E. ENTEREG REMS Program mandates hospitals to assure that hospital staff using the drug are aware of its specific and narrow indications and dosage limitations.
Quality of the evidence
The quality of evidence for all primary and secondary outcomes (except for initiation of total parenteral nutrition within 30 days, for which we found no evidence) was rated as moderate according to GRADE. We downgraded for study limitations (time to tolerance of a solid diet and documented bowel movements).
Potential biases in the review process
We conducted this systematic review in accordance with Cochrane's current standards. Nevertheless, the following potential limitations deserve mention.
Despite a rigorous search for published and unpublished studies, we cannot be entirely certain that we found all trials that could have informed these focused clinical questions.
We contacted the study authors and original manufacturer of alvimopan on several occasions, and they provided feedback to our queries. However, we were unable to obtain the results of an unadjusted analysis for the time‐to‐tolerance of a solid diet and documented bowel movements. We therefore used available adjusted analyses but downgraded for study limitations.
Agreements and disagreements with other studies or reviews
We identified one published systematic review (Cui 2016). In contrast to this Cochrane Review, Cui 2016 also included four observational studies that were described as being of a case‐control design (Manger 2014; Tobis 2014; Vora 2012; Vora 2014). The authors assessed the risk of bias of the RCT using the Jadad scale, and the observational studies using the Newcastle–Ottawa scale. The review did not consider other domains that may impact our confidence in the estimates of effect as defined by GRADE; and chose to pool data for select outcomes such as time to discharge across study designs. For the latter outcome, the pooled HR was 1.17 (95% CI 1.10 to 1.25; I² = 0%). The study by Lee 2014a contributed 46% of the study weight. Not unexpectedly, the most rigorously conducted study — Lee 2014a — showed a more modest effect size than three of the four observational studies (HR range 1.13 to 1.28). This systematic review did not include treatment‐related adverse events among its outcomes.
A Cochrane Review from 2008 addressed the role of systemic‐acting prokinetic drugs to treat postoperative adynamic ileus in patients undergoing abdominal surgery (Traut 2008). This review identified 39 RCTs, including six trials of alvimopan. In their conclusions, the authors state that "alvimopan may prove to be beneficial" but also highlight methodological shortcomings of the supporting body of evidence. This review has not been updated.
Meanwhile, a number of recent systematic reviews taking a broader perspective of the use of μ‐opioid antagonists in the perioperative setting of abdominal surgery exist (Drake 2016; Xu 2016; Nguyen 2015). Drake 2016 included a total of 17 studies across all types of gastrointestinal surgery. They found alvimopan shortened the duration of ileus, but also emphasized the limited methodological and reporting quality. Focused on open abdominal surgery, Xu 2016 included nine randomized controlled trials with 4075 participants who were enrolled in this study. The pooled effect estimates suggested enhanced bowel recovery, shortened length of stay and reduced length of stay as well as less serious adverse events. They did not provide a quality of evidence rating, though. Focused on laparoscopic gastrointestinal surgery, Nguyen 2015 included five trials. They found a clinically meaningful shortening of postoperative ileus duration, but only a marginal reduction in the length of hospitalization. Rates of readmission appeared similar. The quality of evidence was not explicitly rated.
Authors' conclusions
Implications for practice.
In patients undergoing radical cystectomy and urinary diversion, the use of alvimopan administered as part of an enhanced recovery pathway for a limited duration (up to 15 doses for up to seven days) probably reduces the time to tolerance of solid food, time to hospital discharge and rates of major adverse events.
Readmission rates, rates of cardiovascular events and narcotic pain requirements are probably similar. The need for reinsertion of nasogastric tubes is probably reduced. We found no evidence for the impact on rates of parenteral nutrition. All outcome data was limited to 30 days postoperatively.
Implications for research.
Despite the existence of only a single trial, we are moderately confident in the effect size estimates reported, which likely lie close to the true effect. These findings are specific to short‐term, time and dose‐limited use of this agent in the perioperative setting that has since become the basis of alvimopan prescribing in the United States as mandated by the FDA. Additional longer‐term follow‐up data from this trial would be helpful to provide further assurance of safety. The additional regulatory burden placed upon prescribers of this drug was based on long‐term users of the drug for chronic pain who appeared to have experienced higher rates of myocardial infarction, although a definitive causal implications has been implied. Future cohort studies specifically designed for the assessment of this potential treatment‐related harm, possibly drawing upon existing data from the E.A.S.E. ENTEREG REMS Program may help clarify this issue further.
Notes
We have based parts of the Methods section of the protocol on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We thank the Cochrane Urology Group editorial staff as well as the peer reviewers for their support.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
1 alvimopan.mp.
2 entereg.mp.
3 alvimopanum.mp.
4 (677C126AET or 170098‐38‐1 or 156053‐89‐3 or 11227‐0010‐31 or 11227‐0010‐30 or 67919‐020‐10).rn.
5 adl 8‐2698.mp.
6 or/1‐5
7 exp urologic surgical procedures/
8 exp Cystectomy/
9 cystectom*.tw.
10 (bladder* adj3 (surg* or resect* or remov*)).tw.
11 or/7‐10
12 6 and 11
Appendix 2. Embase (Ovid) search strategy
1 exp alvimopan/
2 entereg.mp.
3 alvimopanum.mp.
4 (677C126AET or 170098‐38‐1 or 156053‐89‐3 or 11227‐0010‐31 or 11227‐0010‐30 or 67919‐020‐10).rn.
5 entereg.tn.
6 adl 8‐2698.af.
7 or/1‐6
8 exp urologic surgery/
9 exp cystectomy/
10 cystectom*.tw.
11 (bladder* adj3 (surg* or resect* or remov*)).tw.
12 or/8‐11
13 7 and 12
14 7 and 12
Appendix 3. PubMed search strategy
| #6 | Add | Search (#1 and #5) |
| #5 | Add | Search (#2 or #3 or #4) |
| #4 | Add | Search cystect* |
| #3 | Add | Search cystectomy[MeSH Terms] |
| #2 | Add | Search urologic surgical procedures[MeSH Terms] |
| #1 | Add | Search (alvimopan OR entereg) |
Appendix 4. Cochrane Library search strategy
#1 alvimopan:ti,ab,kw or entereg:ti,ab,kw (Word variations have been searched) 33
#2 MeSH descriptor: [Urologic Surgical Procedures] explode all trees 6511
#3 MeSH descriptor: [Cystectomy] explode all trees 166
#4 cystectom*:ti,ab,kw 405
#5 bladder*:ti,ab,kw N/3 (surg* or resect* or remov*):ti,ab,kw 18
#6 #2 or #3 or #4 or #5 6748
#7 #1 and #6 0
Appendix 5. LILACS search strategy
(tw:(alvimopan or entereg)) AND (tw:(surg* or cystectom*)) AND (tw:(bladder*))
Appendix 6. Web of Science search strategy
| #6 | #5 AND #1 |
| #5 | #3 OR #2 |
| #4 | TS=(677C126AET or 170098‐38‐1 or 156053‐89‐3 or 11227‐0010‐31 or 11227‐0010‐30 or 67919‐020‐10) |
| #3 | TS=(bladder* NEAR/3 (surg* or resect* or remov*)) |
| #2 | TS=(cystectom*) |
| #1 | TS=(alvimopan or entereg) |
Appendix 7. BIOSIS search strategy
| # 6 | #5 AND #1 |
| # 5 | #4 OR #3 |
| # 4 | TS=(bladder* NEAR/3 (surg* or resect* or remov*)) |
| # 3 | TS=(cystectom*) |
| # 2 | TS=(677C126AET or 170098‐38‐1 or 156053‐89‐3 or 11227‐0010‐31 or 11227‐0010‐30 or 67919‐020‐10) |
| # 1 | TS=(alvimopan or entereg) |
Appendix 8. Scopus search straegy
( TITLE‐ABS‐KEY ( ( alvimopan OR entereg ) ) ) AND ( ( TITLE‐ABS‐KEY ( cystect* ) ) OR ( TITLE‐ABS‐KEY ( bladder AND surg* ) ) )
Appendix 9. BioMed Central search strategy
((alvimopan or entereg)[TIAB] AND (cystectom* or surg* or bladder*)[TIAB])
Appendix 10. ASCO abstracts search strategy
Advanced search
Text/abstract/title
1. alvimopan
2. Entereg
results scanned for in scope studies.
Appendix 11. ICTRP search portal strategy
In advanced search
alvimopan in title or intervention
entereg in title or intervention
results scanned for in scope studies.
Appendix 12. Clinicaltrials.gov search strategy
Clinicaltrials.gov
In basic search
alvimopan and (bladder or cystectomy)
entereg and (bladder or cystectomy)
results scanned for in scope studies.
Data and analyses
Comparison 1. Alvimopan versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time‐to‐tolerance of a solid diet AND documented bowel movements | 1 | 280 | Hazard Ratio (Random, 95% CI) | 1.77 [1.41, 2.23] |
| 2 Time‐to‐hospital discharge | 1 | 238 | Hazard Ratio (Random, 95% CI) | 1.67 [1.38, 2.01] |
| 3 Major adverse events | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.18, 0.44] |
| 4 Nasogastric tube (re‐)placement within 30 postoperative days | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.59] |
| 5 Readmission | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.33] |
| 6 Narcotic pain medication use | 1 | 277 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐14.08, 14.08] |
| 7 Any cardiovascular event | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.05] |
1.1. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 1 Time‐to‐tolerance of a solid diet AND documented bowel movements.
1.2. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 2 Time‐to‐hospital discharge.
1.3. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 3 Major adverse events.
1.4. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 4 Nasogastric tube (re‐)placement within 30 postoperative days.
1.5. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 5 Readmission.
1.6. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 6 Narcotic pain medication use.
1.7. Analysis.
Comparison 1 Alvimopan versus placebo, Outcome 7 Any cardiovascular event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lee 2014a.
| Methods | Randomized, parallel group trial | |
| Participants | Adults undergoing radical cystectomy (n = 280) Inclusion criteria: ‐ At least 18 years of age. ‐ American Society of Anesthesiologists (ASA) Physical Status Score of I ‐ III. ‐ Scheduled for radical cystectomy. ‐ Scheduled to receive postoperative pain management with intravenous patient‐controlled opoid analgesia. ‐ Scheduled to have the nasogastric tube removed by the morning of the the first postoperative day (POD). ‐ Able to understand the study procedures, agreed to participate in the study program and voluntarily provided informed consent. Exclusion criteria: ‐ Scheduled for a partial cystectomy. ‐ Previous total colectomy, gastrectomy, or gastric bypass, or functional colostomy or ileostomy. ‐ Ongoing history of short bowel syndrome, chronic constipation (less than three spontaneous bowel movements per week), or chronic diarrhea. ‐ More than three doses of opioids (oral or parenteral) within 7 days before the day of surgery. ‐ Radiation therapy to the abdomen or pelvis within 3 months of scheduled surgery. ‐ Chemotherapy for bladder cancer within 1 month of scheduled surgery; prior neoadjuvant chemotherapy was allowed. ‐ Chemotherapy‐ or radiation‐induced bowel dysfunction (e.g. radiation‐induced colitis). ‐ Pregnant (identified by a positive serum pregnancy test) or lactating, or not postmenopausal (no menses for at least 1 year) and of childbearing potential and not using an accepted method of birth control (i.e. surgical sterilization; intrauterine contraceptive device; oral contraceptive, diaphragm, or condom in combination with contraceptive cream, jelly, or foam; or abstinence). ‐ Participated in another investigational drug or medical device study within 30 days of surgery or planning to be enrolled in another investigational drug or medical device study or any study in which active patient participation was required outside normal hospital data collection during the course of this study ‐ Clinically significant laboratory abnormalities at screening that would have resulted in the cancellation of surgery ‐ Using illicit drugs or abusing alcohol. ‐ History of previous surgeries, illness, or behavior (e.g. depression, psychosis) that in the opinion of the investigator might have confounded the study results or might have posed additional risk in administering the study procedures. |
|
| Interventions | Intervention arm: 12 mg alvimopan plus standardized postoperative care pathway (n = 143). Control arm: Matching placebo plus standardized postoperative care pathway (n = 137). The standardized postoperative care pathway included: Ambulation encouraged on POD1, a liquid was offered on POD3 and solid food on POD4. Epidural anesthesia or analgesia was not permitted and the routine use of opioid‐sparing techniques (ketorolac or cyclooygnease‐2 inhibitors) was restricted to a 2 dose maximum. |
|
| Outcomes | Primay endpoint: ‐ Time to GI‐2 recovery (composite endpoint of the later of upper (first toleration of solid food) and lower (first bowel movement) GI function). Secondary endpoints: ‐ Time from end of surgery (last suture or staple) to time to discharge order written, censored after 10‐day observation period. ‐ Postoperative length of stay in calendar days, not censored at 10 days. ‐ Hospital readmissions ‐ Postoperative ileus related morbidity (postoperative NGT insertion, prolonged stay due to postoperative ileus or readmission ≤ 7 days due to postoperative ileus). ‐ Incidences of nausea, vomiting, abdominal bloating and antiemetic use. |
|
| Funding sources | Cubist Pharmaceuticals held, designed and conducted the study, managed, analyzed, and interpreted the data and reviewed the study. It also provided financial support for medical editorial assistance. | |
| Declarations of interest | Cheryl T. Lee receives grants to institution and travel support from Cubist Pharmaceuticals. Ashish M. Kamat receives consultancy remuneration from Archimedes; grants to institution, and travel support from Cubist Pharmaceuticals; grants/grants pending to institution from FKD; and other remuneration from Photocure, Endo Pharmaceutical, Sanofi, Taris, Cubist Pharmaceuticals, and Allergan. Gilad Amiel receives travel support and provision of writing assistance, medicines, equipment, or administrative support from Cubist Pharmaceuticals. Timothy L. Beard receives speakers’ bureau remuneration from Cubist Pharmaceuticals. Venu Menon receives remuneration for CEC adjudication of blinded events to Cleveland Clinic. Wade J. Sexton receives consultancy remuneration from Endo Pharmaceuticals and Archimedes, payment for lectures including service on speakers’ bureaus from Endo Pharmaceuticals, and payment for development of educational presentations from Oakstone Medical Publishing. Joel W. Slaton receives travel support to institution from Cubist Pharmaceuticals. Robert S. Svatek receives consulting fees or honorariums and travel support from Adolor. Shandra S. Wilson receives remuneration to institution for employment and expert testimony not related to current submission one or two times per year. Lee Techner receives employment and stock/stock options from Cubist Pharmaceuticals. Gary D. Steinberg receives consultancy remuneration from Endo Pharmaceuticals, Photocure, TARIS Biomedical, Predictive Biosciences, Abbott Molecular, and DuPont, and payment for lectures including service on speakers’ bureaus from Endo Pharmaceuticals and Photocure. Michael Koch receives travel support (expenses only, no honorarium) from Adolor. The remaining authors have nothing to disclose. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "On day 0 (surgery), patients were randomized 1:1 (sponsor‐generated random allocation sequence) to receive single‐ dose oral alvimopan (Entereg, Cubist Pharmaceuticals, Inc.) 12 mg or matching placebo between 30 min and 5 h before surgery start and twice‐daily oral doses postoperatively until hospital discharge or a maximum of 7 d (15 in‐hospital doses). Patient randomization was stratified by site and the presence or absence of CV disease to minimize assignment bias." |
| Allocation concealment | Unclear risk | Judgement comment: Allocation concealment not explicitly described. |
| Blinding of participants and personnel All outcomes | Low risk | Quote: "matching placebo" Judgement comment: Study described as double‐blinded, placebo‐controlled. |
| Blinding of outcome assessors All outcomes | Low risk | Judgement comment: Personnel and AE's adjudicators blinded. |
| Blinding of outcome assessors Subjective outcomes (time to GI recovery, Major AE, Any CV event) | Low risk | Judgement comment: Study personnel and AE adjudicators described as blinded. |
| Blinding of outcome assessors Objective outcomes (objective (time to discharge, NG replacement, TPN initiation, readmission, pain meds) | Low risk | Judgement comment: Study personnel and AE adjudicators described as blinded. |
| Incomplete outcome data All outcomes | Low risk | Judgement comment: All patients included in efficacy (except 3 patients who did not undergo surgery) and safety analysis (all). |
| Selective outcome reporting | Low risk | Judgement comment: Not detected. |
| Other sources of bias | Low risk | Judgement comment: Not detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Delaney 2005 | Wrong patient population; not performed in cystectomy patients. |
| Kauf 2014 | Secondary economic analysis. |
| Vora 2012 | Not an RCT. |
Differences between protocol and review
This review is based on a published protocol (Sultan 2016). We did not deviate from the protocol.
Contributions of authors
Bernadette Coles performed the systematic literature search.
Shahnaz Sultan and Philipp Dahm independently screened the studies for eligibility and perform data abstraction.
Shahnaz Sultan and Philipp Dahm independently assessed the risk of bias and rate the quality of evidence (GRADE).
Shahnaz Sultan and Philipp Dahm wrote the discussion and conclusion.
Sources of support
Internal sources
Minneapolis VA Health Care System, USA.
University of Minnesota Department of Urology, USA.
External sources
No sources of support supplied
Declarations of interest
Shahnaz Sultan: none known.
Bernadette Coles: none known.
Philipp Dahm: none known.
New
References
References to studies included in this review
Lee 2014a {published data only}
- Kamat AM, Chang SS, Lee C, Amiel G, Beard T, Fergany A, et al. Alvimopan, a peripherally acting mu‐opioid receptor antagonist, accelerates gastrointestinal recovery and decreases length of hospital stay after radical cystectomy. Journal of Urology 2013;189(4 suppl. 1):e767. [DOI: 10.1016/j.juro.2013.02.2289] [DOI] [Google Scholar]
- Kauf TL, Svatek RS, Amiel G, Beard TL, Chang SS, Fergany A, et al. Alvimopan, a peripherally acting mu‐opioid receptor antagonist, is associated with reduced costs after radical cystectomy: Economic analysis of a phase 4 randomized, controlled trial. Journal of Urology 2014;191(6):1721‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Lee CT, Chang SS, Kamat AM, Amiel G, Beard TL, Fergany A, et al. Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo‐controlled trial. European Urology 2014;66(2):265‐72. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Delaney 2005 {published data only}
- Delaney CP, Weese JL, Hyman NH, Bauer J, Techner L, Gabriel, K, et al. Phase III trial of alvimopan, a novel, peripherally acting, Mu opioid antagonist, for postoperative ileus after major abdominal surgery. Diseases of the Colon and Rectum 2005;48(6):1114‐29. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kauf 2014 {published data only}
- Kauf TL, Svatek RS, Amiel G, Beard TL, Chang SS, Fergany A, et al. Alvimopan, a peripherally acting μ‐opioid receptor antagonist, is associated with reduced costs after radical cystectomy: economic analysis of a phase 4 randomized, controlled trial. Journal of Urology 2014;191(6):1721‐7. [DOI] [PubMed] [Google Scholar]
Vora 2012 {published data only}
- Vora AA, Harbin A, Rayson R, Christiansen K, Ghasemian R, Hwang J, et al. Alvimopan provides rapid gastrointestinal recovery without nasogastric tube decompression after radical cystectomy and urinary diversion. Canadian Journal of Urology 2012;19(3):6293‐8. [PubMed] [Google Scholar]
Additional references
Arumainayagam 2008
- Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU International 2008;101(6):698–701. [PUBMED: 18190646] [DOI] [PubMed] [Google Scholar]
Bauer 2004
- Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterology and Motility 2004;16(Suppl 2):54‐60. [DOI] [PubMed] [Google Scholar]
Cerantola 2013
- Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations. Clinical Nutrition (Edinburgh, Scotland) 2013; Vol. 32, issue 6:879‐87. [DOI] [PubMed]
CTCAE 2010
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 28 May 2009 (v4.03: 14 June 2010) accessed 25 February 2016. [http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf]
Cui 2016
- Cui Y, Chen H, Qi L, Zu X, Li Y. Effect of alvimopan on accelerates gastrointestinal recovery after radical cystectomy: A systematic review and meta‐analysis. International Journal of Surgery (London, England) 2016;25:1‐6. [DOI] [PubMed] [Google Scholar]
Daneshmand 2014
- Daneshmand S, Ahmadi H, Schuckman AK, Mitra AP, Cai J, Miranda G, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. Journal of Urology 2014;192(1):50‐5. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drake 2016
- Drake TM, Ward AE. Pharmacological management to prevent ileus in major abdominal surgery: a systematic review and meta‐analysis. Journal of Gastrointestinal Surgery 2016;20(6):1253‐64. [DOI] [PubMed] [Google Scholar]
Erowele 2008
- Erowele GI. Alvimopan (Entereg), a peripherally acting mu‐opioid receptor antagonist for postoperative ileus. P&T 2008;33(10):574‐83. [PMC free article] [PubMed] [Google Scholar]
FDA 2013a
- FDA. Alvimopan prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2013/021775s010lbl.pdf (accessed prior to 25 April 2017).
FDA 2013b
- NDA 21‐775 Entereg (alvimopan): risk evaluation and mitigation strategy (REMS). www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm129511.pdf (accessed 2 December 2014).
FDA 2013c
- U.S. Food, Drug Administration. NDA 021775/S‐010 supplement approval: fulfillment of postmarketing requirement. www.accessdata.fda.gov/drugsatfda_docs/appletter/2013/021775Orig1s010ltr.pdf (accessed 2 December 2014).
GRADEpro GDT [Computer program]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kraft 2010
- Kraft M, MacLaren R, Du W, Owens G. Alvimopan (Entereg) for the management of postoperative ileus in patients undergoing bowel resection. P&T 2010;35(1):44‐9. [PMC free article] [PubMed] [Google Scholar]
Manger 2014
- Manger JP, Nelson M, Blanchard S, Helo S, Conaway M, Krupski TL. Alvimopan: A cost‐effective tool to decrease cystectomy length of stay. Central European Journal of Urology 2014;67(4):335‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2008
- McNicol ED, Boyce D, Schumann R, Carr DB. Mu‐opioid antagonists for opioid‐induced bowel dysfunction. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006332.pub2] [DOI] [PubMed] [Google Scholar]
Melnyk 2011
- Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice?. Journal de l'Association des Urologues du Canada [Canadian Urological Association Journal] 2011;5(5):342‐8. [PUBMED: 22031616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monn 2014
- Monn MF, Cary KC, Kaimakliotis HZ, Flack CK, Koch MO. National trends in the utilization of robotic‐assisted radical cystectomy: an analysis using the Nationwide Inpatient Sample. Urologic Oncology 2014;32(6):785‐90. [DOI] [PubMed] [Google Scholar]
Nguyen 2015
- Nguyen DL, Maithel S, Nguyen ET, Bechtold ML. Does alvimopan enhance return of bowel function in laparoscopic gastrointestinal surgery? A meta‐analysis. Annals of Gastroenterology 2015;28(4):475‐80. [PMC free article] [PubMed] [Google Scholar]
Patel 2014
- Patel HR, Cerantola Y, Valerio M, et al. Enhanced recovery after surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy?. European Urology 2014;65(2):263‐6. [DOI] [PubMed] [Google Scholar]
Pruthi 2010
- Pruthi RS, Nielsen M, Smith A, Nix J, Schultz H, Wallen EM. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. Journal of the American College of Surgeons 2010;210(1):93–9. [PUBMED: 20123338] [DOI] [PubMed] [Google Scholar]
Ramirez 2013
- Ramirez JA, McIntosh AG, Strehlow R, Lawrence VA, Parekh DJ, Svatek RS. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: a systematic review. European Urology 2013;64(4):588‐97. [DOI] [PubMed] [Google Scholar]
Raynor 2014
- Raynor MC, Pruthi RS. Postoperative ileus after radical cystectomy: looking for answers to an age‐old problem. European Urology 2014;66(2):273‐4. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non‐randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49‐62. [DOI] [PubMed] [Google Scholar]
Sultan 2016
- Sultan S, Modh R, Coles B, Dahm P. Alvimopan for recovery of bowel function after radical cystectomy. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD012111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taguchi 2001
- Taguchi A, Sharma N, Saleem RM, Sessler DI, Carpenter RL, Seyedsadr M, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. New England Journal of Medicine 2001;345(13):935‐40. [DOI] [PubMed] [Google Scholar]
Tan 2016
- Tan WS, Khetrapal P, Tan WP, Rodney S, Chau M, Kelly JD. Robotic Assisted Radical Cystectomy with Extracorporeal Urinary Diversion Does Not Show a Benefit over Open Radical Cystectomy: A Systematic Review and Meta‐Analysis of Randomised Controlled Trials. PLoS One 2016;11(11):e0166221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobis 2014
- Tobis S, Heinlen JE, Ruel N, Lau C, Kawachi M, Wilson T, et al. Effect of alvimopan on return of bowel function after robot‐assisted radical cystectomy. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part a 2014;24(10):693‐7. [DOI] [PubMed] [Google Scholar]
Toren 2009
- Toren P, Ladak S, Ma C, McCluskey S, Fleshner N. Comparison of epidural and intravenous patient controlled analgesia in patients undergoing radical cystectomy. Canadian Journal of Urology 2009;16(4):4716‐20. [PUBMED: 19671221] [PubMed] [Google Scholar]
Traut 2008
- Traut U, Brügger L, Kunz R, Pauli‐Magnus C, Haug K, Bucher H, et al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004930.pub3] [DOI] [PubMed] [Google Scholar]
Vora 2014
- Vora A, Marchalik D, Nissim H, Kowalczyk K, Bandi G, McGeagh K, et al. Multi‐institutional outcomes and cost effectiveness of using alvimopan to lower gastrointestinal morbidity after cystectomy and urinary diversion. Canadian Journal of Urology 2014;21(2):7222‐7. [PubMed] [Google Scholar]
Xu 2016
- Xu LL, Zhou XQ, Yi PS, Zhang M, Li J, Xu MQ. Alvimopan combined with enhanced recovery strategy for managing postoperative ileus after open abdominal surgery: a systematic review and meta‐analysis. Journal of Surgical Research 2016;203(1):211‐21. [DOI] [PubMed] [Google Scholar]


