Abstract
Sec61p is the channel-forming subunit of the heterotrimeric Sec61 complex that mediates co-translational protein import into the endoplasmic reticulum (ER). In yeast, proteins can also be post-translationally translocated by the hetero-heptameric Sec complex, composed of the Sec61 and the Sec63 complexes. The Sec61 channel is also a candidate for the dislocation channel for misfolded proteins from the ER to the cytosol during ER-associated degradation (ERAD). The structure of the Sec61 complex is highly conserved, but the roles of its N-terminal acetylation and its amphipathic N-terminal helix are unknown so far. To gain insight into the function of the Sec61p N-terminus, we mutated its N-acetylation site, deleted its amphipathic helix, or both the helix and the N-acetylation site. Mutation of the N-acetylation site on its own had no effect on protein import into the ER in intact cells, but resulted in an ERAD defect. Yeast expressing sec61 without the N-terminal amphipathic helix displayed severe growth defects and had profound defects in post-translational protein import into the ER. Nevertheless the formation of the hetero-heptameric Sec complex was not affected. Instead, the lack of the N-terminal amphipathic helix compromised the integrity of the heterotrimeric Sec61 complex. We conclude that the N-terminal helix of Sec61p is required for post-translational protein import into the ER and Sec61 complex stability, whereas N-terminal acetylation of Sec61p plays a role in ERAD.
Introduction
Secretory proteins and organelle proteins of the secretory pathway are translocated into the endoplasmic reticulum (ER) during biogenesis [1]. In the ER lumen, imported proteins have to acquire a functional conformation before their delivery to specific cellular destinations via the secretory pathway [2]. Proteins that fail to fold in the ER are retrotranslocated to the cytosol in order to be degraded by proteasomes, a process known as ER-associated degradation (ERAD) [2, 3]. Transport of newly synthesized proteins across the ER membrane can occur either co- or post-translationally [4]. Both modes of translocation require the heterotrimeric Sec61 channel, which consists of three proteins, Sec61p, Sbh1p, and Sss1p in yeast (Sec61α, β, γ in mammals) [5]. The Sec61 complex is sufficient to mediate co-translational import on its own, while it associates with the heterotetrameric Sec63 complex (Sec62p, Sec63p, Sec71p, Sec72p) for post-translational protein import into the yeast ER [5]. Post-translational import generally occurs for soluble proteins that carry only mildly hydrophobic signal sequences, whereas membrane proteins use the signal recognition particle (SRP)-mediated cotranslational pathway [6]. The Sec61 complex is also a candidate channel for the dislocation of ERAD substrates to the cytosol [2, 3, 7, 8].
Sec61p is the channel-forming subunit of the Sec61 complex [9, 10]. The protein is characterized by a compact bundle of 10 transmembrane helices spanning the ER membrane with both termini in the cytoplasm [9, 10]. The two symmetrical halves of Sec61p form an aqueous pore in the ER membrane and a lateral gate facing the lipid bilayer [11]. Sbh1p and Sss1p are tail-anchored membrane proteins with single transmembrane spans [9]. Two evolutionarily conserved large loops of Sec61p, L6 and L8, protruding from the cytoplasmic side of the ER membrane are involved in ribosome binding during co-translational import into the ER [12]. The cytosolic C-terminus of Sec61p has also been shown to contact the ribosome and is functionally important [13, 14]. The cytosolic face of the Sec61 channel also interacts with proteasomes in an ATP-dependent manner [15]. Proteasomes bind the Sec61 channel via the AAA-ATPases of the 19S regulatory particle and in vitro compete with ribosomes for ER membrane binding [16]. The AAA-ATPase Cdc48p, involved in the delivery of both misfolded ERAD substrates and partially translocated proteins to the proteasome, can also bind to the Sec61 channel [2, 17]. The specific cytosolic domains of the Sec61 channel responsible for the interaction with AAA-ATPases, however, still remain to be determined [16, 18]. The Sec61 complex also interacts with other transmembrane protein complexes via its small subunits: the mammalian orthologue of Sbh1p, Sec61β, mediates interaction with the signal peptidase complex, and its yeast homologue, Sbh2p, binds to the SRP receptor [19, 20]. Sbh1p and Sss1p also make contact with the oligosaccharyl transferase complex [21, 22].
Despite the fact that Sec61p structure and function have been extensively characterized, the role of its N-terminus is still unknown. The N-terminal region of Sec61p is likely to be functionally important, given that a 6-histidine tag at the Sec61p N-terminus in combination with point mutations elsewhere in the protein interferes with import, but the phenotype is less severe in the absence of the tag [23]. The N-terminus of Sec61p is oriented towards the cytosol and residues 3–21 have the potential to form an amphipathic α-helix [24]. Together with the Sbh1p N-terminus, the Sec61p N-terminus is exposed at one side of the transmembrane helix bundle forming the transmembrane channel and thus poised to make contact with other proteins [14]. In addition, the starting methionine of Sec61p is cleaved and the serine at position 2 acetylated by the NatA complex (S1 Fig) [25]. N-terminal acetylation occurs co-translationally, and in yeast about 50% of proteins are N-terminally acetylated, but the significance of this modification is only beginning to be understood [26]. Roles for N-terminal acetylation include protein stability, interaction ability, and subcellular targeting [27].
To gain insight into the function of the Sec61p N-terminus, we investigated the effects of altering the sequence context or position of the N-acetylation site by changing the serine at position 2 to tyrosine (sec61S2Y) or deleting N-terminal residues 4–22 forming the amphipathic helix (sec61ΔH1). In addition, we generated a mutant lacking both the N-terminal acetylation site and the N-terminal residues 4–22 (sec61ΔN21). We investigated the effects of these mutations on co- and post-translational import into the ER and ERAD. Furthermore, we asked whether the deletion of the N-terminal helix affects heptameric Sec complex formation and integrity of Sec61 complexes.
Materials and methods
Yeast strains and construction of N-terminal sec61 mutants
The sec61-32 point mutant [28] had been previously cloned into the yeast plasmid pRS315 [29]. The sec61S2Y mutant was obtained by site-directed mutagenesis of a pRS315 plasmid carrying the SEC61 gene using the QuikChange kit (Agilent); the second codon of the SEC61 gene was mutated from TCC to TAC, resulting in a serine to tyrosine amino acid substitution. sec61ΔH1 and sec61ΔN21 were obtained by PCR-mediated DNA deletion of a pRS315 plasmid carrying the SEC61 gene [30]; deletions of the N-terminal residues 4–22 and 2–22 of Sec61p, respectively, were confirmed by DNA sequencing. Plasmids were individually transformed into the KRY461 strain (SEC61::HIS3 ade2-1 leu2-3, 112 trp1-1 prc1-1 his3-11, 15 ura3-1 [pGALSEC61-URA3]), selected on minimal medium without leucine at 30ºC, and subsequently selected on minimal medium without leucine at 30ºC in the presence of 1 g/l 5-fluoroorotic acid (Sigma).
As the first two amino acids determine N-acetylation specificity both wildtype Sec61p and SecΔH1p are substrates for NatA which acetylates S2 in both proteins after initiator methionine cleavage [26]. In Sec61S2Yp the N-terminus begins with MY which is a substrate for N-acetylation on M1 by NatE [26]. Sec61ΔN21 begins with MP and proteins with proline in first or second position have never been found N-acetylated [26].
Growth conditions
S. cerevisiae cells were grown at 30°C in YPD with continuous shaking at 200 rpm or on YPD plates at 30°C. The parental strain KRY461 was grown on YPGalactose. To test temperature sensitivity, 10-fold serial dilutions were prepared and 5 μl of each dilution containing 104−10 cells were dropped onto YPD or YPGal plates and incubated for 6 (20ºC), 3.5 (sec61ΔH1, sec61ΔN21 and corresponding wildtype; 24ºC, 30ºC, 37ºC), or 3 days (sec61S2Y, sec61-32 and corresponding wildtype; 30ºC, 37ºC). To test tunicamycin (Tm) sensitivity, serial dilutions were prepared and 5 μl of each dilution containing 104−10 cells were dropped onto YPD or YPGal (±0.25 μg/ml Tm, ±0.5 μg/ml Tm) plates. Plates were incubated at the indicated temperatures for 3.5 (sec61ΔH1, sec61ΔN21 and corresponding wildtype) or 3 days (sec61S2Y, sec61-32 and corresponding wildtype).
β-galactosidase assay
Wildtype, sec61ΔH1, sec61ΔN21 and sec61S2Y were transformed with the plasmids pJC31, a pRS314 plasmid carrying the UPRE-LacZ reporter construct, and the control plasmid pJC30, a pRS314 plasmid carrying the LacZ gene [31]. Cells were grown in synthetic complete medium without tryptophan to an OD600 of approximately 0.5 and aliquots of 1 ml were harvested by centrifugation and resuspended in 1 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 0.27% β-mercaptoethanol). Subsequently, 100 μl of chloroform and 50 μl of 0.1% SDS were added to each sample; after 10 s of vortexing, samples were pre-incubated for 5 min at 28°C in a water bath, and reactions were induced with 200 μl of 4 mg/ml 2-nitrophenyl-β-D-galactopyranoside Z buffer. After 20 min of incubation at 28°C, reactions were stopped by adding 500 μl of 1 M Na2CO3, samples centrifuged, and supernatants analyzed photometrically at 420 nm to calculate β-galactosidase units.
Western blot analysis
Proteins were resolved by gel electrophoresis using NuPAGE Novex 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific). Proteins were transferred to nitrocellulose and bands detected by immunoblotting with specific rabbit polyclonal antisera. Antibodies against Sec61p, Sbh1p, CPY [29], Rpn12 [16], and preproalpha factor (ppαF) [29] had been previously raised in our laboratory and were used at a dilution of 1:2000; antibodies against Sss1p and Sec63p were kindly provided by Randy Schekman and used at a dilution of 1:2500. Signals were developed by enhanced chemiluminescence using the SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific) according to the manufacturer´s instructions.
Cycloheximide chase
Cells expressing chromosomal CPY* [32] were grown overnight in YPD to an OD600 of 1 and treated with 200 μg/ml of cycloheximide (t = 0). An equal amount of cells (1 OD600) was taken at the indicated time points, washed with sterile water, resuspended in 50 μl of 2x SDS-buffer and lysed with glass beads in a Mini-Beadbeater-16 (BioSpec Products, Bartlesville, OK, US; two 1 min disruption cycles at 4ºC with 1 min of incubation at 4ºC between cycles). Lysates were heated for 10 min at 65ºC and analyzed by Western blotting as described above.
Pulse-labeling and immunoprecipitation
Cells were grown overnight in YPD to an OD600 of 0.5–1 and washed 2 times with labeling medium (5% glucose or galactose, 0.67% yeast nitrogen base without amino acids and ammonium sulfate) supplemented with auxotrophy-complementing amino acids and concentrated to 6 OD/ml. Each 250 μl cell suspension was preincubated at 30ºC under shaking at 600 rpm for 15 min, and subsequently labeled for 5 min adding 55 μCi of EXPRE35S35S Protein Labeling Mix (PerkinElmer). Labeling was stopped by adding 750 μl of ice-cold Tris-azide (20 mM Tris, pH 7.5, 20 mM sodium azide); cells were subsequently washed with 1 ml of Tris-azide and incubated for 10 min at room temperature in resuspension buffer (100 mM Tris, pH 9.4, 10 mM DTT, 20 mM sodium azide). After centrifugation, cells were lysed with glassbeads in 150 μl of lysis buffer (20 mM Tris, pH 7,5, 2% SDS, 1 mM DTT, 1 mM PMSF) and the lysate denatured for 10 min at 65ºC. Glassbeads were washed and the collected supernatant was used for immunoprecipitation with 60 μl of a 20% suspension of Protein A Sepharose beads CL-4B (GE Healthcare) and 10 μl of polyclonal rabbit antisera against CPY or DPAPB in IP buffer (15 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM sodium azide) for 2 h at 4ºC. DPAPB antibodies were kindly provided by Tom Stevens. Precipitates were washed in IP buffer, urea wash (100 mM Tris, pH 7.5, 2 M urea, 200 mM NaCl, 1% Triton X-100, 2 mM sodium azide), Con A wash (20 mM Tris, pH 7.5, 500 mM NaCl, 1% Triton X-100, 2 mM sodium azide), Tris-NaCl wash (10 mM Tris, pH 7.5, 50 mM NaCl, 2mM sodium azide), heated for 10 min in 2x SDS-buffer and resolved by gel electrophoresis. Signals were detected by autoradiography using a Typhoon Trio imager (GE Healthcare).
In vitro post-translational translocation assay
Microsomes were prepared as described in Pilon et al. (1997) [28]. ppαF was translated and 35S-methionine-labeled in vitro using the Rabbit Reticulocyte Lysate System (Promega) according to the manufacturer’s instructions (2 μg RNA per 50 μl reaction) and translocated into wildtype and mutant microsomes at 20ºC for the indicated times. Individual reactions were set up as follows: 3 μl of B88 (20 mM HEPES-KOH, pH 6.8, 250 mM sorbitol, 150 mM KOAc, 5 mM Mg(OAc)2), 1 μl of 10x ATP mix (10 mM ATP, 400 mM creatine phosphate, 2 mg/ml creatine kinase in B88), 5 μl of translation reaction product, 0.6 eq of microsomes (1 μl). To investigate translocation in the presence of limiting amounts of membranes adjusting for equal amounts of Sec61p, 0.3 eq of wildtype and 0.85 eq of sec61S2Y microsomes were used for the experiment. After incubation, samples were resolved by gel electrophoresis and signals acquired by autoradiography. Quantitation was performed using the ImageQuant TL software (GE Healthcare).
Fractionation of Sec complex
Fractionation of Sec complex was performed as described in Pilon et al. (1998) [23]. Briefly, microsomes (50 eq) were resuspended on ice in 100 μl of solubilization buffer (50 mM HEPES-KOH, pH 7.4, 400 mM KOAc, 5 mM MgAc, 10% glycerol, 0.05% β-mercaptoethanol) containing the cOmplete Mini EDTA-free Protease Inhibitor (PI) Cocktail (Roche) and 0.1 mM PMSF; subsequently, 400 μl of solubilization buffer containing 3.75% digitonin were added, resulting in a final 3% digitonin concentration, and samples were incubated on ice for 30 min and subsequently centrifuged at 110,000 g in a TLA100.3 (Beckman Instruments, Palo Alto, CA, US) rotor for 30 min at 4°C. The Sec63 complex was precipitated from the resulting supernatant at 4°C for 1 h with 100 μl of Concanavalin A (Con A) Sepharose 4B (GE Healthcare). Remaining beads were cleared from the supernatant by centrifugation to obtain the free fraction. Con A beads were washed twice with equilibration buffer (1% digitonin, 50 mM HEPES-KOH pH 7.4, 10% glycerol, 0.05% β-mercaptoethanol, 0.1 mM PMSF and PIs) and glycoproteins were subsequently released in 2x SDS-buffer for 10 min at 65ºC. To obtain the ribosome-associated membrane protein (RAMP) fraction, the pellet from the 110,000 g spin was dissolved in 100 μl of 50 mM HEPES-KOH (pH 7.8), 1 M KAc, 17.5 mM MgAc, 2.5% digitonin, 1 mM puromycin, 0.2 mM GTP, 5 mM dithiothreitol, 0.1 mM PMSF containing PIs. After 30 min on ice and 30 min at 30°C, RAMPs including the Sec61 complex were recovered from the supernatant after centrifugation at 100,000 g for 30 min at 4°C. Equal amounts of each fraction were analyzed by gel electrophoresis on NuPAGE Novex 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific) and immunoblotting with the indicated antibodies.
Sucrose gradient centrifugation of Sec61 complex
Sucrose gradients were prepared in 13 x 51 mm polycarbonate centrifuge tubes (Beckman) using 1 ml of 15%, 10%, 5% and 0% sucrose in 50 mM HEPES-KOH, pH 7.5, 500 mM potassium acetate, 1 mM EDTA, 0.1% Triton X-100, 0.05% β-mercaptoethanol, 1 mM PMSF containing PIs. Microsomes (50 eq) were centrifuged and the pellet was resuspended in 100 μl of solubilization buffer (50 mM HEPES-KOH, pH 7.5, 500 mM KOAc, 1% Triton X-100, 10 mM EDTA, 0.05% β-mercaptoethanol, 1 mM PMSF and 5x PIs) and incubated for 15 min on ice. Solubilized microsomes were loaded onto 0–15% sucrose gradients and ultracentrifuged (200,000 g, 4°C, 16 h) in a TLA 100.3 rotor. After centrifugation, 13 fractions were collected from top to bottom of the gradients, precipitated with TCA, and analyzed by gel electrophoresis and immunoblotting with the indicated antibodies.
Results
Viability and ER stress induction are affected in sec61 N-terminal mutants
To investigate the function of the Sec61p N-terminus, we used sec61 mutants carrying a deletion of the N-terminal helix but preserving the N-terminal acetylation site in its original sequence context, sec61ΔH1, lacking both the N-terminal acetylation site and the N-terminal helix, sec61ΔN21, or carrying a mutation in the N-acetylation acceptor site, sec61S2Y (Fig 1A). The serine to tyrosine substitution in position 2 is predicted to result in a non-cleavable and acetylated initiator methionine, and thus in a substantially more bulky N-terminus than in the wildtype protein (S1 Fig) [33, 34]. Mutants were introduced into a strain bearing a chromosomal deletion of SEC61 by plasmid shuffling [29]. All sec61 mutant proteins were stable, and expressed at wildtype level (sec61ΔN21) or approximately 40% of wildtype (sec61S2Y and sec61ΔH1; see below and S2 Fig) which is not limiting for ER import (S2 Fig). We first tested the effects of these mutations on cell growth at various temperatures and in the presence or absence of tunicamycin, which interferes with N-linked glycosylation in the ER and hence protein folding [35]. Translocation-defective sec61 mutants frequently accumulate misfolded proteins in the ER and are therefore tunicamycin-sensitive [29]. The sec61S2Y mutant, together with the ERAD-defective cold-sensitive sec61-32 strain as a control, was grown at 20°C, 30°C, and 37°C without tunicamycin, and at 30°C in the presence of 0.25 or 0.5 μg/ml tunicamycin (Fig 1B). The sec61S2Y mutant did not grow at 37°C (Fig 1B, top panel), suggesting a possible role for the acetylation of S2 in Sec61p function. In addition, growth of the strain was affected in the presence of the higher concentration of tunicamycin, indicating perturbations in ER homeostasis. Growth of the sec61ΔH1 and sec61ΔN21 mutants was analyzed at 20°C, 30°C, and 37°C, both in the presence and absence of 0.25 μg/ml tunicamycin (Fig 1C). The strains exhibited severe growth defects under all conditions tested and no growth at all at 20°C in the presence of tunicamycin (Fig 1C). These results indicate that the N-terminal domain of Sec61p is highly important for function.
Fig 1. Viability and growth of sec61 N-terminal mutants under stress conditions.
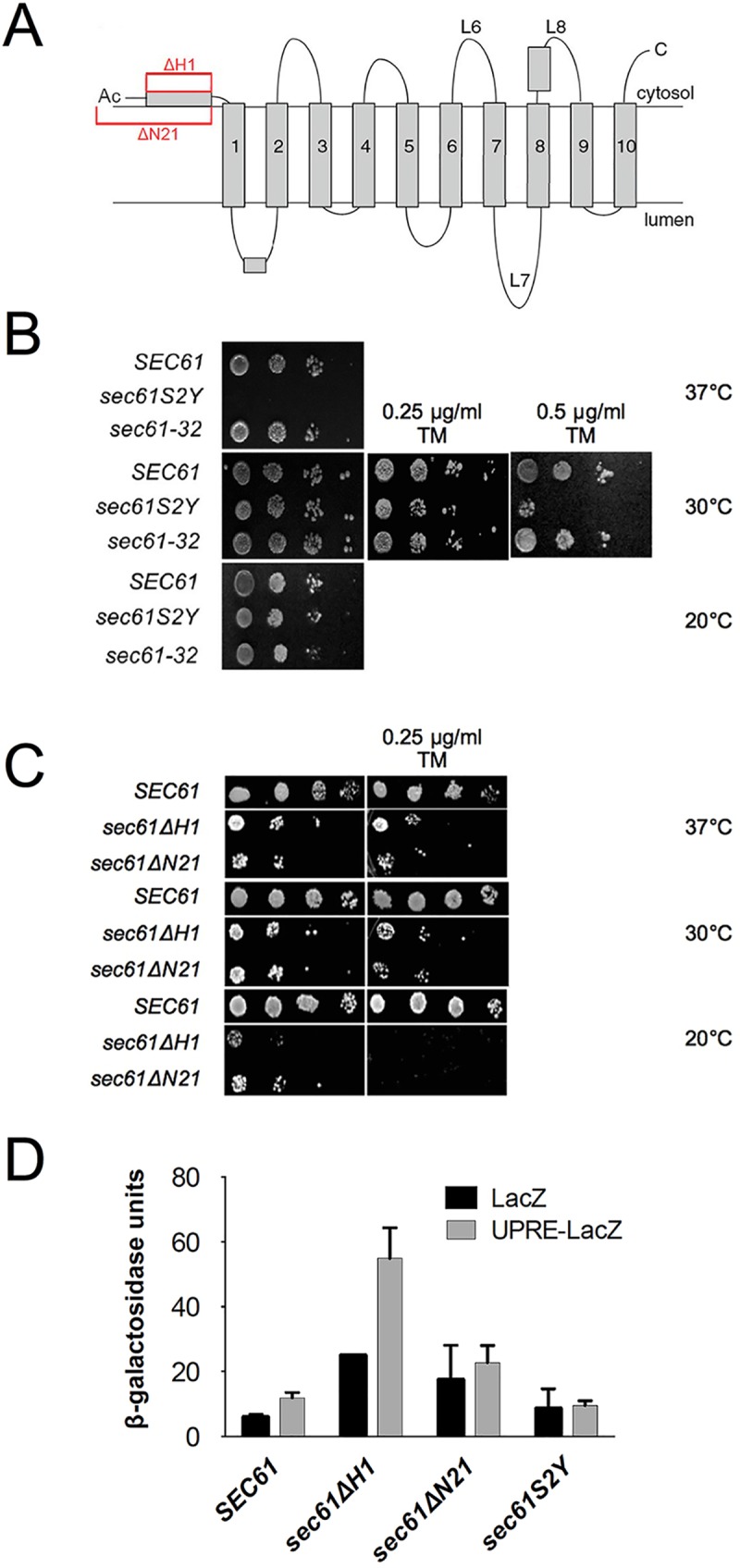
A: Topology of Sec61p. N-terminal mutations characterized in this paper are highlighted in red. B: Temperature- and tunicamycin-sensitivity of sec61S2Y. Cells (104−10) expressing sec61S2Y, sec61-32, or wildtype SEC61 from the wildtype promoter were grown at 20°C (6 d), 30°C (without or with 0.25 μg/ml and 0.5 μg/ml tunicamycin) and 37°C (3 d) on YPD plates. C: Temperature- and tunicamycin-sensitivity of sec61ΔH1 and sec61ΔN21. Cells (104−10) expressing sec61ΔH1, sec61ΔN21 from the wildtype promoter, or the parental strain (KRY461, expressing wildtype SEC61 from the GAL1 promoter) were grown for 3.5 days on YPD or YPGal plates, respectively, without or with 0.25 μg/ml tunicamycin at the indicated temperatures. D: Liquid β-galactosidase assay with sec61 N-terminal mutants. The wildtype, sec61ΔH1, sec61ΔN21, and sec61S2Y strains were transformed with the plasmids pJC31 (UPRE-LacZ) and the control plasmid pJC30 (LacZ). Cells were harvested in early exponential phase, lysed, and β-galactosidase activity was analyzed photometrically in duplicate samples. The experiment was performed 2 times with duplicate samples. Error bars represent standard deviation.
To directly investigate UPR induction of the sec61ΔH1, sec61ΔN21 and sec61S2Y strains, we transformed each of the mutants and the corresponding wildtype with plasmids carrying the β-galactosidase gene under the control of a promoter without or with a UPR element [31]. Transformants were grown at 30°C, lysed, and β-galactosidase activity monitored using a chromogenic substrate [31]. The UPR was strongly induced in the sec61ΔH1 strain, but although the β-galactosidase activity was higher in sec61ΔN21 than in wildtype cells, this was independent of the UPRE (Fig 1D). Despite its temperature- and tunicamycin-sensitivity, in the sec61S2Y mutant the UPR was not induced (Fig 1D). Collectively, our data suggest that repositioning of the N-terminal acetylation site in sec61ΔH1 is more detrimental to ER protein homeostasis than the absence of the N-acetylation site in the sec61ΔN21 mutant.
N-terminal acetylation at S2 of Sec61p plays a role in ERAD
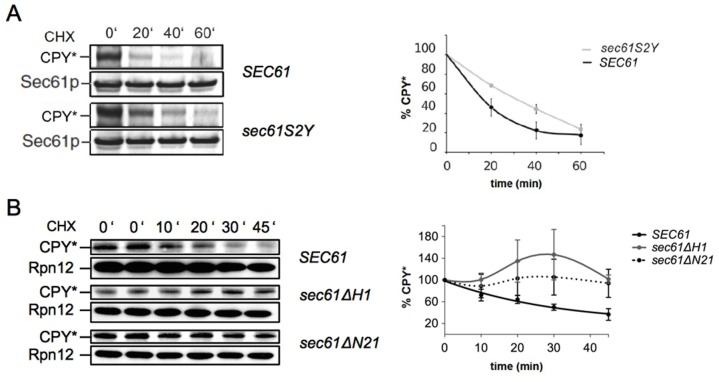
Tunicamycin-sensitivity is often associated with defects in export of misfolded proteins from the ER to the cytosol [36]. We therefore conducted cycloheximide chase experiments to monitor the decline of the steady state levels of the commonly used soluble ERAD substrate CPY* in the sec61S2Y, sec61ΔH1, and sec61ΔN21 strains [32]. We found that the half-life of CPY* increased approximately 2-fold in sec61S2Y compared to the wildtype strain (Fig 2A). In the helix deletion mutants sec61ΔN21 and especially sec61ΔH1 CPY* accumulated in the ER during the chase, suggesting that post-translational import of CPY* into the ER was still taking place after protein biosynthesis had been inhibited with cycloheximide (Fig 2B). Whether or not ERAD was also affected in these mutants remained unclear, even when we extended the chase to 90 min. We conclude that N-acetylation of Sec61p at S2 is required for ERAD of misfolded soluble proteins.
Fig 2. Sec61p N-acetylation at S2 is important for ERAD.
ERAD of CPY* was investigated in sec61S2Y (A), sec61ΔH1, and sec61ΔN21 (B). Cells were grown at 30°C to an OD600 = 1, translation was inhibited by adding 200 μg/ml cycloheximide, extracts were prepared by bead-beating, and samples resolved by SDS-PAGE. The 0' samples were taken in duplicate in (B). Proteins were transferred to nitrocellulose and CPY* was detected with polyclonal rabbit antiserum and enhanced chemiluminescence. Bands were quantified using the ImageQant TL software. Sec61 (A) and Rpn12 (B) were used as loading controls. Band intensities were quantified relative to loading controls. Curves represent the averages of three independent experiments. Error bars represent standard deviations.
Deletion of the N-terminal amphipathic helix of Sec61p severely impairs post-translational protein import into the ER
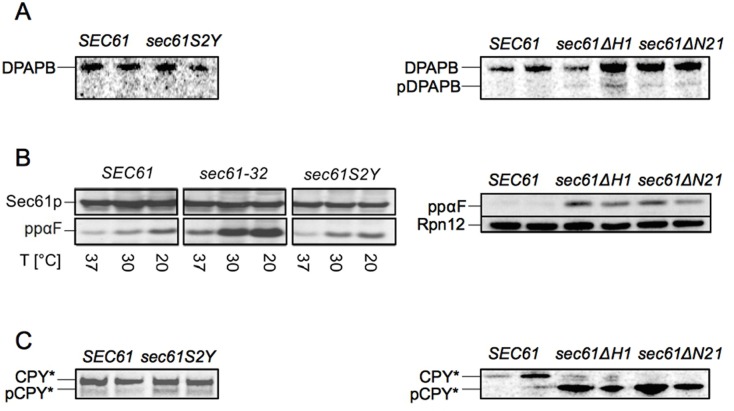
As the results shown in Fig 2B suggest an ER import defect or an ERAD defect for sec61ΔH1 and sec61ΔN21, we decided to investigate import directly. We first monitored co-translational membrane integration of diaminopeptidase B (DPAPB) in the sec61S2Y, sec61ΔH1, and sec61ΔN21 strains by pulse-labeling with 35S-Met/Cys for 5 min, lysing the cells, and immunoprecipitation with specific polyclonal antibodies. We barely detected the cytosolic precursor form of DPAPB (pDPAPB) in sec61ΔH1 and sec61ΔN21 (Fig 3A, right panel), and mutation of the N-terminal acetylation site had no effect at all on pDPAPB import (Fig 3A, left panel). These data show that none of the investigated N-terminal sec61 mutations affects co-translational translocation into the ER.
Fig 3. The Sec61p N-terminal helix is important for post-translational protein import into the ER in vivo.
A: Co-translational ER import of newly synthesized DPAPB in sec61S2Y (left panel), sec61ΔH1, and sec61ΔN21 (right panel). Cells were grown at 30°C to early exponential phase, labeled with 35S-Met/Cys for 5 min and lysed. Cytosolic precursor (pDPAPB) and ER-membrane integrated DPAPB were immunoprecipitated, resolved by SDS-PAGE, and detected by autoradiography. B: Post-translational ER import of ppαF in sec61S2Y (left panel), sec61ΔH1, and sec61ΔN21 (right panel). Cells were grown at 30°C to an OD600 = 1 and shifted to the indicated temperatures for 3h, extracts were prepared by bead-beating, and samples resolved by SDS-PAGE. Proteins were transferred to nitrocellulose and the accumulation of cytosolic ppαF was analyzed by immunoblotting. C: Post-translational ER import of newly synthesized pCPY* in sec61S2Y (left panel), sec61ΔH1, and sec61ΔN21 strains (right panel). Cells were grown at 30°C to early log phase, labeled with 35S-Met/Cys for 5 min and lysed; cytosolic pCPY* and ER-lumenal CPY* were immunoprecipitated, resolved by SDS-PAGE and detected by autoradiography. Duplicate samples were taken for experiments shown in panels A, B (right), and C. Experiments were performed three times.
We next investigated the sec61S2Y, sec61ΔH1, and sec61ΔN21 mutants for post-translational import defects, monitoring the cytoplasmic accumulation of the post-translational import substrate preproalpha factor (ppαF) in cell lysates by Western Blotting with specific polyclonal antisera. Substitution of the N-acetyl acceptor serine in position 2 of Sec61p had no effect on post-translational import of ppαF in vivo (Fig 3B, left panel). In addition, cytosolic accumulation of ppαF in sec61S2Y was not increased at 37°C, despite the temperature sensitivity of the sec61S2Y mutant (see Fig 1B). In the cold-sensitive sec61-32 control strain, however, ppαF levels were increased at 20°C (Fig 3B, left panel). As shown in Fig 3B (right panel), post-translational import of ppαF was profoundly affected in both sec61ΔH1 and sec61ΔN21. Strong post-translational import defects were also displayed by the sec61ΔH1 and sec61ΔN21 mutants in a pulse-labeling experiment with 35S-Met/Cys for 5 min followed by immunoprecipitation with antibodies against carboxypeptidase Y (CPY) to monitor the efficiency of post-translational ER import of newly synthesized proteins (Fig 3C, right panel). No post-translational import of cytosolic precursor pCPY* could be detected in sec61ΔN21 cells during the 5 min pulse, and the sec61ΔH1 mutant only allowed minimal pCPY* import into the ER (Fig 3C, right panel). In contrast, the sec61S2Y substitution led only to a marginal accumulation of pCPY* in the cytosol (Fig 3C, left panel). Our data suggest that the N-terminal helix of Sec61p, but not its N-terminal acetylation site, is essential for post-translational import into the ER.
Post-translational protein import into sec61S2Y, sec61ΔH1 and sec61ΔN21 microsomes is impaired in vitro
As subtle translocation defects can be masked by the abundance of Sec61 channels in intact cells, to further explore a potential impact of the sec61S2Y mutation on protein import into the ER, we investigated the ability of sec61S2Y microsomes to import ppαF in vitro [18, 37]. The ppαF mRNA was translated in vitro in the presence of 35S-labeled methionine, and the resulting radiolabeled ppαF subsequently incubated at 20°C in the presence of ATP and limiting amounts of wildtype or mutant microsomes for the indicated periods of time (Fig 4A). In vitro the sec61S2Y mutation led to a reduction in the import of ppαF into yeast microsomes (Fig 4A), and this post-translational import defect was more substantial compared to the one found in intact cells (compare Fig 4A vs. Fig 3C, left panel). We also attempted to investigate ppαF import into sec61ΔH1 and sec61ΔN21 microsomes (Fig 4B). Even after 2 hours of incubation, however, we detected no glycosylated 3gpαF in the sec61ΔN21 samples, and only a very limited amount of 3gpαF in the presence of sec61ΔH1 membranes (Fig 4B). These results confirm the critical role of the Sec61p N-terminus in post-translational soluble protein import into the ER. Our data suggest a requirement for the Sec61p N-terminal amphipathic helix for post-translational protein import into the ER with a possible contribution by N-acetylation at the N-terminus of the helix.
Fig 4. N-acetylation of Sec61p at S2 and its N-terminal helix are required for ER import in vitro.
In vitro post-translational import of ppαF into limiting amounts of sec61S2Y (A), sec61ΔH1, and sec61ΔN21 microsomes (B). The mRNA for ppαF was translated in the presence of 35S-methionine in vitro and radiolabeled ppαF was translocated into wildtype and mutant microsomes at 20°C in the presence of ATP and a regenerating system. At the indicated time points (min), 2x SDS-buffer was added and samples were resolved by SDS-PAGE and cytosolic ppαF and translocated, signal-cleaved glycosylated 3gpαF detected by autoradiography. Bands were quantified using the ImageQuant TL software. Experiments were performed four times. The graph in A represents the average of two independent experiments. Error bars represent standard deviation.
The Sec61 complex is unstable in the absence of the N-terminal helix
To test whether the severe defects in post-translational import found for sec61ΔH1 and sec61ΔN21 both in vivo and in vitro (Fig 3 and Fig 4) were due to an impaired formation of heptameric Sec complexes, we solubilized wildtype, sec61ΔH1, and sec61ΔN21 microsomes in digitonin, ultracentrifuged lysates, and precipitated Sec complexes from the supernatant using Concanavalin A beads, which bind to the N-glycans of Sec71p [23]. The solubility of Sec61ΔH1p and Sec61ΔN21p was dramatically reduced compared to wildtype, suggesting that Sec61p without its N-terminal helix is prone to aggregation (Fig 5A, Sol. fractions). Although we could only solubilize small amounts of Sec61ΔH1p and Sec61ΔN21p, we were able to detect amounts of both variants in the Con A fractions although the ratios of Sec61ΔH1p and Sec61ΔN21p to Sec63p in the ConA fractions were lower compared to wildtype (Fig 5A, ConA fractions). In contrast, we observed a dramatic loss of both mutant Sec61 protein variants from the ribosome-associated membrane protein (RAMP) fractions (Fig 5A). We found Sec63p in comparable amounts in the Con-A and RAMP fractions of wildtype, sec61ΔH1, and sec61ΔN21 membranes, but there was more Sec63p in the “Free” fraction in the mutants compared to wildtype (Fig 5A, bottom panels). Our data suggest that despite the strong post-translational import defects shown by both the N-terminal helix deletion mutants, their heptameric Sec complexes are largely intact.
Fig 5. The N-terminal helix of Sec61p is required for Sec61 complex stability.
A: Stability of heptameric Sec complexes in sec61ΔH1 and sec61ΔN21 membranes. Wildtype, sec61ΔH1, and sec61ΔN21 microsomes were solubilized in solubilization buffer (see Methods) containing 3% digitonin and lysates centrifuged at 110,000 g to sediment ribosome-bound Sec61 complexes (ribosome-associated membrane proteins, RAMP). From the supernatants, heptameric Sec complexes were precipitated with Concanavalin A Sepharose (Con A). Sec61p and Sec63p not bound to either Con A or ribosomes were detected in the “Free” fraction. In all fractions, Sec61p and Sec63p were detected by immunoblotting with polyclonal antisera. Loss of Sec61p from the sec61ΔH1 and sec61ΔN21 Soluble fractions is likely due to the formation of SDS-resistant aggregates in digitonin. B: Stability of Sec61 complexes in sec61ΔH1 and sec61ΔN21 strains. Microsomes were solubilized in 1% Triton X-100 and layered onto a 0–15% sucrose gradient. After centrifugation at 200,000 g for 16 h, fractions were collected from top to bottom, and proteins resolved by SDS-PAGE. Sec61p, Sbh1p, and Sss1p were detected by immunoblotting. For both A and B the experiment was performed 4 times.
By contrast, either the stability of the Sec61 complex or its interaction with ribosomes seemed to be compromised in the absence of the N-terminal helix, as indicated by the reduced amount of mutant Sec61p in the RAMP fractions (Fig 5A, top panels). Next, we therefore investigated the stabilities of the sec61ΔH1 and sec61ΔN21 trimeric complexes directly. We solubilized wildtype, sec61ΔH1, and sec61ΔN21 microsomes in 1% Triton X-100 and centrifuged solubilized Sec61 complexes through a shallow 0–15% sucrose gradient [38]. Fractions were collected from top to bottom, proteins precipitated with trichloroacetic acid, resolved by SDS-PAGE, and Sec61 complex subunits detected in each fraction by blotting with antibodies against the C-termini of Sec61p and Sss1p, and the N-terminus of Sbh1p. We observed a distinct loss of colocalization of Sec61p, Sbh1p, and Sss1p in gradient fractions from both sec61ΔH1 and sec61ΔN21 complexes (Fig 5B). A reduced number of fractions contained all three proteins for the two deletion mutants (wildtype: fractions 4–10; sec61ΔH1: fraction 7; sec61ΔN21: fractions 5–7). The destabilization of trimeric Sec61 complexes was more pronounced in sec61ΔH1 microsomes compared to sec61ΔN21 microsomes. In contrast, solubilization and fractionation of microsomes derived from sec61S2Y cells showed that the interaction of Sec61S2Yp with Sbh1p and Sss1p was not significantly altered. Our data suggest that the N-terminal amphipathic helix of Sec61p is required for stability of the Sec61 complex.
Discussion
The Sec61 complex mediates protein import into the ER, and is also a candidate channel for the dislocation of ERAD substrates [3, 5]. To investigate the function of the Sec61p N-terminus in these processes in yeast, we characterized a set of sec61 N-terminal mutants. We have shown here that N-acetylation site of Sec61p at S2 is important for ERAD (Fig 2A), and may contribute to post-translational import into the ER (Fig 4A), whereas its N-terminal amphipathic helix is essential for post-translational import into the ER and is required for stability of the Sec61 complex (Fig 3C, Fig 5B).
Role of the Sec61p N-acetylation site
We investigated Sec61p function in the sec61S2Y mutant, in which Sec61p can no longer be acetylated at its canonical NatA consensus serine at position 2 after methionine cleavage but is predicted to be acetylated at the uncleaved initiator methionine (S1 Fig), and in two new sec61 mutants: one carrying a deletion of the N-terminal helix but preserving the N-terminal acetylation site in its original sequence context, sec61ΔH1, and one lacking both the N-terminal acetylation site and the N-terminal helix, sec61ΔN21 (Fig 1A). The mutant strains stably expressed Sec61p at wildtype level (sec61ΔN21) or approximately 40% of wildtype (sec61S2Y and sec61ΔH1; Fig 3B and S2 Fig). In a GAL shut-off experiment ER translocation defects only occurred when Sec61p levels fell below 20% of wildtype, and we observed no effect of sec61S2Y on cotranslational and only a marginal effect on posttranslational import in vivo, confirming that Sec61p was not limiting in our N-terminal sec61 mutant cells (S2 Fig; Fig 3A, 3B and 3C, left panels). N-acetylation at the initiator methionine can target soluble proteins for degradation by the Ac/N-end rule pathway, but since Sec61S2Yp was stable in a cycloheximide chase over 3 h this does not seem to be the case for our mutant protein [39]. Since mutation of the N-terminal acetylation site did not influence the stability of Sec61p, but resulted in lower expression levels, our data suggest that N-acetylation at S2 may play a role in biosynthesis of Sec61p.
Although sec61S2Y cells were temperature-sensitive, incubation at higher temperature did not affect co- or post-translational import (Fig 3B, left panel). In in vitro experiments with limiting amounts of microsomes and adjusting for equal amounts of Sec61p and Sec61S2Yp, however, we detected a reduced post-translational import of ppαF into sec61S2Y microsomes (compare Fig 4A vs. Fig 3C, left panel). This difference between the sec61S2Y effect in intact cells and in the cell-free assay might be due to an increase in mutation-associated instability of Sec61p as a consequence of the microsome preparation procedure. N-terminal acetylation has been shown to increase N-terminal helicity and affinity for physiological membranes, and although N-acetylation in the sec61S2Y mutant is likely preserved, its helix stabilizing effect is will be lost due to the increased bulk of the N-terminus generated by the presence of both the initiator methionine and the tyrosine at position 2 (S1 Fig) [40]. Thus, the S2Y mutation will lead to fraying of the N-terminal helix, which is essential for post-translational import (below).
Fig 2A shows that CPY* retrotranslocation to the cytosol is considerably delayed in the sec61S2Y mutant. Since the mutant has no protein import defect in vivo (Fig 3), this effect is likely direct. The defect in ERAD of CPY* was not exacerbated in sec61S2Y cells at higher temperature, thus the temperature-sensitivity of the mutant remains unexplained. Although the perturbance of ER homeostasis in the sec61S2Y mutant caused tunicamycin-sensitivity even at low concentration (Fig 1B), on its own it did not induce the UPR (Fig 1D) suggesting that the mutant was able to cope with a physiological load of protein misfolding. Since protein-protein interactions are often dependent on N-terminal acetylation in a specific sequence context, our data may indicate that Sec61p N-acetylation at S2 is required for interaction with components of the ERAD machinery like the Hrd1 ligase, or the Cdc48 complex (Fig 6, left) [16, 17, 33, 41].
Fig 6. Model: Role of the Sec61 N-terminal helix in posttranslational ER import.
Left: TMD1 of Sec61 (red) is anchored at its N-terminus in the ER membrane by the strongly hydrophobic, amphipathic N-terminal helix H1 which positions Sec61TMD1 such that it can interact closely with TMD3 of Sec63 (blue) in wildtype S. cerevisiae. Middle: Deletion of the N-terminal acetylation site (yellow) and H1 of Sec61 in Sec61ΔN21p reduces Sec61TMD1 anchoring to the membrane and thereby changes its tilt angle and/or mobility, thus reducing the interaction between Sec61TMD1 with Sec63TMD3. Right: Deletion of H1 in Sec61ΔH1p additionally increases the distance between Sec61TMD1 and Sec63TMD3 because of the repositioning of the acetyl group (which serves as an anchor for a putative ERAD factor) (green) close to the Sec63-binding residues of Sec61TMD1, thereby blocking Sec61p-Sec63p interaction. Both middle and right scenarios affect the opening of the Sec61 lateral gate by Sec63p and hence posttranslational import.
The sec61ΔH1 mutant was the only N-terminal sec61 mutant that displayed a significant UPR induction, although it preserves the N-acetylation site (Fig 1D). Since the mutant protein is stable, this is unlikely to be a result of Sec61ΔH1p eliciting the UPR itself as a misfolded protein. The N-acetylation of Sec61ΔH1p, however, may recruit an interacting factor to its N-terminus, which in the mutant protein due to the absence of the helix ends up in the wrong position and further interferes with function of Sec61ΔH1p (Fig 6, right) [41].
Role of the Sec61p N-terminal amphipathic helix
Growth of the sec61ΔH1 and sec61ΔN21 mutants was compromised in all conditions tested, suggesting an important role for the N-terminal amphipathic helix in Sec61p function (Fig 1C). The growth defect was caused by a strong post-translational import defect (Fig 3B and 3C, right panels), which—due to the overlap between import and export during the chase—made it difficult to evaluate possible ERAD defects in the helix deletion mutants (Fig 2B). In addition, we found that all sec61 N-terminal mutants including sec61S2Y were synthetically lethal with an inefficiently translocating post-translational ER import substrate that clogs the channel, suggesting that the Sec61p N-terminus enhances efficiency of post-translational protein import into the ER [42].
A possible explanation for the almost exclusive post-translational import defects exhibited by the sec61ΔH1 and sec61ΔN21 mutants was that the interaction of Sec61p with the Sec63 complex required for post-translational import was compromised in the absence of the N-terminal helix. We examined this by Con A precipitation of solubilized Sec complexes (Fig 5A), but to our surprise found no reduction in Sec complex formation in the mutants (Fig 5A). Instead, we found a dramatic loss of Sec61p from the RAMP fractions of sec61ΔH1 and sec61ΔN21 membranes, suggesting reduced affinity of mutant Sec61 complexes for ribosomes or reduced Sec61 complex stability (Fig 5A).
Gradient fractionation experiments with solubilized Sec61 complexes corroborated the hypothesis that the N-terminal helix of Sec61p is important for Sec61 complex stability (Fig 5B). Membranes were solubilized with Triton X-100 because Sec61 complexes are only marginally stable in this detergent, thus even minor perturbations result in dissociation of the subunits [38]. The distribution of Sec61 complex subunits in the gradient fractions suggests that the Sec61p N-terminal helix is required for Sec61 complex stability, and that interaction of Sbh1p with Sec61p is more strongly dependent on the Sec61p N-terminal helix than that of Sss1p with Sec61p, as Sbh1p colocalized with Sec61ΔH1p in only 1 and with Sec61ΔN21p in only 3 fractions, respectively (Fig 5B). Sbh1p consists of a single transmembrane helix that is close to transmembrane domain 4 of Sec61p in the crystal structure, and its cytosolic domain is physically close to the cytosolic N-terminus of Sec61p [10, 43]. Upon solubilization, the interaction between the cytosolic domains of Sec61p and Sbh1p might become more important, and the dissociation of Sbh1p from Sec61ΔH1p and Sec61ΔN21p after Triton X-100 solubilization suggests that the Sec61p N-terminal helix might be the cytosolic Sbh1p interacting partner (Fig 5B). The growth defects of sec61ΔH1 and sec61ΔN21 cells were, however, not rescued upon overexpression of SBH1, suggesting that Sbh1p interaction is not limiting for mutant channel stability. Sss1p makes contact with Sec61p at multiple sites, including the transmembrane helices H6, H7, and H8 [44]. The reduced interaction of Sss1p with Sec61p lacking its N-terminal helix (see Fig 5B; wildtype: fractions 4–13; sec61ΔH1: fractions 7–12; sec61ΔN21: fractions 7–12) might therefore be caused by structural changes in the overall conformation of Sec61p that affect some of its interaction sites with Sss1p. Structure of the N-terminal half of Sec61p might be stabilized by the association of the amphipathic N-terminal helix with the cytosolic ER membrane surface and thus coordinated movement of the N-terminal half of Sec61p during channel opening might be compromised if the helix is missing [10]. Given that we observed no DPAPB import defects in sec61ΔH1 and sec61ΔN21 cells (Fig 3A, right panel), the Sec61 complex lacking the N-terminal helix seems to be sufficiently stabilized by the ribosome to function during co-translational protein import into the ER. Our data suggest that, in the absence of the N-terminal helix of Sec61p, the Sec61 complex on its own is unstable, and that its interactions with the Sec63 complex are insufficient to allow post-translational import.
A recent paper from the Rapoport lab [45] provides an explanation for our observations: The cryo-electron microscopy structure of the Sec complex reported by Wu et al. revealed three interactions between Sec63 and the Sec61 channel [45]. The interaction between the N-terminus of Sec63, Sss1 and the loop 5 hinge of Sec61 in the ER lumen is not essential for posttranslational import into the ER, but Sec63-Sec61 contacts in the cytosol and in the membrane allow Sec63 to partially open the lateral gate of the channel [45]. The interaction of the cytosolic Brl domain of Sec63 with loops 6 and 8 of Sec61 acts as a static anchor point for Sec63 whereas binding of Sec63 transmembrane domain (TMD) 3 to Sec61 TMD1 and the TMDs of Sbh1 and Sss1 pry open the cytosolic end of the lateral gate of Sec61 [45]. Combined these interactions allow Sec63 to function as a scaffold for the gate-opened conformation of Sec61 and enable insertion of low-hydrophobicity signal sequences of post-translational import substrates into the partially open lateral gate [45]. The cytosolic and lumenal Sec63-Sec61 interactions are unlikely to be affected by the deletion of the N-terminal amphipathic helix of Sec61 which explains why Sec63 still binds to our mutant Sec61 complexes (Fig 5A). The interaction of Sec63 TMD3 with Sec61 TMD1, however, will likely be affected by the deletion of the amphipathic helix from the N-terminus of Sec61 TMD1 (Fig 6). The missing helix may alter the tilt angle of Sec61 TMD1 and/or its mobility (Fig 6), thus weakening its interaction with the TMDs of Sbh1 and Sss1 and explaining the instability of the solubilized Sec61 complex in our gradient fractionation (Fig 5B). In addition, the absence of the N-terminal helix likely weakens the interaction of Sec61 TMD1 with Sec63 TMD3 (Fig 6) which would prevent the opening of the lateral gate and hence result in the exclusively posttranslational ER protein import defect that we observe (Fig 3). Thus the Sec complex structure by Wu et al. [45] bolsters our data on the functional importance of N-terminal helix of Sec61.
Conclusion
In summary, we have shown here that the Sec61p N-terminus contains two structural elements that are functionally important: The N-acetylation site at S2 plays a role in ERAD, likely stabilizes the N-terminal helix, and may play a role in Sec61p biogenesis. The Sec61p N-terminal amphipathic helix is essential for post-translational import into the ER and Sec61 complex stability.
Supporting information
(PDF)
(EPS)
Acknowledgments
Antibodies against Sss1p and Sec63p were kindly provided by Randy Schekman, DPAPB antibodies by Tom Stevens. A first draft of the manuscript was written by the MSc Infectious Disease Biology class of 2016. We thank Sonja Hess at the Caltech Proteome Exploration Lab for communicating data on the full N-acetylation of the Sec61p N-terminus.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
KR received core funding from the Saarland University for this work.
References
- 1.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. 10.1146/annurev-biophys-050511-102312 [DOI] [PubMed] [Google Scholar]
- 2.Xie W, Ng DT. ERAD substrate recognition in budding yeast. Semin Cell Dev Biol. 2010. July;21(5):533–9. 10.1016/j.semcdb.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Zattas D, Hochstrasser M. Ubiquitin-dependent protein degradation at the yeast endoplasmic reticulum and nuclear envelope. Crit Rev Biochem Mol Biol. 2015. Jan-Feb;50(1):1–17. 10.3109/10409238.2014.959889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann R, Eyrisch S, Ahmad M, Helms V. Protein translocation across the ER membrane. Biochim Biophys Acta. 2011. March;1808(3):912–24. 10.1016/j.bbamem.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 5.Johnson AE, van Waes MA. The translocon: a dynamic gatewayat the ER membrane. Annu Rev Cell Dev Biol. 1999;15:799–842. 10.1146/annurev.cellbio.15.1.799 [DOI] [PubMed] [Google Scholar]
- 6.Ast T, Cohen G, Schuldiner M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell. 2013. February 28;152(5):1134–45. 10.1016/j.cell.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 7.Schäfer A, Wolf DH. Sec61p is part of the endoplasmic reticulum-associated degradation machinery. EMBO J. 2009. October 7;28(19):2874–84. 10.1038/emboj.2009.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012. December 7;151(6):1163–7. 10.1016/j.cell.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandon EC, Trueman SF, Gilmore R. Protein Translocation across the Rough Endoplasmic Reticulum. Cold Spring Harb Perspect Biol. 2013. February 1;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voorhees RM, Hegde RS. Structure of the Sec61 channel opened by a signal sequence. Science. 2016. January 1;351(6268):88–91. 10.1126/science.aad4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackinnon AL, Paavilainen VO, Sharma A, Hegde RS, Taunton J. An allosteric Sec61 inhibitor traps nascent transmembrane helices at the lateral gate. Elife. 2014;3:e01483 10.7554/eLife.01483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z, Jiang Y, Mandon EC, Gilmore R. Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation. J Cell Biol. 2005. January 3;168(1):67–77. 10.1083/jcb.200408188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harty C, Römisch K. Analysis of Sec61p and Ssh1p interactions in the ER membrane using the split-ubiquitin system. BMC Cell Biol. 2013. March 11;14:14 10.1186/1471-2121-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer S, Burbaum L, Unverdorben P, Pech M, Chen Y, Zimmermann R, et al. Structure of the native Sec61 protein-conducting channel. Nat Commun. 2015. September 28;6:8403 10.1038/ncomms9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalies KU, Allan S, Sergeyenko T, Kröger H, Römisch K. The protein translocation channel binds proteasomes to the endoplasmic reticulum membrane. EMBO J. 2005. July 6;24(13):2284–93. 10.1038/sj.emboj.7600731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng W, Sergeyenko T, Zeng N, Brown JD, Römisch K. Characterization of the proteasome interaction with the Sec61 channel in the endoplasmic reticulum. J Cell Sci. 2007. February 15;120(Pt 4):682–91. 10.1242/jcs.03351 [DOI] [PubMed] [Google Scholar]
- 17.Braunstein I, Zach L, Allan S, Kalies KU, Stanhill A. Proteasomal degradation of preemptive quality control (pQC) substrates is mediated by an AIRAPL-p97 complex. Mol Biol Cell. 2015. November 1;26(21):3719–27. 10.1091/mbc.E15-02-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser ML, Römisch K. Proteasome 19S RP binding to the Sec61 channel plays a key role in ERAD. PLoS One. 2015. February 6;10(2):e0117260 10.1371/journal.pone.0117260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalies KU, Rapoport TA, Hartmann E. The beta subunit of the Sec61 complex facilitates cotranslational protein transport and interacts with the signal peptidase during translocation. J Cell Biol. 1998. May 18;141(4):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Cheng Z, Mandon EC, Gilmore R. An interaction between the SRP receptor and the translocon is critical during cotranslational protein translocation. J Cell Biol. 2008. March 24;180(6):1149–61. 10.1083/jcb.200707196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavan M, Lennarz W. The molecular basis of coupling of translocation and N-glycosylation. Trends Biochem Sci. 2006. January;31(1):17–20. Epub 2005 Dec 13. 10.1016/j.tibs.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Scheper W, Thaminy S, Kais S, Stagljar I, Römisch K. Coordination of N-glycosylation and protein translocation across the endoplasmic reticulum membrane by Sss1 protein. J Biol Chem. 2003. September 26;278(39):37998–8003. 10.1074/jbc.M300176200 [DOI] [PubMed] [Google Scholar]
- 23.Pilon M, Römisch K, Quach D, Schekman R. Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol Biol Cell. 1998. December;9(12):3455–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson BM, Critchley AJ, Stirling CJ. Determination of the transmembrane topology of yeast Sec61p, an essential component of the endoplasmic reticulum translocation complex. J Biol Chem. 1996. October 11;271(41):25590–7. [DOI] [PubMed] [Google Scholar]
- 25.Helbig AO, Rosati S, Pijnappel PWWM, van Breukelen B, Timmers MHTH, et al. Perturbation of the yeast N-acetyltransferase NatB induces elevation of protein phosphorylation levels. BMC Genomics 2010;11:685 10.1186/1471-2164-11-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta. 2016. October;1864(10):1372–401. 10.1016/j.bbapap.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Aksnes H, Drazic A, Marie M, Arnesen T. First Things First: Vital Protein Marks by N-Terminal Acetyltransferases. Trends Biochem Sci. 2016. September;41(9):746–60. 10.1016/j.tibs.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Pilon M, Schekman R, Römisch K. Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J. 1997. August 1;16(15):4540–8. 10.1093/emboj/16.15.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tretter T, Pereira FP, Ulucan O, Helms V, Allan S, Kalies KU, et al. ERAD and protein import defects in a sec61 mutant lacking ER-lumenal loop 7. BMC Cell Biol. 2013. December 6;14:56 10.1186/1471-2121-14-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson MD, Rzeznicka K, Rosenbäck M, Hansson M, Sirijovski N. PCR-mediated deletion of plasmid DNA. Anal Biochem. 2008. April 15;375(2):373–5. 10.1016/j.ab.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 31.Cox J, Shamu C, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993. June 18;73(6):1197–206. [DOI] [PubMed] [Google Scholar]
- 32.Knop M, Finger A, Braun T, Hellmuth K, Wolf DH. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 1996. February 15;15(4):753–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman I, Munro S. An N-terminally acetylated Arf-like GTPase is localised to lysosomes and affects their motility. J Cell Sci. 2006. April 15;119(Pt 8):1494–503. 10.1242/jcs.02958 [DOI] [PubMed] [Google Scholar]
- 34.Soromani C, Zeng N, Hollemeyer K, Heinzle E, Klein M-C, Tretter T, et al. N-acetylation and phosphorylation of Sec complex subunits in the ER membrane. BMC Cell Biol. 2012. December 13;13:34 10.1186/1471-2121-13-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao H, Smeekens JM, Wu R. Quantification of tunicamycin-induced protein expression and N-glycosylation changes in yeast. Analyst. 2016. June 21;141(12):3737–45. 10.1039/c6an00144k [DOI] [PubMed] [Google Scholar]
- 36.Travers KJ, Patil CK, Wodicka L, Lockhardt DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000. April 28;101(3):249–58. [DOI] [PubMed] [Google Scholar]
- 37.Feng D, Zhao X, Soromani C, Toikkanen J, Römisch K, Vembar SS, et al. The transmembrane domain is sufficient for Sbh1p function, its association with the Sec61 complex, and interaction with Rtn1p. J Biol Chem. 2007. October 19;282(42):30618–28. 10.1074/jbc.M701840200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falcone D, Henderson MP, Nieuwland H, Coughlan CM, Brodsky JL, Andrews DW. Stability and function of the Sec61 translocation complex depends on the Sss1p tail-anchor sequence. Biochem J. 2011. June 1;436(2):291–303. 10.1042/BJ20101865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shemorry A, Hwang CS, Varshavsky A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol Cell. 2013. May 23;50(4):540–51. 10.1016/j.molcel.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dikiy I, Eliezer D. N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound α-synuclein and increases its affinity for physiological membranes. J Biol Chem. 2014. February 7;289(6):3652–65. 10.1074/jbc.M113.512459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starheim KK, Gevaert K, Arnesen T. Protein N-terminal acetyltransferases: when the start matters. Trends Biochem Sci. 2012. April;37(4):152–61. 10.1016/j.tibs.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 42.Ast T, Michaelis S, Schuldiner M. The Protease Ste24 Clears Clogged Translocons. Cell. 2016. January 14;164(1–2):103–14. 10.1016/j.cell.2015.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao X, Jäntti. Functional characterization of the trans-membrane domain interactions of the Sec61 protein translocation complex beta-subunit. BMC Cell Biol. 2009. October 26;10:76 10.1186/1471-2121-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson BM, Esnault Y, Craven RA, Skiba F, Fieschi J, Kepes F, et al. Molecular architecture of the ER translocase probed by chemical crosslinking of Sss1p to complementary fragments of Sec61p. EMBO J. 1997. August 1;16(15):4549–59. 10.1093/emboj/16.15.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Cabanos C, Rapoport TA. Structure of the post-translational protein translocation machinery of the ER membrane. Nature 2018, December 31 (Epub ahead of print) 10.1038/s41586-018-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(EPS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.