Abstract
Pancreatic cancer is one of the most aggressive malignancies with an increase in incidence predicted, particularly in African Americans. Pancreatic cancer is considered a silent disease with poor prognosis and a lack of early biomarkers for detection. Proteomics has been applied in many diseases for identifying or discovering biomarkers. It has long been suggested that chronic pancreatitis may be a risk factor for developing pancreatic cancer. This study identified proteins that are altered in expression in pancreatic cancer and pancreatitis compared to normal using proteomic technology. Proteins were extracted from laser captured micro-dissected tissues and separated in 2-DPAGE and imaged. The protein profiles of pancreatic cancer and pancreatitis are similar but differed with the protein profile of normal adjacent tissues. Representative proteins, overexpressed in tumor and pancreatitis but not normal tissues, were excised from gels, subjected to in-gel digestion, and analyzed by MALDI-TOF mass spectrometry. Proteins identified included transferrin, ER-60 protein, proapolipoprotein, tropomyosin 1, alpha 1 actin precursor, ACTB protein, and gamma 2 propeptide, aldehyde dehydrogenase 1A1, pancreatic lipase and annexin A1. Several proteins, which were shown in pancreatic cancer, were also observed in pancreatitis samples. Understanding the role of these specific proteins and their mechanistic action will give insights into their involvement in pancreatic cancers.
Keywords: Pancreatic cancer, Proteomic, Pancreatitis, 2D-gel, Biomarkers, Lipase
Introduction
Pancreatic cancer is one of the leading causes of death in the United States. It is considered the 4th most common cancer among both men and women and is ranked as the 11th most common cause of death globally [1]. Pancreatic cancer accounts for 7.2% (43,090) of cases of death in the United States and 331000 deaths annually worldwide [1]. This cancer is lethal because it lacks early symptoms and results in late- stage detection and a high mortality rate. Several studies have been undertaken to identify biomarkers for early detection of pancreatic cancer. Among these biomarkers is serum carbohydrate antigen (CA19–9),which has been extensively studied and widely used for the diagnosis of pancreatic cancer so far [2]. It has a 90% specificity to pancreatic cancer. However, it is not expressed in Caucasians lacking the Lewis blood group antigen (~5% ofthe population) whereas an elevation can be observed in chronic pancreatitis and obstructive jaundice [3,4] Because of its limitations, CA19–19 is an unreliable screening biomarker and is restricted to the detection of tumor recurrence after surgical resection [3,5,6]. Carcinoembryonic antigen (CEA) is another biomarker that has been used to diagnose pancreatic cancer. Since the protein is lacking in most pancreatic tumors and because studies have shown that CA19–9 has a better specificity and sensitivity compared to CEA, scientists have discontinued using CEA to diagnose pancreatic cancer [7]. Nevertheless, combining them has been common in panels [8]. According to researchers from the Mayo Clinic, methylation markers distinguishing pancreatic cancer from benign controls are detected in pancreatic juice [9]. Kisiel et al. [10] identified a panel of methylated biomarkers CD1D, KCNK12, CLEC11A, NDRG4, IKZF1, PKRCB and KRAS resulting in 75% sensitivity and 95% specificity comparing pancreatic cancer to normal pancreas and pancreatitis.
Due to the limitations of current screening techniques, it is essential to find new biomarkers that would not only better distinguish pancreatic cancer from diseases with similar symptoms such as pancreatitis or benign pancreatic cyst [9] but also aid in reducing the mortality rate of pancreatic cancer. Important technological innovations have been made in the past decades to facilitate biomarker identification [11]. In this study, we employed laser capture microdissection, 2- dimensional electrophoresis, tandem mass spectrometry (MS/MS), and pathway analysis to search for the protein markers in the pancreatic cancer specimens. Several of the identified proteins were further confirmed by immunohistochemistry.
Materials and Methods
Tissue preparation
Ten frozen tissues each of pancreatic adenocarcinoma, normal adjacent pancreatic, and pancreatitis tissues were collected from patients that underwent surgery as part of standard care of their condition at Indiana University School of Medicine (Indianapolis, IN). The specimens were obtained and immediately snap frozen using liquid nitrogen. Indiana University and Purdue University Institutional Review Board Committee approved the study and an informed consent was procured from each patient.
Laser capture microdissection and protein extraction
Frozen sections of fresh healthy pancreas, pancreatitis and pancreatic adenocarcinoma tissues were prepared and cells were micro dissected using ArcturusXT™ Laser Capture Microdissection (LCM) System. Briefly, the sections 6 μm were cut and mounted on glass slides and fixed in 75% alcohol for 30 s, and stained with haematoxylin. Following staining, the sections were then air-dried and micro dissected. Approximately 200,000 to 250,000 cells were micro dissected and more than one LCM caps were pooled. The captured cells were then visualized by a microscopic to ensure that each cell population to be 95% homogeneous (Figure 1). The micro dissected cells were then lysed in lysis buffer (7 mol/L urea, 2 mol/L thiourea, 100 mmol/L DTT, 4% CHAPS, 0.5 mmol/L EDTA, 40 mmol/L Tris, 2% NP40, 1% Triton X-100, 5 mmol/L phenylmethylsulphonyl fluoride) at 4°C for 1 h, and then centrifuged at 12,000 rpm for 30 min at 4°C. The supernatants were transferred to a new tube and protein concentration was determined with Amersham 2D Quant kit (GE Healthcare, Piscataway, NJ). 2.5 mg protein was transferred into glass tubes, and precipitated using TCA/acetone. The resulted pellets were solubilized in 0.4 mL solution containing 9 M urea, 4% Igepal, 1% DTT and 2% carrier ampholytes (pH 8– 10.5).
Figure 1:
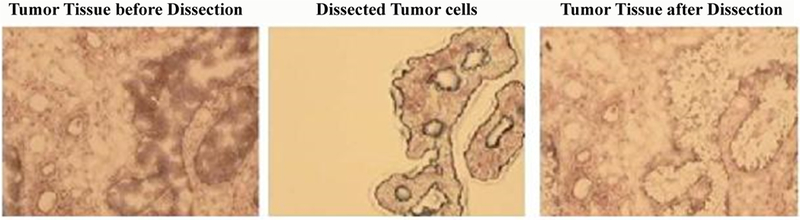
Representative images of frozen pancreatic cancer section specimens before and after laser capture microdissection.
2-Dimmensional gel electrophoresis and image analysis
Two-dimensional gel electrophoresis was carried out in triplicate for a total of 30 gels according to a modified protocol from Bell et al. [12]. Five hundred micrograms of each sample were loaded onto a 24 cm IPG strip (linear pH 3–10) (Bio-Rad, Hercules, CA) for 24 h passive rehydration at room temperature and focused to 100,000 VH (approximately 21 h) using the Bio-Rad Protean IEF Cell. IPG strips were reduced first using 6 M urea, 0.375 M Tris (pH 8.8) (Fischer, Pittsburgh, PA), 4% SDS, 20% glycerol (Fischer, Pittsburgh, PA), and 2% DTT. Alkylation was carried out using 6 M urea, 0.375 M Tris (pH 8.8), 4% SDS, 20% glycerol, and 2.5% iodoacetamide (Sigma, St. Louis, MO). The reduced and alkylated IPG strips were loaded across the top of 11%−19% gradient slab gels. Using the Bio-Rad Protean plus Dodeca Cell, gels were run in parallel at 145 V for 18 h at 8°C for separation by molecular weight. Gels were fixed overnight in 50% ethanol, 2% phosphoric acid (85%) (Fischer, Pittsburg, PA), 48% milli-Q water, and then washed 3 times for 30 min each in milli-Q water. Gels were equilibrated in a buffer of 10% phosphoric acid (85%), 10% ammonium sulfate (Sigma-Aldrich, St. Louis, MO), and 20% methanol (Mallinckrodt, Hazelwood, MO) for 1 h. Colloidal Coomassie G-250 stain (Bio- Rad, Hercules, CA) was added (0.12%) and allowed to stain for 96 h. Gels were washed several times in milli-Q water and scanned on the GS-800 Calibrated Imaging Densitometer (Bio-Rad, Hercules, CA) for analysis by PDQuest software (Bio-Rad, v.7.1). The PDQuest software was used to subtract background, normalize and match gels, and to assign an identification and intensity number to each protein spot. Each gel was assigned to a match set and matched to a selected reference gel. Spots shared between all gels were used as landmarks to improve match rate between gels. The resulting raw data, given in ppm, were exported and used for two-way ANOVA statistical analysis to identify differentially expressed spots.
MALDI-TOF-TOF analysis
Differentially expressed protein spots were manually excised from the gels and placed in a 96-well plate. Gel plugs were destained with 25 mM ammonium bicarbonate and 50% acetonitrile (ACN) then digested with 5 μg/ml trypsin and incubated overnight at 37°C. Formic acid (0.3%) was added to deactivate trypsin digestion. To extract tryptic peptides from the gel, 60% ACN and 5% TFA were added to each digested gel plug and sonicated in a water bath sonicator on ice for 15 min. The supernatant was collected following 1 min centrifugation. This procedure was completed 3 times. The supernatant was pooled, dried in a speed vacuum apparatus, and resolubilized in 0.1 μl TFA. C18 Zip Tips were used following the manufacturer’s (Millipore, Billerica, MA) protocol to remove salts and other contaminants. Purified peptides were completely dried in a speed vacuum apparatus then reconstituted in 1 μl of 0.1% TFA. An equal volume of sample (0.5 μl) and alpha cyano-4-hydroxycinnamic were mixed and spotted onto a MALDI target plate. Sigma peptide mix standards were prepared for calibration. Once the sample/matrix was air dried completely, peptide masses were analyzed via MS/MS using the AB4700 MALDI- TOF/TOF (Applied Biosystems, Foster City, CA). GPS Explorer software (Applied Biosystems, Foster City, CA) was used for analysis. Peptide searches were performed using the Mascot protein identification software (Matrix science) and the NCBI database. The following parameters were set for database searching: human species, mass ranges within 600–4000 Da of measured values, pI range 3–10, two missed trypsin cleavages, and 100 ppm mass tolerance with 1+ peptide change and an MS/MS fragment tolerance of 0.15 Da carboxyamidomethylation of cysteine residues (+57.0215) was set as a fixed modification and oxidation of methionine residues (+16) was set as a variable modification.
Immunohistochemistry
Thirty each pancreatic cancer, normal adjacent, and pancreatitis tissue sections (5 μm) were cut from formalin-fixed paraffin-embedded surgically removed tissues, and mounted on positively charged Superfrost slides (Fisher Scientific, Chicago, IL). The tissue sections were then deparaffinized and rehydrated in graded alcohols and were subjected to immunohistochemical staining according to the manufacturer’s protocol (Biocare Medical, Concord, CA, USA). In brief, the hydrated sections were heated in Biocare Gene retrieval steamer 45 min in 10 mM citrate buffer, pH 6.0, to unmask the epitopes. Then we used 3% hydrogen peroxide to block endogenous peroxidase activity. Nonspecific binding was blocked with background polisher and slides were incubated for 10 min at room temperature. The slides were then incubated 30 min with primary mouse monoclonal antibody or rabbit polyclonal antibodies as descripted. The primary antibody complexes were then visualized with a biotin-labelled secondary antibody (Universal Goat Link) for 15 min; and finally, with HRP for 15 min and stained for 5 min with 3, 3’-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin, dehydrated, and mounted. Negative control slides were treated with no antibody. The intensity of each protein immunostaining was graded on a scale of 0 to 3, where 0, no staining; 1, equivocal staining; 2, moderate to intense staining; and 3, highest intensity staining.
Statistical analysis
For a selected spot in the gels, two-sample paired t-test was used to compare invasive pancreatic carcinoma and their paired non-neoplastic tissues. The analyzed variable is the log of the spot intensity. In case the spot was not present, ‘0’was used to signify the lack of intensity. Bonferroni correction was used to adjust for multiple comparisons, where the cut-off value is 0.05. Bonferroni correction is a multiple- comparison correction used when several dependent or in-dependent statistical tests are being performed simultaneously. Significantly different spots between the two tissues types were selected for further consideration as potential biomarkers. The most significant proteins were reported.
Results
The purpose of this study was to characterize and compare the protein expression profiles of non-neoplastic pancreas, pancreatitis, and pancreatic adenocarcinoma tissues to determine alterations in proteins expression that can be identified as biomarkers for early detection and/ or therapeutic intervention of pancreatic adenocarcinoma. However, comparing these tissue samples to identify the molecular characteristics is often hindered by tissue heterogeneity. Therefore, we obtained relatively pure cell populations from these tissue samples by laser capture microdissection (LCM). Tissues were dissected as described in the Materials and Methods, and visual inspection indicated that homogeneous populations of epithelial cells were obtained from each tissue (Figure 1). After cells solubilization from the LCM cap to quantitatively analyze the proteins that were differentially expressed, the protein concentration of all samples was determined by modified Lowry method using Amersham 2D Quant kit. Then a 200 mg protein from each cell lysates was loaded per gel of each sample normal, pancreatitis, and pancreatic cancer (n=10 each). Ampholyte with a pH range of 4–8 was used for the 1-D tube gel to obtain a wide-range protein profile, while large format gradient slab gels were used for better separation.
Using this approach, routinely, more than 1500 spots could be determined. However, to alleviate the difficultly in quantitatively analyzing weak spots near background level and identifying these spots with MS unambiguously, the sensitivity threshold of the PDQuest spot detector was adjusted so that only approximately 1000–1400 spots were detected and analyzed. Despite overall similarities in expression profiles, the levels of several major polypeptides were highly altered in the tumor tissues compared normal tissues. The images were analyzed by comparing normal pancreas, pancreatitis, and invasive pancreatic adenocarcinoma tissues of each individual cancer patient and across patients and on an average at least 70% of total spots matched. Using the Student’s t-test incorporated in the PDQuest software as well as manually, we showed many proteins to be differentially expressed. Figure 2 shows representative gels of normal pancreas and pancreatic carcinoma tissues, which represent their proteome expression map. Thirty-three proteins were found to be significantly (p=0.05) altered in expression (Table 1). Statistical analysis using Bonferroni correction (p=0.005) was performed to eliminate possible false positives and give a more stringent p-value. Among the proteins significantly altered in expression were pancreatic lipase, Annexin, Protein PP4-X, Aldehyde dehydrogenase 1A1, and Calreticulin. Proteins with a Z-score lower than 1.65, were noted as unambiguous identification by comparison with results from the previous experiments.
Figure 2:
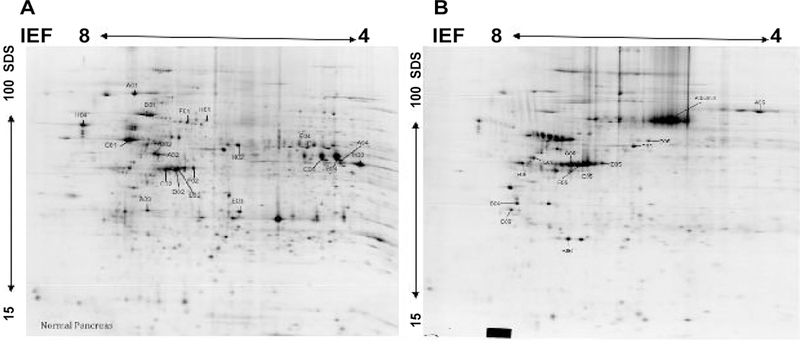
Representative 2-DE gel images of protein profiles of normal pancreas tissues (A) and pancreatic carcinoma (B). Proteins were separated by IEF as first dimension, using 24 cm tube gels pH 4–8 and linear gradient gel 11.5 to 19% as a second dimension. The protein spots were cut from the gel, trytically digested, and identified via MALDI-MS. Significantly expressed spots are posted in Table 1 along with their individual PD-Quest spot number assignment and other information.
Table 1:
Proteins altered in expression between pancreatic adenocarcinoma and pancreatitis and normal pancreas tissues.
| ID Number | Protein Name | Access Number | PI | M.W. |
|---|---|---|---|---|
| A01 | Tumor rejection antigen-1 (gp96) | 4507677 | 4.8 | 92.7 |
| A02 | Protein-disulfide isomerase-related protein 5 | 1710248 | 5 | 46.5 |
| A03 | Annexin V | 999926 | 5 | 35.8 |
| A04 | L-Arginine: Glycine Amidino-transferase | 6730020 | 6.4 | 44.6 |
| B01 | BiP protein | 6470150 | 5.2 | 71 |
| B02 | ATP synthase | 32189394 | 5.3 | 56.5 |
| B04 | Lipase | 226753 | 6.3 | 50.2 |
| C01 | Prolyl 4-hydroxylase, beta subunit | 20070125 | 4.8 | 57.5 |
| C02 | Keratin 8, type II cytoskeletal | 105815 | 5.4 | 53.7 |
| C02 | Actin, gamma 1 pro-peptide | 4501887 | 5.3 | 42.1 |
| C04 | Pancreatic lipase | 10835000 | 6.3 | 51.9 |
| D02 | Unknown (protein for IMAGE:3538275) | 16924319 | 5.8 | 40.8 |
| E02 | ACTB protein | 15277503 | 5.6 | 40.5 |
| E03 | Protein PP4-X | 189617 | 5.6 | 36.3 |
| E04 | Aldehyde dehydrogenase 1A1 | 21361176 | 6.3 | 55.5 |
| F01 | Heat shock 70kDa protein 8 isoform 1 | 5729877 | 5.4 | 71.1 |
| F02 | Cytokeratin 18 (424 AA) | 30311 | 5.3 | 47.3 |
| H01 | Heat shock 70kDa protein 9B precursor | 24234688 | 5.9 | 74 |
| H02 | ER-60 protein | 2245365 | 5.9 | 57.2 |
| H03 | L-arginine- glycine amidinotransferase | 1085329 | 6.6 | 45.3 |
| H04 | Calreticulin precursor | 4757900 | 4.3 | 48.3 |
| A05 | Transferrin | 4557871 | 6.9 | 77 |
| B05 | ER-60 protein | 2245365 | 5.9 | 57.2 |
| B06 | Pro-apolipoprotein | 178775 | 5.4 | 28.9 |
| C05 | Keratin 8 | 4504919 | 5.5 | 53.7 |
| C06 | Tropomyosin 4 | 4507651 | 4.7 | 28.6 |
| D05 | Cytokeratin 18 | 30311 | 5.3 | 47.3 |
| D06 | Tropomyosin 1 | 27597085 | 4.7 | 32.9 |
| E05 | Alpha 1 actin precursor | 4501881 | 5.2 | 42.4 |
| F05 | ACTB protein | 15277503 | 5.6 | 40.5 |
| F06 | Vimentin | 340234 | 4.7 | 35.1 |
| G05 | Actin, gamma 2 pro-peptide | 4501889 | 5.3 | 42.3 |
| G06 | Unnamed protein product | 21757045 | 5 | 52.5 |
| H06 | Vimentin | 2119204 | 5.1 | 53.7 |
Immunohistochemistry protein validation
Limited validation study was carried on several significantly differentially expressed proteins between normal, pancreatitis and invasive pancreatic adenocarcinoma, Table 1 that included ANXA1, pancreatic lipase, and ALDH1A1. These markers were selected because they were either identified previously as pancreatic biomarkers or associated with invasive cancer resistance to therapy [13,14]. The immunohistochemistry of Aldehyde dehydrogenase 1A1 (ALDH1A1), pancreatic lipase and Annexin (ANXA1) was classified according to the score methods described in the Materials and Methods section. Representative photographs of each protein staining in tissues are shown in Figure 3. The immunoreactivity is determined by taking into account the intensity of the signal and the percentage of marker- stained cells. None of the normal or tissue sections incubated with no antibody showed immunoreactivity. However, strong staining is shown in the cytoplasm of cancer as well as pancreatitis tissues. Overall expression of ANXA1, Lipase and ALDH1A were significantly increased in cancer cells compared to normal tissues (Figure 3).
Figure 3:
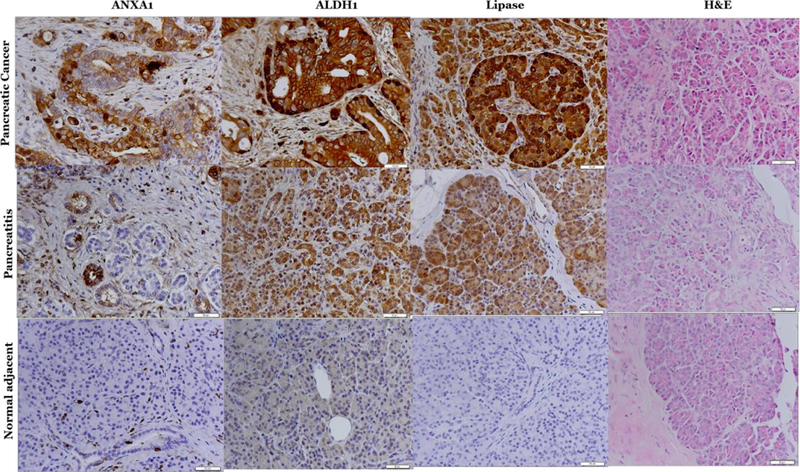
Immunohistochemistry validation of Annexin, ALDH1A1 and pancreatic lipase in pancreatic cancer, pancreatitis and normal tissues.
Discussion
Pancreatic cancer, now the fourth leading cause of cancer deaths in the United States for which the 5-year survival rates are 3% [15], is often dubbed the silent killer because it seldom causes symptoms until advanced. As a result, few pancreatic cancers are operable at diagnosis and surgical excision cures only 10–15% of patients [16]. Furthermore, pancreatic cancer is often resistant to conventional radiotherapy or chemotherapy [17,18]. Not surprisingly, only 1–4% of patients with pancreatic adenocarcinoma survive 5 years past diagnosis [17]. Recent clinical trials with gemcitabine hydrochloride produced only modest clinical benefits and marginally increased survival in patients with advanced pancreatic cancer [19]. Much has been learned about the genetics and pathogenesis of pancreatic cancer from studies of biopsy samples from cancer patients, cell lines, and mouse models. However, successful treatment requires more reliable biomarkers of the disease.
In this study, we used LCM, 2-dimensional gel electrophoresis and data analysis using normal, pancreatitis, and pancreatic adenocarcinoma to identify specific and sensitive biomarkers for detection of pancreatic cancer. Our study has improved on the limited proteomic-based approach in pancreatic cancer used previously [20]. We have use LCM (AutoPix automated system) to procure homogeneous tumor cells from pancreatic carcinoma and cells from normal or inflamed pancreas. Proteins extracted from dissected cells were separated in 2D-PAGE and altered proteins in expression were subsequently identified by mass spectrometry and database search. Using this approach, we have identified several proteins that were overexpressed in pancreatitis and tumor compared to normal pancreas tissues. To our surprise the proteomic landscape of pancreatitis is similar to the landscape of cancer. A previous proteomic study by Chen [21] found that about 40% of the 116 differentially expressed proteins identified in chronic pancreatitis were also involved in pancreatic cancer. Upon validation of these differentially expressed proteins between pancreatitis and cancer, cathepsin D, integrin 1, and plasminogen were found to over express in both pancreatic cancer and chronic pancreatitis, while annexin A2 and insulin-like growth factor-binding protein 2 were overexpressed in cancer but not in chronic pancreatitis. In a different study comparing the proteome ofpancreatic intraepithelial neoplasia tissues with chronic pancreatitis, more than 25% of the overexpressed proteins identified were also overexpressed in chronic pancreatitis [22]. These studies and our study suggest that pancreatitis and pancreatic cancer share many protein signatures.
Due to the close similarities, our study did not examine makers that deregulated in pancreatitis vs. cancer; however, we identified those differentially expressed between pancreatic adenocarcinoma vs. normal pancreas tissues. We identified 34 proteins to be differentially expressed (Table 1). We validated proteins in normal, pancreatitis, and pancreatic cancer tissues using immunohistochemistry. One of the proteins we validated is pancreatic lipase. Pancreatic lipase is an enzyme that catalyzes the breakdown of fatty acids and glycerol and it is routinely used for diagnosis of pancreatic cancer [23]. In patients with intraductal papillary mucinous neoplasm of pancreas without a history of pancreatitis, serum pancreatic enzymes outside of the normal range are associated with a greater risk of malignancy. A positive correlation between the levels of serum pancreatic lipase in patient with history of pancreatitis and the presence of malignancy were found [24]. The findings of this study in addition to previous reports suggest that pancreatic enzymes such as lipase may serve as useful markers in patients with pancreatitis to denote high risk of pancreatic cancer.
The second marker we selected to further investigate is Annexin A (ANXA). ANXA is a Ca2+-binding protein over-expressed in pancreatic cancer. Annexins are cytosolic or associated with the membrane or the cytoskeleton in a calcium-dependent manner [25]. Annexin I is a steroid-regulated protein and therefore involved in some actions of glucocorticoids, such as inhibition of cell proliferation, anti-inflammatory effects, the regulation of cell migration, differentiation, death and the hypothalamic-pituitary axis [26–30] Recently, it was reported that extracellular ANXA1 mediates pancreatic cancer cell motility acting on formyl peptide receptors [31]. Its expression was found to be increased by 325% in micro dissected pancreatic cancer nests compared to normal pancreatic tissues [32]. A study by Xiao- Feng Bai et al. suggested that because annexin I is a substrate protein of EGFR, activated EGFR pathway promotes the annexin I up-regulation and might associate with pancreas malignant transformation [25].
The third protein we identified is aldehyde dehydrogenase 1A1 (ALDH1A1). ALDH1A1 is a member of a family of enzymes that oxidize a wide range of endogenous and exogenous aldehydes to their corresponding carboxylic acids [33]. The high expression of ALDH1A1, one of the isoforms, is associated with more aggressive tumors and worse outcomes in bladder, ovarian, lung, prostate and pancreatic cancers [34–37]. In pancreatic cancer patients, the expression of ALDH1A1 in tumors is associated with a poorer survival rate [37,38]. Recently, ALDH1A1 has been described as a prognostic marker in a pancreatic cancer tissue microarray (38). Additionally, a recent study from Surendra Singh et al. revealed that ALDH1B1 is also expressed at very high levels in human pancreatic cancer and contributes to the proliferation in these tumor cells [39]. ALDH1A1 has also been suggested as a cancer stem cell (CSC) marker in a number of cancers [37,38]. CSCs are a population of tumor cells that are known to drive malignant growth, chemo resistance, and metastases. It is thought that high levels of ALDH1A1 can provide drug protection and radiation resistance to CSCs [39]. Therefore, ALDH1A1 specific targeted therapy may be a useful therapeutic target in pancreatic cancer.
Conclusion
In conclusion, the significant factor for the poor prognosis of pancreatic cancer may be attributed to its biological aggressiveness, the difficulty of early diagnosis, and poor response to conventional therapeutics. Additionally, pancreatic masses are sometimes indistinguishable from chronic pancreatitis or benign pancreatic cysts when biopsy is obtained from the lesion. For these reasons, we decided to identify markers differentially expressed between cancer vs. normal pancreas tissues. The comparisons of protein expression profiles of pancreatic cancer and pancreatitis and normal pancreas using laser capture microdissection, 2- dimensional electrophoresis, tandem mass spectrometry (MS/MS) provided important information about the molecular characteristics and revealed some new specific or associated biomarkers of pancreatic cancer including pancreatic Lipase, annexin A1 and ALDH1A1. Our study confirmed these proteins as biomarkers for early detection of pancreatic cancer. Understanding the role of these specific proteins and their mechanistic action will give insights into their involvement in pancreatic cancer.
Acknowledgements
The authors thanks Dr. Frank Weizmann, Cellular and Integrative Physiology, Indiana University School of Medicine for performing the MALDI-TOF analysis.
Funding
The study is funded by NIH Grant number 5r03CA119310–2.
References
- 1.Ilic M, Ilic I (2016) Epidemiology of pancreatic cancer. World J Gastroenterol 22: 9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong D, Ko AH, Hwang J, Venook AP, Bergsland EK, et al. (2008) Serum CA19–9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas 37: 269–274. [DOI] [PubMed] [Google Scholar]
- 3.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, et al. (2006) ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 24: 5313–5327. [DOI] [PubMed] [Google Scholar]
- 4.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, et al. (2009) CA19–9 serum levels in obstructive jaundice: Clinical value in benign and malignant conditions. Am J Surg 198: 333–339. [DOI] [PubMed] [Google Scholar]
- 5.Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 3: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, et al. (2008) Postresection CA 19–9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. J Clin Oncol 26: 5918–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duraker N, Hot S, Polat Y, Hobek A, Gengler N, et al. (2007) CEA, CA 19–9 and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol 95: 142–147. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Yang J, Li H, Wu Y, Zhang H, et al. (2015) Tumor markers CA19–9, CA242 and CEA in the diagnosis of pancreatic cancer: A meta-analysis. Int J Clin Exp Med 8: 11683–11691. [PMC free article] [PubMed] [Google Scholar]
- 9.Herreros-Villanueva M, Bujanda L (2016) Non-invasive biomarkers in pancreatic cancer diagnosis: What we need vs. what we have. Ann Transl Med 4: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisiel JB, Raimondo M, Taylor W, Yab TC, Mahoney DW, et al. (2015) New DNA methylation markers for pancreatic cancer: Discovery, tissue validation, and pilot testing in pancreatic juice. Clin Cancer Res 28: 2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat K, Wang F, Ma Q, Li Q, Mallik S, et al. (2012) Advances in biomarker research for pancreatic cancer. Curr Pharm Des 18: 2439–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Belle W, Anensen N, Haaland I, Bruserud O, H0gda KA, et al. (2006) Correlation analysis of two-dimensional gel electrophoretic protein patterns and biological variables. BMC bioinfo 7: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruckert F, Pilarsky C, Grutzmann R (2010) Serum tumor markers in pancreatic cancer: Recent discoveries. Cancers (Basel) 2: 1107–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SK, Kim H, Lee DH, Kim TS, Kim T, et al. (2013) Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy- resistant cancer cells. PloS one 8: 78130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 16.Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, et al. Treatment and survival in 13560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: An epidemiological study. Br J Surg 82: 111–115. [DOI] [PubMed] [Google Scholar]
- 17.Wolff RA, Chiao P, Lenzi R, Pisters PW, Lee JE, et al. Current approaches and future strategies for pancreatic carcinoma. Invest New Drugs 18: 43–56. [DOI] [PubMed] [Google Scholar]
- 18.Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, et al. (2001) Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: Treatment variables and survival duration. Ann Surg Oncol 8: 123–132. [DOI] [PubMed] [Google Scholar]
- 19.Alberts SR, Schroeder M, Erlichman C, Steen PD, Foster NR, et al. (2004) Gemcitabine and ISIS-2503 for patients with locally advanced or metastatic pancreatic adenocarcinoma: A north central cancer treatment group phase II trial. J Clin Oncol 22: 4944–4950. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Person MD, Zhu J, Abbruzzese JL, Li D (2004) Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res 64: 9018–9026. [DOI] [PubMed] [Google Scholar]
- 21.Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, et al. (2007) Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics 6: 1331–1342. [DOI] [PubMed] [Google Scholar]
- 22.Pan S, Brentnall TA, Kelly K, Chen R (2013) Tissue proteomics in pancreatic cancer study: Discovery, emerging technologies and challenges. Proteomics 13: 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hameed AM, Lam VW, Pleass HC (2015) Significant elevations of serum lipase not caused by pancreatitis: A systematic review. HPB 17: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roch AM, Parikh JA, Al-Haddad MA, DeWitt JM, Ceppa EP, et al. (2014) Abnormal serum pancreatic enzymes, but not pancreatitis, are associated with an increased risk of malignancy in patients with intraductal papillary mucinous neoplasms. Surgery 156: 923–930. [DOI] [PubMed] [Google Scholar]
- 25.Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, et al. (2004) Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol 10: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flower RJ, Rothwell NJ (1994) Lipocortin-1: Cellular mechanisms and clinical relevance. Trends Pharmacol Sci 15: 71–76. [DOI] [PubMed] [Google Scholar]
- 27.Parente L, Solito E (2004) Annexin 1: More than an anti-phospholipase protein. Inflammation Res 53: 125–132. [DOI] [PubMed] [Google Scholar]
- 28.Perretti M, Gavins FN (2003) Annexin 1: An endogenous anti-inflammatory protein. Physiol Sci 18: 60–64. [DOI] [PubMed] [Google Scholar]
- 29.De Coupade C, Solito E, Levine JD (2003) Dexamethasone enhances interaction of endogenous Annexin 1 with L-selectin and triggers shedding of L-selectin in the monocytic cell line U-937. Br J Pharmacol 140: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckingham JC, Flower RJ (1997) Lipocortin 1: A second messenger of glucocorticoid action in the hypothalamo-pituitary-adrenocortical axis. Mol Med Today 3: 296–302. [DOI] [PubMed] [Google Scholar]
- 31.Belvedere R, Bizzarro V, Forte G, Dal Piaz F, Parente L, et al. (2016) Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of formyl peptide receptor pathway. Sci Rep 6: 29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, Zhang D, Jia Q, Li T, Zhang W, et al. (2009) Proteomic and tissue array profiling identifies elevated hypoxia-regulated proteins in pancreatic ductal adenocarcinoma. Cancer Invest 27: 747–755. [DOI] [PubMed] [Google Scholar]
- 33.Vasiliou V, Pappa A, Petersen DR (2000) Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact 129: 1–9. [DOI] [PubMed] [Google Scholar]
- 34.Deng S, Yang X, Lassus H, Liang S, Kaur S, et al. (2010) Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PloS one 5: 10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, et al. (2009) Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 7: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, Su Y, Mei Y, Leng Q, Leng B, et al. (2010) ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Investig 90: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, et al. (2010) Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst 102: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahlert C, Bergmann F, Beck J, Welsch T, Mogler C, et al. (2011) Low expression of aldehyde deyhdrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer. BMC Cancer 11: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Arcaroli JJ, Orlicky DJ, Chen Y, Messersmith WA, et al. (2016) ALDH1B1 as a modulator of pancreatic adenocarcinoma. Pancreas 45: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


