Figure 1. A schematic illustration of the expression, glycosylation modification, lysosomal targeting and secretion of human lysosomal acid lipase (LAL) based on experimental evidence and knowledge on other soluble lysosomal hydrolases.
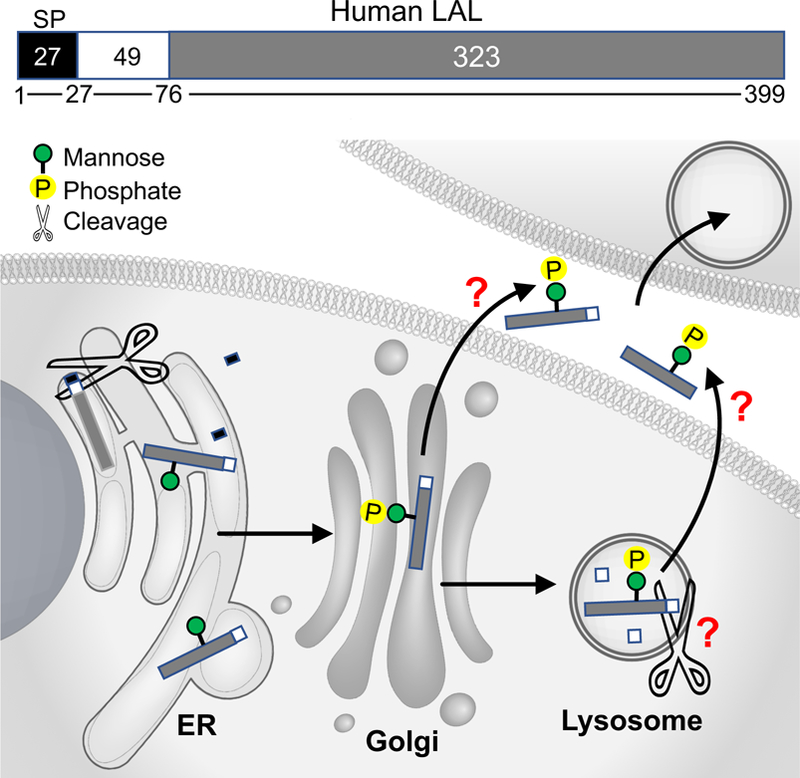
Human LAL consists of 399 amino acids (AAs).9 The first 27 AAs are predicted to be the signal peptide (SP) sequence.9 In many lysosomal proteins, the signal peptide directs their co-translational translocation to the endoplasmic reticulum (ER) for cleavage by the signal peptidase,11 although the role of signal peptide sequence in LAL has not been experimental confirmed. The proprotein of 372 AAs after signal peptide cleavage undergo co-translational/post-translational glycosylation modification in the ER,9, 48 and then transport to Golgi for the addition of mannose 6-phophate (M6P),15 which is important for lysosomal targeting of the hydrolase. It is postulated that the 372 AA proprotein undergoes cleavage of the first N-terminal 49 AAs to form the mature lipase of 323 AAs, which is enzymatically active as suggested by size-exclusion chromatography.9 Extracellular LAL with M6P modification can be taken up by other cells through M6P receptor-mediated endocytosis, and remain catalytically active.15–17 The functional significance of signal peptide sequence, the intracellular location where the proprotein cleavage occurs, and the secretory pathway of mature LAL remain to be further characterized.
