Figure 2. LAL–mediated lysosomal lipolysis links lipid metabolism to diverse cellular functions.
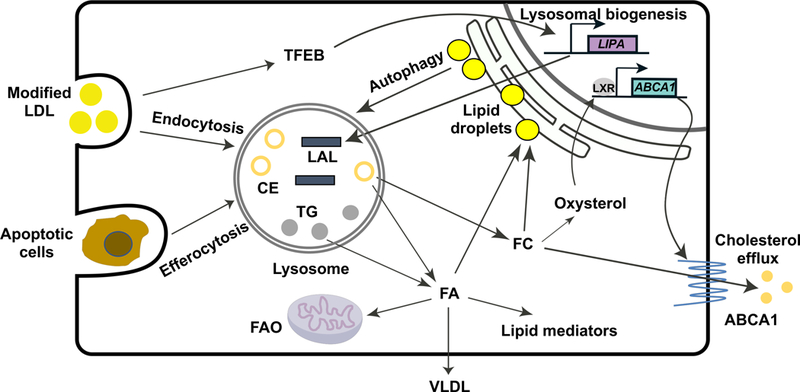
LAL hydrolyzes cholesteryl ester (CE) and triglyceride (TG) in the lysosome to release fatty acids (FAs) and free cholesterol (FC). Modified low-density lipoprotein (LDL) internalized through scavenger receptor-mediated endocytosis is an important source of CE and TG for lysosomal hydrolysis. The hydrolyzed FAs and FC can be re-esterified and form lipid droplets in the endoplasmic reticulum (ER) for storage. Lipid droplets can be delivered to the lysosome for LAL-mediated hydrolysis via autophagy to provide energy supply and maintain cellular homeostasis. The engulfed apoptotic cells by macrophages through a process called efferocytosis also deliver neutral lipids to the lysosome, and LAL is essential for maintaining the efferocytosis capacity of macrophages.29 The lipolytic products of LAL have active biological roles. Hydrolyzed FAs are substrates for fatty acid oxidation (FAO) and synthesis of very low-density lipoprotein (VLDL).32 CE-derived FAs also provide precursors for the systhesis of lipid mediators that have a broad spectrum of functional impact on inflammatory response and resolution.26 Lysosomal flux of FC is essential for the production of oxysterol that triggers the activation of liver-X-receptor (LXR),28 resulting in increased ABCA1 expression and cholesterol efflux to remove cholesterol from the cells. How is LIPA expression regulated remains incompletely understood. Under conditions of lysosomal stress induced by atherogenic lipids such as oxidized-LDL, transcription factor EB (TFEB) triggers lysosomal biogenesis, including increased expression of the LIPA gene.42 ABCA1, ATP-binding cassette transporter A1.
