Abstract
Purpose
To investigate compositional changes of knee cartilage at the site of newly appearing cartilage lesions and the surrounding cartilage 1–4 years prior to lesion onset using quantitative T2-measurements.
Methods
Fifty-seven cartilage plates with newly appearing cartilage lesions from 45 knees (cases) and 52 plates from 26 control knees from the OAI cohort (controls) were evaluated. Using MRI T2-mapping, composition of local (the site of future lesions) and surrounding cartilage (remainder of the cartilage plate) was assessed 1–4 years prior to lesion onset. Analogous cartilage ROIs in control plates without cartilage lesions were assessed over 1–4 years. Mixed models were used to compare T2-means and change rates between local and surrounding cartilage within cases and controls, and to compare change rates in local and surrounding cartilage between cases and controls, adjusting for covariates.
Results
Four years prior to lesion onset, we found that local cartilage ROIs had higher T2-values compared to the surrounding cartilage. No such differences were found in control plates. In cases mean local T2-values were persistantly elevated compared to the surrounding cartilage prior to lesion onset reaching significance 1 year prior (+2.94ms, p=0.012). T2-values of the surrounding cartilage were also persistantly higher in cases compared to controls, reaching significance 2 years prior to lesion onset (+3.61ms, p=0.003).
Conclusion
The findings of our study support the concept of compositional cartilage changes as a mechanism for cartilage degradation and that both diffuse and focal changes of cartilage composition within a cartilage plate precede the development of cartilage lesions.
Keywords: Osteoarthritis, knee, cartilage, T2 mapping, MRI
Introduction
Knee osteoarthritis (OA) is a chronic degenerative joint disease involving all anatomical components of the joint.1 The osteochondral unit, however, plays a crucial role in the onset and progression of joint degeneration.2 Due to the low regenerative capacity of cartilage tissue 3, cartilage lesions progress over time once they are macroscopically present. Therefore, preventive interventions such as modification of risk factors or correction surgery 4 would ideally be initiated before the onset of macroscopic lesions. Early changes in cartilage composition such as content and integrity of the collagen component can be analyzed with quantitative MRI T2 relaxation time measurements 5–7. Increased T2 relaxation time measurements have been shown to be associated with risk factors for the development of OA such as age, sex, obesity and injuries 8–10 and are predictive of the clinical onset of radiographic OA and knee pain.11–13
While the cited data 8–13 were based on average values of T2 of completely segmented cartilage of the individual compartmental regions (e.g. medial tibial plate) of a knee joint, little is known about the regional distribution of compositional changes within a compartment during the onset and progression of OA. Cross-sectional studies describing the physiological laminar pattern of T2 relaxation time in the cartilage layers of knees with OA found a heterogenous distibution of T2 values whitin an articular plate with focal increases in T2 within macroscopic cartilage lesions14–16. In a recently published study, visually assessed focal morphological signal abnormalities of cartilage in intermediate weighted morphological sequences, equivalent to grade 1 lesions of the Whole-Organ Magnetic Resonance Imaging Score (WORMS), were found to be associated with a significantly increased risk for the later onset of macroscopic lesions.17 No published in vivo data, however, exists about the regional distribution of quantitative cartilage T2 values prior to the onset of focal cartilage lesions and whether the onset of cartilage lesions is preceded by focal elevations of cartilage T2 values.
Therefore the aim of our study was to investigate the extent to which newly appearing cartilage lesions are preceded by: (i) focal cartilage structural breakdown indicated by local changes of cartilage T2 composition at the site of the future focal lesion; (ii) changes in cartilage composition in the plate surrounding the site of the future focal lesion; and (iii) to compare the regional heterogeneity and rate of change in cartilage T2 in plates that develop new lesions with corresponding control plates that do not develop cartilage structural lesions in the same time interval.
Materials and Methods
Patient selection
We studied knees from the Osteoarthritis Initiative (OAI), a longitudinal, observational multicenter study of the natural evolution of knee OA with 4796 participants sponsored by the National Institutes of Health. The study was compliant with the Helsinki Declaration. All subjects included in this study provided informed consent. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards. For the present study, we used our database of WORMS scorings of 1217 right knees of OAI participants. Complete WORMS scorings of the time points baseline, 12, 24, 36, 48 and 72 months follow up contained in the database were performed for several published analyses.10, 18–22 Right knees of the incidence and progression subcohort with a reading of an MRI acquired at the 48 and/or 72 month clinic visit, and a Kellgren-Lawrence grade <=2 to ensure that sufficient cartilage quantity was present for quantitative MRI measurements, were eligible for the present study (See Figure 1 for subject selection diagram).
Figure 1:

Illustration of T2 analysis: The newly appearing partial thickness lesion (b, white arrow) is segmented with a red ROI allways including all layers of the cartilage (d). The surrounding cartilage including the the entire articular plate of the same anatomic region is segmented with the yellow ROIs (d). ROIs of local and surrounding cartilage are than copied to the same slice of the prior examination (c).
We then identified knees with new cartilage lesions in any plate (e.g. lateral tibia, medial tibia, etc.) by comparing cartilage WORMS scores in the reading database from the 48 month and/or 72 month follow-up with the baseline studies. A cartilage lesion was defined as new if the baseline WORMS score changed from 0 (no lesion) or 1 (focal signal change) to a WORMS grade ≥2 at the latest follow up. 107 knees met these criteria. The MRIs from baseline to the latest follow-up were then visually re-assessed by two radiologists (MK with 13 years and BJS with 4 years of experience, respectively) to ensure that the new lesion was the only lesion in the plate and that it was clearly visible in two planes and located in the central and not marginal slices of the articular plate so that partial volume effects were minimized for the signal quantification in T2 analysis. Of these potential cases, 62 were excluded during visual review because lesions were too subtle (e.g. superficial contour irregularities graded as WORMS 2 lesions) to be segmented or because of a marginal position in the articular plate likely to cause a substantial partial volume effect in the T2 maps. Two potential cases were excluded because additional preexisting lesions were located at different sites in the same articular plate. The resulting sample consisted of 45 right knees that developed in total 59 new cartilage lesions in 59 plates during the observation period. Because of imaging artifacts in MESE sequences (movement artifacts, high noise and blurry contours), 2 plates with new lesions were excluded, leaving 57 plates for analysis (Table 1, Suppl. Figure 1). In these knees with a new cartilage lesion we analyzed the MRI studies from all available timepoints (0 (baseline), 12, 24, 36, 48 and 72 months visits as described in the study protocol of the OAI www.oai.ucsf.edu) to determine the follow-up interval in which each new lesion occurred and to account for the fact that some knees may have developed a new lesion in more than one plate during the observation period.
Table 1:
Cartilage plate and subject characteristics of cases and controls at lesion onset
| Characteristic | Plates with new lesions | Control plates without new lesion | p-value for difference |
|---|---|---|---|
| Number of plates | 57 | 52 | |
| Lesion grade (WORMS) | N | N | |
| Grade 0 | 0 | 44 | |
| Grade 1 | 0 | 8 | |
| Grade 2 | 28 | 0 | |
| Grade 2.5 | 1 | 0 | |
| Grade 3 | 18 | 0 | |
| Grade 4 | 4 | 0 | |
| Grade 5 | 6 | 0 | |
| Lesion grade* | 2.80 (0.97) | 0.15 (0.36) | |
| Compartments: | |||
| MFC** | 17 (30) | 15 (29) | |
| MT | 9 (16) | 8 (15) | |
| LFC | 12 (21) | 11 (21) | |
| LT | 14 (25) | 13 (25) | |
| PAT | 3 (5) | 3 (6) | |
| TRO | 2 (3) | 2 (3) | |
| Number of knees/subjects | 45 | 26 | |
| Sex n (%) female | 36 (80) | 21 (80) | |
| Age * | 59.3 (5.9) | 58.7 (5.8) | 0.102 |
| BMI* | 28.7 (4.1) | 23.9 (3.3) | <0.001 |
| KL grade* | 1.13 (1.02) | 0.22 (0.42) | <0.001 |
numbers are mean (SD)
n (%), BMI = body mass index, KL = Kellgren- Lawrence score
To control for a possible bias related to the magic angle effects or loading effects on the T2 quantification of cartilage, we selected control plates with healthy cartilage and a comparable spatial orientation of cartilage from 105 subjects who were participants in the OAI “normal reference” cohort and who had MRI readings at the 48 months clinical visit. Participants in this group at baseline were without knee pain, radiographic knee OA and knee OA risk factors. Since T2 values of healthy cartilage show natural variations between compartments 23 the control plates were frequency matched to cases to control for a compartment related bias (e.g. lateral tibia, patella). They were also frequency matched by sex, and age. This required selection of multiple control plates from some of the knees to achieve similar numbers of each plate in the two groups. We selected 54 control plates without cartilage lesions (WORMS Grade < 2) from 26 knees. Two plates were excluded because of technical insufficient T2 images, leaving 52 from 54 lesion plates for analysis (Table 1).
Radiographs
Radiographs were acquired with a standing postero-anterior fixed flexion knee position as described in detail in the OAI Radiographic Procedure Manual freely accessible at http://www.oai.ucsf.edu. Knee radiographs were analyzed centrally and graded with regard to the degree of joint degeneration using the Kellgren-Lawrence score (KL score).24–26
MR imaging protocol
MR images of the right knee were obtained at one of four sites (Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; and Memorial Hospital of Rhode Island, Pawtucket, RI) using identical 3.0 Tesla scanners (Trio, Siemens) and quadrature transmit-receive coils (USA Instruments, Aurora, Oh, USA). The scan protocol included the following sequences: 1) coronal proton density-weighted fast spin-echo (FSE), echo time (TE) = 29ms, repetition time (TR) = 3700ms, flip angle + 180°; 2) sagittal 3-D dual echo in the steady state (DESS) with selective water excitation TE = 4.7ms, TR = 16.3ms, flip angle = 25°; 3) sagittal intermediate-weighted FSE with fat suppression TE = 30ms, TR = 3200ms, flip angle = 180°; and 4) sagittal T2-weighted multi-echo spin-echo (MESE), TE = 10, 20, 30, 40, 50, 60, 70, TR = 2700. 27 A detailed description of cartilage T2 quantification as performed at our institution was published elsewhere28
Grading of cartilage lesions
Cartilage lesion scores as found in the UCSF-data base were performed for previous studies and obtained through visual morphological grading with a 8-point cartilage subscore of the semi-quantitative Whole-Organ Magnetic Resonance Imaging Score (WORMS) grading system29 in 6 regions (patella, trochlea, the medial and lateral femoral condyle and the medial and lateral tibial plateau). Criteria for the different grades were as follows: 0 = normal thickness and signal intensity, 1 = normal thickness or swelling with abnormal signal on fluid-sensitive sequences, 2 = partial-thickness focal defect < 1 cm in greatest width, 2.5 = full-thickness focal defect < 1 cm in greatest width, 3 = grade 2 defect wider than 1 cm but < 75% of the region, 4 = diffuse (> 75% of region) partial-thickness loss, 5 = multiple areas of full-thickness loss (grade 2.5) or a grade 2.5 lesion wider than 1 cm but < 75% of region, and 6 = diffuse (>75% or region) full-thickness loss. Cartilage showing signal changes without defects (grade 1) was considered as normal.
T2 relaxation time measurements
We used T2 relaxation time measurements to assess the biochemical properties of the “local” focal cartilage area that later degraded to a structural lesion. The “local” cartilage area in plates prior to the development of new lesions was defined as the exact location and spatial extension of the later lesion within the normal appearing cartilage in the preceding MRI examinations. The co-localization of lesion location and extension in MRIs of different time points was performed as follows: Firstly cartilage lesions were segmented by two radiologists (MK and BJS who segmented a comparable number of cases and controls to avoid a segmentor related bias) in all slices on the first echo of the sagittal 2-D multi-echo SE sequence using an in-house developed spline-based, semi-automated software segmentation algorithm in MATLAB (MathworksInc, El Segundo, CA).30,31 In a second step the ROI of the lesion was transferred to the cartilage in the same sequence of the preceding MRIs. This transfer was done without co-registration of images so that in most cases the position of the ROI needed to be manually corrected with visual comparison to the images side by side (Figure 1). The segmentation of the “surrounding” cartilage included all the remaining clearly distinguishable cartilage of the articular plate of one of the following anatomical regions: medial (MFC) or lateral femoral condyle (LFC), medial (MT) or lateral tibia (LT), patella (PAT) or trochlea (TRO) 28 (Figure 1). In control plates the same analysis was performed as described for plates with lesions. We then defined “local” cartilage ROI in the control plates of each anatomic region so that their size and location had a similar distribution as the size and location of the “local” cartilage ROI in the case plates of the same anatomic region. The “surrounding” cartilage in control plates was defined in the same manner as for the case plates.
Cartilage mean T2 relaxation times at the “local” site of the later appearing lesion and the “surrounding” region of the entire articular plate were analyzed in all available MRIs obtained 1 to 4 years prior to the time-point when the lesion first appeared. We limited the maximum number of follow-up to 4 years prior to lesion onset since no reasonable sample size could be obtained beyond this range of years. For the description of the methodology we purposely chose a different nomenclature for the OAI clinical visits (0–72 months according to the OAI protocol) and the follow-up intervals prior to lesion onset (1–4 years) in our study to clearly differentiate between the time axis of the OAI study and the reverse (backward) time axis of our analysis prior to lesion onset. For each knee, the number of prior timepoints with T2 data and the duration of time prior to lesion onset varied depending on the time-point at which the lesion first appeared and the availability of T2 data from prior time-points. For a lesion that first appeared on the 72 months OAI visit the cartilage quality analysis could include MRIs obtained 2 (48 months OAI visit), 3 (36 months OAI visit), and 4 (24 months OAI visit) years prior to the MRI on which the lesion first appeared. For a lesion that first appeared on the 12 months OAI visit, cartilage composition could be analyzed only 1 year (baseline/0 month OAI visit) prior to onset. As a consequence the number of plates in the analysis is different for each time-point.
Statistical analysis
Statistical analysis was performed using JMP version 11 (SAS Institute, Cary, NC, USA) and STATA version 14 software (StataCorp LP, College Station, TX, USA). Descriptive statistics were used to characterize the study groups (cases with newly developing lesions and normal controls). Differences between groups at baseline were tested with the Wilcoxon signed rank test. To account for multiple measurements per knee, mixed random effects models were used to compare mean cartilage T2 values between local cartilage and the surrounding cartilage in plates developing lesions and in control plates and to compare T2 values of the local cartilage and of the surrounding cartilage between plates developing lesions and controls. Mixed random effects models accounting for multiple measurements per knee were used to compare the rates of change in T2 values between the local and surrounding cartilage in each group (case and controls) and between groups. All models were adjusted for age, sex, BMI and KL-grade.
Results
Subject characteristics
As a result of the frequency matching both study groups (subjects with new cartilage lesions vs. controls) had the same gender distribution (80% female), a similar distribution of anatomic location of cartilage plates, and a similar age (59.3±0.59y vs. 58.7±0.59y, p>0.05). Knees of plates with newly appearing lesions had slightly higher KL scores (mean KL score: 1.13±1.02 vs. 0.22±0.42, p<0.05) and subjects with newly appearing lesions had a higher BMI (mean BMI: 28.7±4.1 vs. 23.9±3.3, p<0.05) at the time point of lesion onset compared to the 48 month OAI visit of the controls (Table 1).
Cartilage lesions
Of the 57 new cartilage lesions studied, most occurred in the MFC (30%) and the LT (25%), followed by the LFC (21%),the MT (16%), the patella (5%) and the trochlea (3%) (Table 1). Eleven of 45 knees (24%) showed multiple lesions in different plates, ranging from 2 (10 knees) to 3 lesions (1 knee). The severity of new cartilage lesions ranged from focal partial thickness lesions (grade 2) to large full thickness lesions (grade 5). The majority of lesions (88%) showed a partial thickness pattern (grades 2,3,4), while 12% were full thickness lesions (grades 2.5 and 5). There were no Grade 6 lesions (diffuse full thickness loss). Mean lesion score was 2.80±0.97. Twenty four lesions occurred at 72 months, 10 at 48 months, 10 at 36 months, 5 at 24 months and 8 at 12 months time point. As a result 34 lesions could be followed back for 4 years, 10 for 3 years 5 for 2 years and 8 for 1 year. Thirty-six new lesions (64%) were preceded by focal signal alterations (WORMS grade 1) one year prior. Of the 52 control plates 8 plates (15%) showed focal signal alterations in the cartilage (WORMS grade 1). The mean lesion score of the control plates was 0.15±0.36.
T2 relaxation values in local and surrounding cartilage prior the onset of focal cartilage lesions
In plates developing new lesions, mean local T2 values were persistently elevated compared to the surrounding cartilage with differences ranging from 0.69ms (p=0.488) 4 years prior to 2.94ms (p=0.012) 1 year prior to lesion onset (Table 2, Figures 2 and 3, suppl. Figure 2). In contrast T2 values of the local ROIs of control plates were lower compared to the surrounding cartilage of the controls, a difference which was only significant four years prior to lesion onset. Differences ranged from −2.35ms (p=0.043) four years prior to −0.07ms (p=0.955) two years prior to lesion onset (Table 2). Compared to controls, T2 values of the surrounding cartilage were persistently elevated in the plates with newly appearing lesions. Differences were most pronounced 2 to 1 year prior to lesion onset (2.17ms [1 year prior] to 3.61ms [2 years prior]) reaching significance 2 years prior to lesion onset (p=0.003) (Table 2). Also the local cartilage T2 values of new lesion cases were constantly higher compared to the local cartilage ROI of controls. Differences increased from four years prior (4.24ms, p=0.103) to one year prior (9.50ms, p<0.0001) (Table 2).
Table 2:
Cartilage T2 relaxation times prior to the onset of cartilage lessions
| local vs surrounding cartilage in cases and controls |
||||||||
|---|---|---|---|---|---|---|---|---|
| cases |
controls |
|||||||
| Time point | local | surrounding | difference | p-value | local | surrounding | difference | p-value |
| 1 year prior | 38.75 (1.1)* | 35.79 (1.1)* | 2.94 (0.6−52)** | 0.012 | 33.23 (0.9) | 34.31(0.9) | −1.00 (−3.1−0.9) | 0.289 |
| 2 years prior | 35.67 (1.0) | 34.92 (1.0) | 0.74 (−0.8 − 2.3) | 0.418 | 32.62 (0.9) | 32.69 (0.9) | −0.07 (−2.4−2.3) | 0.955 |
| 3 years prior | 35.68 (1.1) | 35.24 (1.1) | 0.44 (−1.7−2.6) | 0.691 | 31.20 (1.1) | 32.07 (1.1) | −0.86 (−3.3−1.6) | 0.492 |
| 4 years prior | 35.22 (1.2) | 34.52 (1.2) | 0.69 (−1.2−2.6) | 0.488 | 30.82 (1.4) | 33.18 (1.4) | −2.35 (−4.6−0.7) | 0.043 |
| cases vs controls for local and surrounding cartilage |
||||||||
| local |
surroundimg |
|||||||
| Time point | case | controls | difference | p-value | case | controls | difference | p-value |
| 1 year prior | 41.09 (1.0) | 31.58 (1.4) | 9.50 (5.6−13.3) | <0.0001 | 36.11 (1.0) | 33.93 (0.8) | 2.17 (−0.8−5.1) | 0.153 |
| 2 years prior | 36.64 (1.2) | 31.65 (1.1) | 4.99 (1.25−8.74) | 0.009 | 35.59 (0.7) | 31.98 (0.8) | 3.61 (1.2−6.0) | 0.003 |
| 3 years prior | 35.27 (1.3) | 32.05 (1.5) | 3.22 (−1.0−7.5) | 0.137) | 35.08 (0.8) | 32.54 (1.0) | 2.53 (−0.4−5.4) | 0.088 |
| 4 years prior | 35.18 (1.4) | 30.94 (1.5) | 4.24 (−0.86−9.3) | 0.103 | 34.45 (0.9) | 33.77 (1.2) | 0.68 (−2.7−4.1) | 0.693 |
Numbers are least square means (std.err) estimated from Mixed Models and adjusted for multiple measurements (random effects), age, sex, BMI and KL grade).
mean (95% CI).
Figure 2:
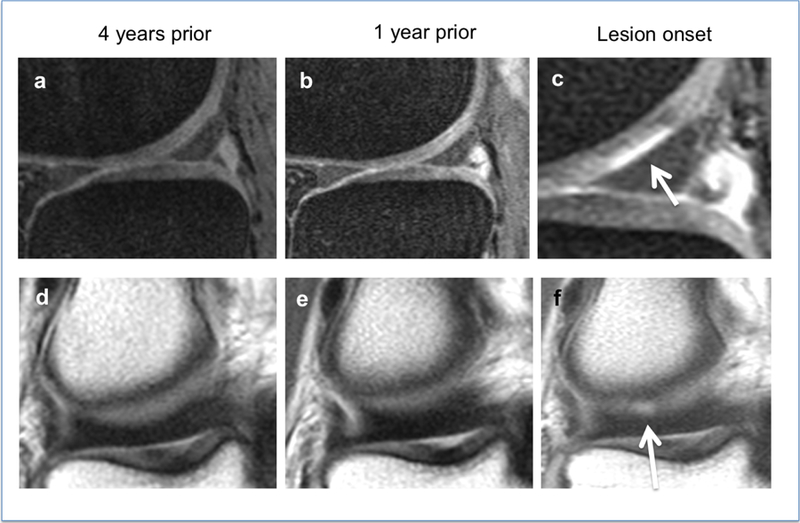
A partial thickness lesion, WORMS grade 2 (white arrows, c and f) develops in the lateral femoral condyle after 4 years. Sagittal intermediate weighted sequences (a–c) and coronal proton-density weighted sequences (d–f).
Figure 3:
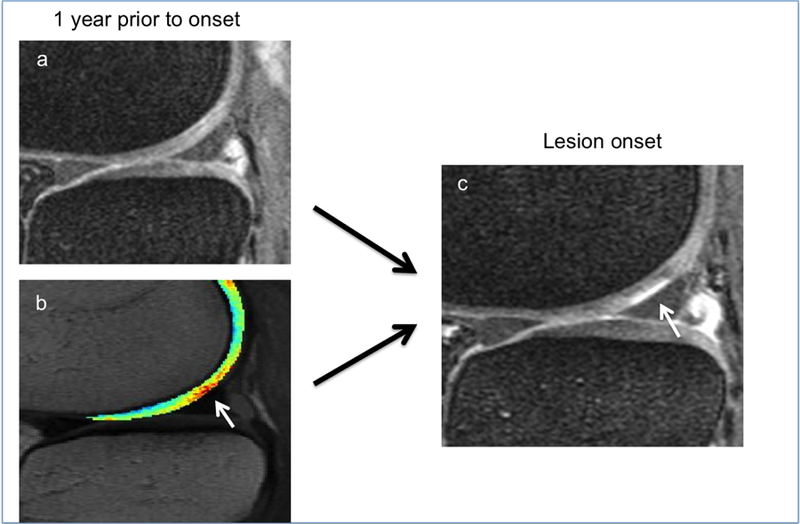
Same lesion as in Figure 2. While the cartilage of the lateral femoral cartilage appears normal in the sagittal fat-saturated intermediate weighted sequence (a), local T2 elevation of the cartilage T2 map (b, white arrow) is demonstrated in the lesion equivalent area 12 months prior to lesion onset (c, white arrow).
Since 64% of cartilage lesions were preceded by focal signal alterations in fluid sensitive sequences (WORMS 1) we compared mean T2 values between local ROIs with WORMS 0 and WORMS 1 grades one year prior lesion onset. Mean T2 of WORMS 1 cartilage was 36.2ms (std.err 1.35, CI=33.51–38.89). Mean T2 of WORMS 0 cartilage was 34.76ms (std.err 0.91, CI=32.94–36.57). The difference of T2 between both WORMS grades was 1.43ms (std.err 1.63, CI=−1.81–4.68), but not significant (p=0.381). The rate of change in WORMS 0 lesions was higher compared to WORMS 1 lesions (0.49ms/y, std.err 0.20, CI=0.08–0.88 vs. 0.29ms/y, std.err 0.27, CI=−0.24–0.83) but differences were not statistically significant (p=0.568).
The rate of change of T2 values in the local cartilage prior to the development of new lesions over 4 years was significantly higher compared to surrounding cartilage of the corresponding plate of controls (0.93ms/y vs. 0.008ms/y, p=0.028) (Table 3, Figure 4). There was no significant difference between the surrounding cartilage of the new lesion cases and surrounding cartilage of controls (0.23ms/y vs. 0.008ms/y, p=0.571) (Table 3). In cases there was a nonsignificant trend for the slope of the T2 change over 4 years to be higher in local cartilage compared to the surrounding cartilage (0.93ms/y vs. 0.23ms/y, p=0.072). In control plates the T2 increase of local ROIs was also higher compared to the surrounding cartilage, but the difference was not significant (0.50ms/y vs. 0.008ms/y, p=0.206).
Table 3:
Comparison of rates of change of cartilage T2 relaxation times from 4 to 1 year prior to the onset of cartilage lesions
| Rate of change of cartilage T2 |
||||
|---|---|---|---|---|
| Study group | case local | case surrounding | control local | control surrounding |
| case local | / | |||
| case surrounding | 0.69 (0.38;–0.06;1.45), 0.072 | / | ||
| control local | 0.42 (0.41;–0.39;1.24), 0.308 | 0.27 (0.41;–0.54;1.10), 0.517 | / | |
| control surrounding | 0.94 (0.42; 0.10:1.77), 0.028 | 0.24 (0.42;–0.59;1.10), 0.571 | 0.51 (0.40;–0.28;1.3), 0.206 | / |
Numbers are differences of rates of change (delta T2 per year) estimated from random effects Mixed Models adjusted for multiple measurements, age, sex, BMI and KL grade. (std.err.;95% CI), p values (level of significance p < 0.05).
Figure 4:
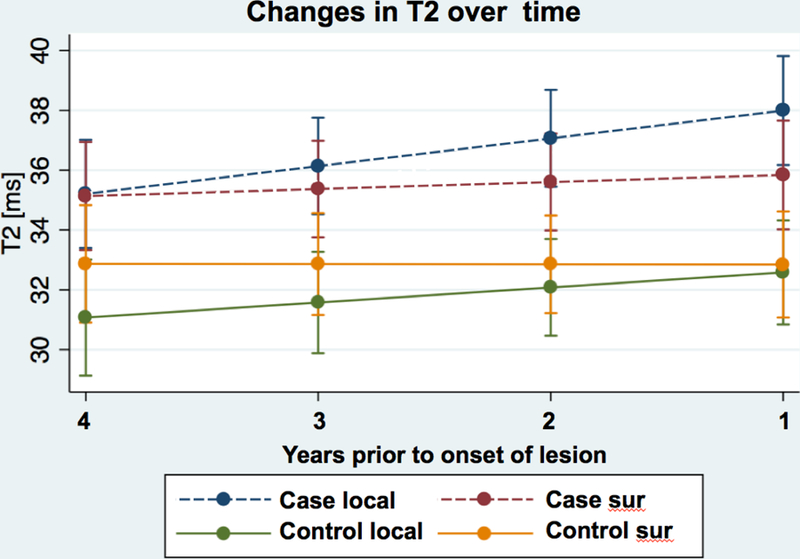
Linear regression of T2 relaxation times of local cartilage and surrounding cartilage in plates with newly appearing cartilage lesions (case) and normal control plates (control) over the course of up to 48 months prior to lesion onset.
Discussion
Analyzing T2 composition of cartilage at the site of newly appearing cartilage lesions one to four years prior to the onset of the lesion, we found that these local cartilage ROIs had higher T2 values prior to the onset of the lesions compared to the surrounding cartilage of the same plate. Moreover, T2 values worsened more rapidly over time prior to lesion onset compared to normal cartilage of controls that did not develop new cartilage lesions.
The value of global T2 measurements of whole compartments to predict clinical onset and progression of knee OA was shown in several studies 11–13. Liebl et al. 11 compared 50 cases with progression of radiographic KL from 0 to ≥ 2 over 4 years with 80 controls showing stable KL 0 grades in the same observation period and found significantly higher T2 values on the compartment level in cases compared to controls. Following 289 subjects of the OAI cohort with risk factors for knee OA over 3 years, Joseph et al. 12 found that increased baseline cartilage T2 of whole compartments predicted longitudinal morphological degeneration of the cartilage, menisci and bone marrow. They also found that baseline heterogeneity of cartilage T2 as measured with texture analysis on the level of compartments predicted progression of joint degeneration. However, these authors did not analyze the regional distribution of cartilage T2 and only used cartilage global T2 quantification as a biomarker for the prediction of OA onset. However, there remains a discrepancy between these global measures of cartilage composition and the fact that structural degeneration of cartilage takes place in a regionally heterogenous pattern with the development of lesions. Our study shows that degradation of already altered cartilage composition is aggravated and accelerated in the location of later appearing structural lesions and thus provides an important link between changes of cartilage composition and the onset of OA.
In a recently published study focusing on morphological cartilage changes prior to the development of cartilage lesions17 Schwaiger et.al. found a significantly increased risk for the development of cartilage lesions (57% in 4 years) in cartilage plates with focal signal inhomogeneities (WORMS 1 lesions) compared to plates with homogenous appearing cartilage (4 %). Similarly, in the present study, we found a substantial higher rate of WORMS grade 1 lesions in plates prior to the development of cartilage lesions (64% 1 year prior lesion onset) compared to controls in the 4 year follow up (15%). Since the influence of WORMS 1 signal alterations in fluid sensitive sequences on T2 quantification is unclear we compared T2 means of WORMS 0 and WORMS 1 graded local cartilage of cases one year prior to lesion onset and found higher T2 values in WORMS 1 lesions, the difference was, however, not statistically significant. A sensitivity analysis with regard to the influence of WORMS 1 signal alterations on the results of our study did not reveal a significant impact.
Findings of ACL tear models suggest a pathophysiological sequence in the development of cartilage lesions 32 showing compositional cartilage changes prior to the onset of macroscopic cartilage lesions 33 followed by structural lesions 2.2 years post ACL tear 34. As the findings of our study indicate that both focal and generalized processes of biochemical cartilage degradation occur during the initiation and progression of cartilage degeneration in OA, we propose the following hypothetical multistage model of cartilage loss in knees with, or at high risk of developing OA (Figure 5):
In response to systemic risk factors such as increased age, obesity, female sex, inflammation, metabolic and other systemic factors, early systemic compositional changes alter the cartilage quality in a diffuse generalized pattern.
Additional local factors lead to further rapid degradation of already impaired cartilage composition in a focal pattern. The etiology of these local factors remains unknown and further analysis e.g. a statistical atlas of lesion location in the context of specific knee conditions may provide more information.
This additional focal deterioration of cartilage biochemical properties may then lead to the development of a macroscopic lesion within a mean time period of one year.
Figure 5:

Graphic illustration of compositional cartilage degradation prior to the onset of a macroscopic lesion.
Biomarkers of cartilage composition may play a crucial role for the selection of patients, that could profit from conservative interventions to protect cartilage from developing non reversible cartilage structural defects. These interventions depend on risk factors and on the distribution of cartilage alterations. For example in an obese patient with diffusely altered cartilage composition a more systemic intervention such as a weight loss program would be recommended. If the cartilage would be additionally altered in specific locations interventions such as gait training, muscular stabilization and correction of limb alignment could help to prevent the onset of cartilage lesions.
Our study has several limitations. 1. The study population included 80% women, so our findings may apply primarily to women. 2. We selected only clearly visible cartilage lesions that were suitable for segmentation. This may have introduced a bias in the way that it excludes many small and subtle changes in the natural progress of cartilage degeneration and may thus reduce the generalizability of our findings. However, our goal was a proof of concept study using unambiguous cartilage lesion as an outcome. 3. We segmented cartilage using ROIs that included all layers of the cartilage. As a consequence our analysis could not differentiate between the deep and superficial cartilage layer. 4.We did not analyze structural lesions other than cartilage lesions such as meniscus or ligament lesions that might have an influence on cartilage composition. Our purpose was, however, to analyze the regional and temporal distribution of cartilage composition during the process of cartilage degeneration and we consider possible structural changes of the knee other than cartilage lesions as local risk factors for cartilage degradation.
In conclusion, the findings of our study support the concept of compositional cartilage changes as a mechanism for the development of cartilage lesions and that both diffuse and focal areas of accelerated degradation of cartilage composition within a cartilage plate precede the development of cartilage loss at that site. These findings are consistent with a proposed multi-stage model of cartilage loss in OA in which accelerated focal cartilage degradation occurs on a cartilage substrate of diffusely compromised compositional integrity. This study therefore provides insights into the development of focal cartilage lesions and how T2 relaxation time measurements of cartilage composition may help elucidate their pathophysiology.
Supplementary Material
Suppl. Figure 1: Selection of Participants
Suppl. Figure 2: Sagittal fat-saturated intermediate weighted sequence with a focal signal alteration (WORMS grade 1) (a, white arrow,) 1 year prior onset of a WORMS grade 2 lesion (c, white star). Sagittal fat-saturated intermediate weighted sequence with superimposed T2 map of cartilage (b): focally elevated T2 values (b white arrow) are detectable at the local cartilage area in lateral tibia plateau 12 months prior to lesion onset (white arrow)
ACKNOWLEDGMENTS
The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) with research conducted by the Osteoarthritis Initiative Study Investigators. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants U01-AR059507, P50-AR060752 and R01-AR064771. M.K. received grants from the Gottfried and Julia Bangerter-Rhyner Foundation, Basel, Switzerland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Brandt KD, Radin EL, Dieppe PA, van de Putte L, Yet more evidence that osteoarthritis is not a cartilage disease., in Ann Rheum Dis 65, 1261–64 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lories RJ & Luyten FP The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7, 43–49 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Tuan RS, Chen AF, Klatt BA Cartilage Regeneration. J Am Acad Orthop Surg 21:5, 303–11 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Hodgson R Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin North Am 40:4, 699–710 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB Spatial variation of T2 in human articular cartilage. Radiology 205:2, 546–50 (1997) [DOI] [PubMed] [Google Scholar]
- 6.Liess C, Lusse S, Karger N, Heller M, Gluer CC Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage 10:907–913 (2002) [DOI] [PubMed] [Google Scholar]
- 7.David-Vaudey E, Ghosh S, Ries M, Majumdar S T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging 22:673–682 (2004) [DOI] [PubMed] [Google Scholar]
- 8.Joseph GB, McCulloch CE, Nevitt MC, Heilmeier U, et al. A reference database of cartilage 3 T MRI T2 values in knees without diagnostic evidence of cartilage degeneration: data from the osteoarthritis initiative. Osteoarthritis Cartilage (2015). [DOI] [PMC free article] [PubMed]
- 9.Baum T, Joseph GB, Nardo L, Virayavanich W, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res (Hoboken) 65, 23–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging 35, 370–378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebl H, Joseph G, Nevitt MC, Singh N, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis 74, 1353–1359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 20:7, 727–35 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage 21:1, 69–76 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14, 50–55 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Mosher TJ, Dardzinski BJ & Smith MB Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology 214, 259–266 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA & Smith MB Spatial variation of T2 in human articular cartilage. Radiology 205, 546–550 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, et al. Can Signal Abnormalities Detected with MR Imaging in Knee Articular Cartilage Be Used to Predict Development of Morphologic Cartilage Defects? 48-Month Data from the Osteoarthritis Initiative. Radiology 152308 (2016). [DOI] [PMC free article] [PubMed]
- 18.Joseph GB, Baum T, Carballido-Gamio J, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis research & therapy 2011;13(5):R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. Jun 2010;18(6):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kretzschmar M, Lin W, Nardo L, et al. Association of Physical Activity Measured by Accelerometer, Knee Joint Abnormalities, and Cartilage T2 Measurements Obtained From 3T Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. Arthritis care & research September 2015;67(9):1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gersing AS, Solka M, Joseph GB, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage January 30 2016. [DOI] [PMC free article] [PubMed]
- 22.Yu A, Heilmeier U, Kretzschmar M, Joseph GB, et al. Racial differences in biochemical knee cartilage composition be-tween AfricaneAmerican and CaucasianeAmerican women with 3Tesla MR-based T2 relaxation time measurements e data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2015. [DOI] [PMC free article] [PubMed]
- 23.Joseph GB, McCulloch CE, Nevitt MC, Heilmeier U, Nardo L, Lynch JA, Liu F, Baum T, Link TM A reference database of cartilage 3 T MRI T2 values in knees without diagnostic evidence of cartilage degeneration: data from the osteoarthritis initiative. Osteoarthritis Cartilage (2015), 10.1016/j.joca.2015.02.006 [DOI] [PMC free article] [PubMed]
- 24.KELLGREN JH & LAWRENCE JS Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16, 494–502 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felson DT & Nevitt MC Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am 30, 783–97, vii (2004). [DOI] [PubMed] [Google Scholar]
- 26.Felson DT, Niu J, Guermazi A, Sack B & Aliabadi P Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 70, 1884–1886 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterfy CG, Schneider E & Nevitt M The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 16, 1433–1441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, Majumdar S, Link TM A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 19:8, 984–9 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterfy CG, Guermazi A, Zaim S, Tirman PF, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12, 177–190 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 12, 120–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carballido-Gamio J & Majumdar S Atlas-based knee cartilage assessment. Magn Reson Med 66, 574–583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Ginckel, Verdonk A, Witvrouw P, E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage 21, 1009–1024 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Theologis AA. Kuo, Cheng D, Bolbos J, Carballido-Gamio RI, J. Ma CB et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1rho) magnetic resonance imaging: 1-year cohort study. Arthroscopy 27, 65–76 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X Kuo D Theologis A Carballido-Gamio J Stehling C Link TM et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2einitial experience with 1-year follow-up. Radiology 258 505–14 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1: Selection of Participants
Suppl. Figure 2: Sagittal fat-saturated intermediate weighted sequence with a focal signal alteration (WORMS grade 1) (a, white arrow,) 1 year prior onset of a WORMS grade 2 lesion (c, white star). Sagittal fat-saturated intermediate weighted sequence with superimposed T2 map of cartilage (b): focally elevated T2 values (b white arrow) are detectable at the local cartilage area in lateral tibia plateau 12 months prior to lesion onset (white arrow)


