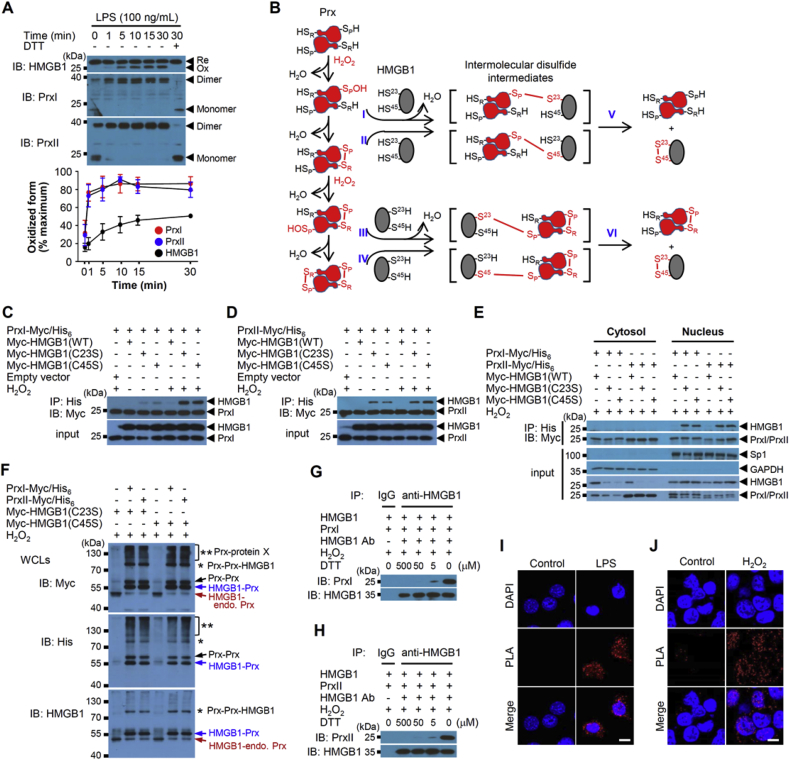
Fig. 1.
HMGB1 oxidation mediated by PrxI and PrxII in the nucleus. (A) Mouse BMDMs were treated with 100 ng/mL LPS for various times, after which the nuclear fraction of cell lysates was isolated and subjected to nonreducing SDS-PAGE and immunoblotting. The intensities of bands corresponding to oxidized (Ox) forms of HMGB1 and to oxidized (dimeric) forms of PrxI and PrxII were determined. Means ± SEM (n = 4). Re, reduced form. (B) Mechanistic scheme for the formation of disulfide HMGB1 catalyzed by PrxI or PrxII. I-VI indicate the mechanistic process of reactions for HMGB1 disulfide formation by Prxs. (C, D) IP of Myc–tagged human HMGB1(WT), HMGB1(C23S) or HMGB1(C45S) as well as with those for Myc/His6-tagged human PrxI or PrxII after 50 μM H2O2 for 30 min stimulation in HEK293T cells. (E) WCLs prepared in (C) and (D) were also separated into cytosolic and nuclear fractions before IP. Sp1 and GAPDH were used as nucleus and cytoplasmic marker, respectively. (F) HEK293T cells were transfected with Myc-HMGB1(C23S) or Myc-HMGB1(C45S) and Myc/His6-tagged PrxI or PrxII, and stimulated with 50 μM H2O2 at 30 min. WCLs were subjected to 8% nonreducing SDS-PAGE. Black, blue, and red arrows show (Prx-Myc/His6)2, Myc-HMGB1-Prx-Myc/His6, Myc-HMGB1-endogenous Prx complexes, respectively. *, (Prx-Myc/His6)2-Myc-HMGB1; **, Prx-Myc/His6-protein X complexes. Endo.: endogenous. (G, H) A mixture (total of 1 ml) of HMGB1 (0.5 μg/ml) with either PrxI (0.4 μg/ml) or PrxII (0.4 μg/ml) proteins was treated with various concentrations of DTT and 50 μM H2O2. The mixtures were immunoprecipitated with anti-HMGB1 Ab and immunoblotted with anti-PrxI/II Abs. Amounts of HMGB1-PrxI/II disulfide intermediate were analysed with reducing SDS-PAGE. IgG, irrelevant Ab. (I, J) PLA assay for detection of the interaction between endogenous HMGB1 and PrxII. PLA signal (red fluorescence) were detected with antibodies HMGB1 and PrxII after1 μg/mL LPS for 1 h in MEFs or 50 μM H2O2 for 30 min in HEK293T cells. Scale bars, 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)