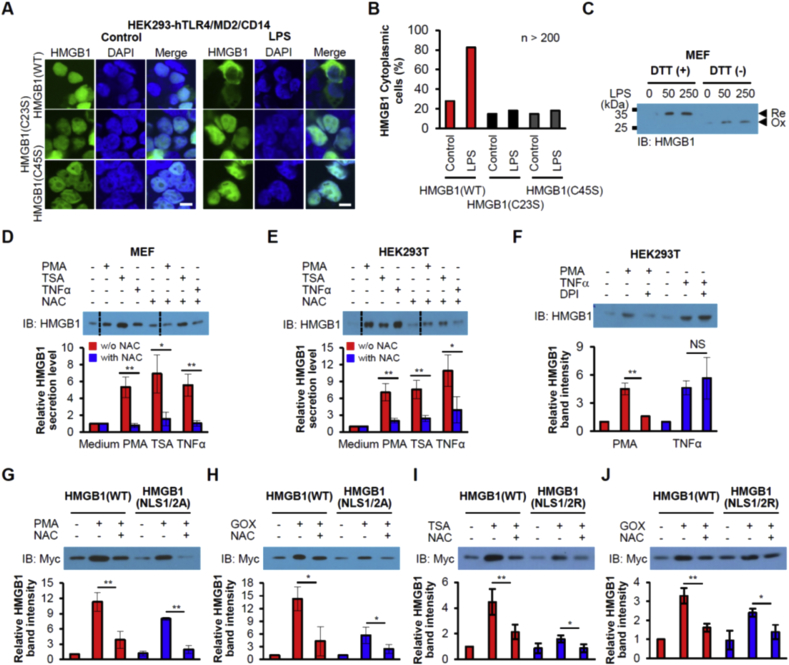
Fig. 3.
Oxidation of HMGB1 is required for nucleocytoplasmic translocation and secretion. (A, B) HEK293-hTLR4/MD2/CD14 cells expressing Myc/EGFP-tagged WT, C23S, or C45S forms of HMGB1 were incubated with 1 μg/mL LPS for 2 h, and examined for EGFP fluorescence by confocal microscopy (A). Scale bars, 10 μm. The percentage of cytoplasmic EGFP was determined (B). n > 200. (C) MEF cells were stimulated with LPS (50 or 250 ng/mL) for 24 h, after which culture supernatants were harvested, and subjected to nonreducing SDS-PAGE with/without DTT. (D-F) MEF or HEK293T cells were incubated in the presence of 250 nM PMA, 10 ng/mL TSA, 50 ng/mL TNFα, 2 mM NAC or 10 μM DPI for 24 h, after which culture supernatants were subjected to IB analysis. Means ± SEM (n = 3). *P < 0.05, **P < 0.01, t-test. NS, not significant. Dotted line is cut line of same membrane. (G-J) Oxidation effect on phosphorylation or acetylation-defective HMGB1. HMGB1 secretions of the phosphorylation-defective mutant HMGB1(NLS1/2A) and the acetylation-defective mutant HMGB1(NLS1/2R) were tested after PMA, TSA, or GOX treatments. HMGB1 knockout MEF cells (I, J) were transfected with each plasmid for 36 h and stimulated for 24 h. Culture supernatants were harvested and IB analyses were performed using an anti-Myc Ab. Means ± SEM (n = 3). *P < 0.05, **P < 0.01, t-test.