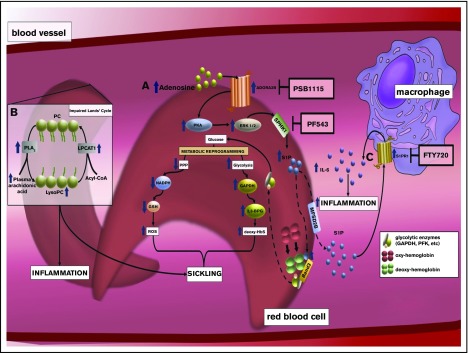
Figure 3.
The discovery of an elevation of S1P and an impaired Lands' cycle in erythrocytes promoting sickling reveals multiple potential therapeutics to treat SCD. (A) Elevated plasma adenosine signaling via ADORA2B receptor underlies activation of SphK1 and increased S1P production in sickle RBCs in a PKA- and extracellular signal-regulated kinase (ERK)–dependent manner. Elevated intracellular S1P directly binds to deoxyHbS, which promotes deoxyHbS anchoring to membrane-bound band 3. This enhances the release of membrane-bound glycolytic enzymes to the cytosol, inducing metabolic reprogramming by accelerating glucose metabolism toward glycolysis instead of PPP, which induces 2,3-BPG production and nicotinamide adenine dinucleotide phosphate–mediated glutathione synthesis reduction. As such, elevated SphK1 mediates intracellular S1P production in sickle cells, which results in an increase of 2,3-BPG production and reactive oxygen species (ROS) generation. (B) Elevated erythrocyte membrane lysophosphatidylcholine (LysoPC) content and circulating erythrocyte arachidonic acid in sickle RBCs is the result of an impaired Lands’ cycle. Correcting an imbalanced Lands’ cycle by interfering with the activity of cytosolic PLA2 or inducing activation of lysophosphatidycholine acyltransferase 1 (LPCAT1), 2 key enzymes of the Lands’ cycle, led to a reduction of elevated RBC membrane LysoPC content and circulating arachidonic acid levels, which attenuated sickling. (C) Elevated plasma S1P signaling via S1PR1 contributes to a sustained inflammatory response and disease progression in SCD by reciprocal upregulation of IL-6 and S1PR1 expression in macrophages.