Table 8.
Comparison of solvents in [2+2] cascade formation of dienes (Eyckens et al., 2016a).
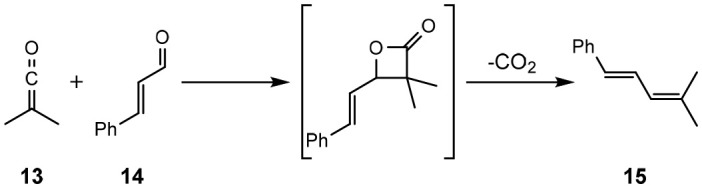 | ||||
|---|---|---|---|---|
| Entry | Solvent | Time (h) | Temp. (°C) | Yield (%)a |
| 1 | CHCl3 | 6 | r.t. | <5 |
| 2 | 5.0 M LPDE | 6 | r.t. | ~20 |
| 3 | [Li(G3)]TFSI | 6 | r.t. | 56 |
| 4 | [Li(G4)]TFSI | 6 | r.t. | 41 |
| 6b | [Li(G3)]TFSI | 6 | r.t. | 60 |
| 7 | [Li(G3)]TFSI | 6c | 80 | 70 |
Isolated yield.
Activated molecular sieves (100 mg) were used throughout the reaction.
The reaction mixture was heated for the finally hour of the specified time.
