Abstract
Cryptococcosis is the opportunistic infection usually seen in immunocompromised individuals. Among human immunodeficiency virus (HIV)–seropositive participants, cryptococcal meningitis is the second most common cause of opportunistic neuro-infection, but at times, it occurs in non-HIV patients who are immunodeficient due to other reasons like chronic glucocorticoid use, organ transplantation, malignancy, and sarcoidosis and has rarely been described in diabetic patients. We present a fatal case of Cryptococcus gattii meningitis in a 56-year-old HIV-negative male patient with diabetes mellitus.
Keywords: Cryptococcosis, Cryptococcus gattii, diabetes mellitus, meningitis
Introduction
Cryptococcus neoformans and Cryptococcus gattii are encapsulated, heterobasidiomycetous fungi that have progressed from being rare human pathogens, with just over 300 cases of cryptococcosis reported in the literature before 1955, to becoming a common worldwide pathogen as immunocompromised human populations have dramatically increased over the past two decades.[1] There are three variants—C. neoformans var. neoformans (serotype D), C. neoformans var. grubii (serotype A), and C. gattii (serotypes B and C). In 2005, the genome sequence of C. neoformans was released, and several more strains including C. gattii have been sequenced.[2]
Cryptococcosis crosses the entire spectrum of patient populations, from the apparently immunocompetent host without an underlying disease to those severely immunocompromised from infection with the human immunodeficiency virus (HIV), organ transplantation, or a malignancy.
In this article, we report a case of cryptococcal meningitis confirmed by cerebral spinal fluid (CSF) findings, fungal culture, and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) in HIV-negative male patient who succumbed to his illness despite treatment.
Case Report
A 56-year-old retired defense male personnel was admitted to the emergency ward of our tertiary care hospital for a chronic headache. Past history of the patient revealed a history of tubercular pleural effusion 7 years back for which he received anti-tubercular treatment (ATT), and it subsequently resolved. Two months prior to presentation at our hospital, the patient had been evaluated for a severe headache at an outside private clinic where he was provisionally diagnosed as tuberculoma/neurocysticercosis and was re-started on ATT along with a dose of albendazole and steroids; however, the patient had no relief. The patient was currently suffering from unremitting, persistent right frontal headache, vomiting, and diplopia. The patient's relatives denied any recent travel history or exposure to pets.
On physical examination, he was unconscious with a Glasgow Coma Scale score of 7. Neck stiffness and Kernig's sign were positive. All deep tendon reflexes were diminished. Other systemic examination revealed no abnormalities. Initial laboratory investigations showed elevated glucose (250 mg/dL), total leucocyte count of 9,500 cumm with 85% neutrophils, and erythrocyte sedimentation rate (ESR) of 17 mm/hr. Vital signs were the following: temperature = 101.3°F, BP = 130/70 mm Hg, pulse = 79 beats/min, and respiratory rate = 22 breaths/min. Liver function tests (LFT) revealed elevated alanine aminotransferase (ALT) (64 U/L). HIV testing by chemiluminescence was found to be non-reactive, whereas CD4 (cluster of differentiation 4) count showed 148 cells/μL. Other routine tests were unremarkable.
MRI of the brain revealed multiple small enhancing foci in midbrain on the right side, right superior cerebellum, and left cerebral peduncle with mild perilesional edema with features suggestive of infective granuloma. No growth was detected on blood and urine cultures. Lumbar puncture (LP) was performed and revealed a slightly hazy CSF with 65 WBC/mm3 (96% lymphocytes, 4% neutrophils), 0 RBC/mm3, protein 53 mg/dL, and sugar 10 mg/dL. Direct wet mount, Gram staining, and India ink preparation of the CSF showed 4 to 9 μm round-budding capsulated cells [Figures 1 and 2]. GeneXpert for Mycobacterium tuberculosis was negative. On Day 2, the patient was initiated on induction therapy with IV amphotericin B (1 mg/kg) and oral flucytosine (100 mg/day).
Figure 1.
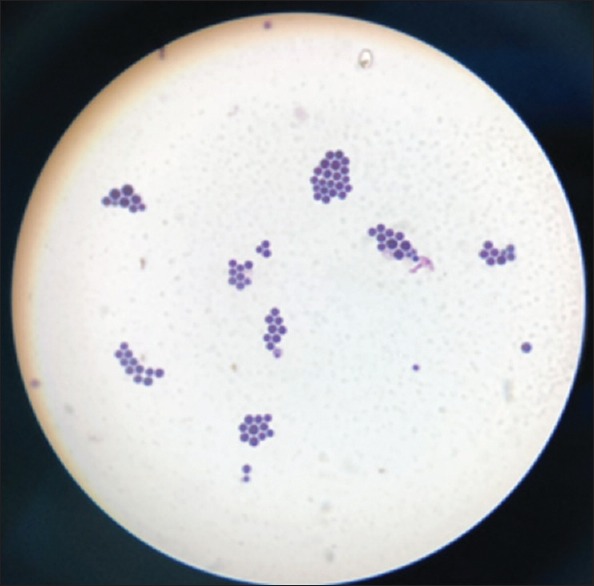
Gram stain showing gram-positive budding yeast cells
Figure 2.

India ink showing capsulated round yeast cells
Over the next two days, the patient became more responsive, and mild improvement in orientation was seen. Fungal culture of CSF on Sabouraud Dextrose Agar (SDA) with antibiotics yielded highly mucoid, cream-colored colonies of yeast after 2 days of incubation at 37°C which were Gram positive, urease positive, and capsulated [Figures 1–3]. In addition, MALDI-TOF-MS (Bruker Biotyper Microflex, Massachusetts) confirmed the isolate as C. neoformans var gattii with a confidence interval of 2.14.
Figure 3.

SDA tube showing mucoid, creamy pasty colonies of Cryptococcus spp.
During this time, his serum glucose remained elevated, ranging between 280 mg/dL and 320 mg/dL with an HbA1C of 6.4%. On the fifth day of hospitalization, the patient's condition deteriorated. Therapeutic LP was done to decrease the raised intracranial pressure. While the LP was being attempted, the patient became cyanotic and developed respiratory failure needing mechanical ventilation. He was intubated immediately, and advanced cardiac life support was started. Despite aggressive attempts at resuscitation, he finally succumbed to his illness on Day 6.
Discussion
The genus Cryptococcus contains more than 39 species; however, only a few are able to cause human disease. Before the AIDS epidemic, it was found that at least 80% of clinical isolates worldwide were C. neoformans serotype A (var. grubii). It is a capsulated fungus that reproduces by budding and has a worldwide distribution causing disease not only in immunocompromised but also in the immunocompetent. Infection occurs primarily via inhalation of the fungi from environmental sources into the lung alveoli where the infection is restricted in the lung in the immunocompetent or may disseminate when immunity is compromised. Occasional cases of direct traumatic inoculation through contaminated objects or laboratory/clinical accidents such as needle sticks have also been reported.
The organism has a special predilection to infect the central nervous system (CNS) (neurotropism) probably due to (i) presence of receptors specific for some ligand on the yeast and (ii) increased dopamine levels in CNS allows melanin production by the phenoloxidase in the yeast contributing to increased fungal virulence.[3,4] As a consequence, meningitis is seen to be the commonest manifestation. However, it would be more accurate to describe the syndrome as meningoencephalitis, as the histopathological examination demonstrates that along with the subarachnoid space, the brain parenchyma is also involved.
It has a wide range of clinical presentations, which can vary from asymptomatic colonization of the respiratory airways to dissemination of infection into any organ of the body. It can lodge in the meninges, bones, adrenals, kidneys, liver, spleen, and so on. In CNS, cryptococcosis presentation varies, which may be acute (few days to a week), sub-acute (over 2-4 weeks), and chronic in nature. Most present with signs and symptoms of subacute meningitis, such as headache, fever, cranial nerve palsies, lethargy, coma, or memory loss over several weeks.
The most important host response to cryptococcal infection is cell-mediated immunity, which explains its increased incidence in HIV-seropositive patients.[5] It is the second most common CNS fungal pathogen causing lethal infections in immunocompromised patients, particularly with AIDS.[6,7,8,9] Other common causes of immunosuppression are use of glucocorticoid, organ transplantation, malignancy, sarcoidosis, and liver failure.[10] In a review of cryptococcosis on non-HIV patients by Kiertiburnankul et al., it was concluded that the most common associated conditions were immunosuppressive drug treatment (41%), SLE (16%), malignancies (16%), and diabetes mellitus (14%).[11] Our case represents this trivial, however significant fraction of diabetic patients.
Uncontrolled diabetics are more liable to infections like cryptococcosis, rhinocerebral mucormycosis, malignant otitis externa, pyelonephritis, acute necrotizing fasciitis, and so on as blood enriched with glucose provides an excellent media for growth leading to poor clinical outcome. Same was observed in our patient. These outcomes reflect defects of the immune system in the diabetic population.
Host response to cryptococcal infection is mostly cell-mediated immunity involving CD4+ and CD8+ (cluster of differentiation 4) lymphocytes, natural killer cells, and activated phagocytes along with presence of cytokines like tumor necrosis factor (TNF), IL-2 (interleukin), IL-12, monocyte chemotactic factor (MCF), and macrophage inflammatory protein-α (MIP-α) which contribute to the development of granulomatous inflammation.[12] As serum levels of these cytokines are elevated in diabetes, it is possible that such patients mount an exaggerated immune response (paradoxical to their defective immune state) to C. neoformans infection leading to a terminal outcome, as seen in our case.[13] Hence, anti-inflammatory measures may also play an adjunctive role in such infections.
Diagnostic procedures like wet mount, India ink examination, and latex agglutination identify that yeasts are effective tools for rapidly diagnosing cryptococcal meningitis, and thus guide the clinician to initiate prompt treatment. The use of MALDI-TOF additionally enabled us to rapidly identify the variant of Cryptococcus giving us a better epidemiological profile of the types/variants of Cryptococcus prevalent in this geographical region. Although the host appears to be the primary factor in outcome, the infecting cryptococcal strain may have some impact on outcome, and in most cases, in developed countries, the immediate mortality rate of cryptococcal meningitis unfortunately still remains at 10% to 25% and, in developing countries, with limited resources, the mortality rate at 6 months can reach 100%.[7,14]
Conclusion
To conclude, this case demonstrates the nuances of an exaggerated inflammatory immune response, which plays an important role in the pathogenesis of CNS cryptococcosis disease in the diabetic population. Diabetic patients are at increased risk for such opportunistic infections due to the defects in the immune system. As a result, high rates of morbidity and mortality are seen in such subjects. Our case also emphasizes the need for clinicians to consider cryptococcosis in the differential diagnosis of neurologic and even cutaneous manifestations in diabetes mellitus. Exogenous anti-cytokine therapy may hold an attractive therapeutic modality to supplement the current anti-microbial agents in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–4. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chander J. Textbook of Medical Mycology. 4th ed. 2016. pp. 434–56. [Google Scholar]
- 4.Forbes BA, Sahm DF, Weisfeld AS. Bailey and Scott's Diagnostic Microbiology. 11th ed. USA: Mosby, Inc; 2002. Laboratory methods in basic mycology; pp. 724–5. [Google Scholar]
- 5.Manoharan G, Padmavathy BK, Vasanthi S, Gopalte R. Cryptococcal meningitis among HIV-infected patients. Indian J Med Microbiol. 2001;19:157–8. [PubMed] [Google Scholar]
- 6.Arora V, Tumbanathan A. Cryptococcal meningitis associated with tuberculosis in a HIV infected person. Ind J Tuberculosis. 1997;44:39–41. [Google Scholar]
- 7.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507–44. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Khanna N, Chandramukhi A, Desai A, Ravi V. Cryptococcal infections of the central nervous system: An analysis of predisposing factors, laboratory findings and outcomes in patients from South India with special reference to HIV infection. J Med Microbiol. 1996;45:376–9. doi: 10.1099/00222615-45-5-376. [DOI] [PubMed] [Google Scholar]
- 9.Khanna N, Chandramukhi A, Desai A, Ravi V, Santosh V, Shankar SK, et al. Cryptococcosis in the immunocompromised host with special reference to AIDS. Indian J Chest Dis Allied Sci. 2000;42:311–5. [PubMed] [Google Scholar]
- 10.Satishchandra P, Mathew T, Gadre G, Nagarathna S, Chandramukhi A, Mahadevan A. Cyptococcal meningitis: Clinical, diagnostic and therapeutic overviews. Neurol India. 2007;55:226–32. doi: 10.4103/0028-3886.35683. [DOI] [PubMed] [Google Scholar]
- 11.Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis. 2006;10:72–8. doi: 10.1016/j.ijid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1998;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Yamagishi S, Adachi H. Circulating advanced glycation end products (AGEs) and soluble form of receptor for monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev. 2007;24:109–14. doi: 10.1002/dmrr.766. [DOI] [PubMed] [Google Scholar]
- 14.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]


