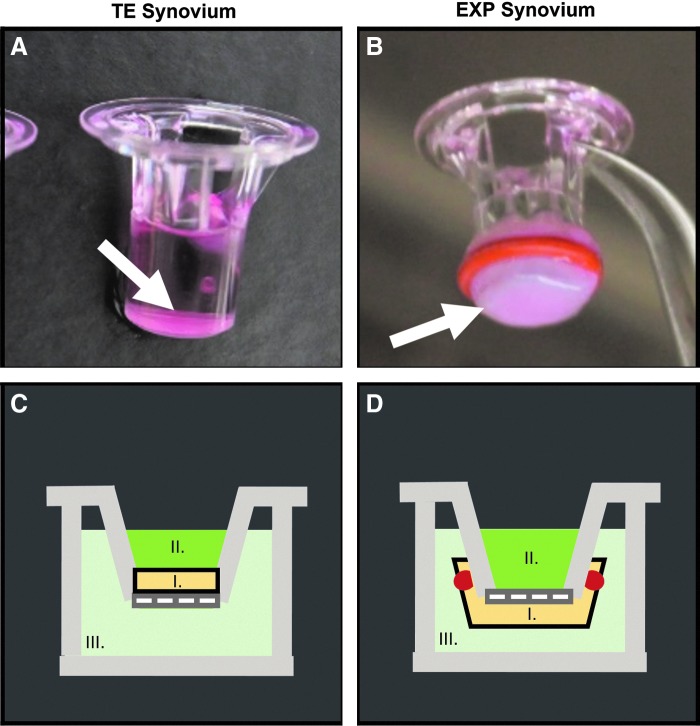
FIG. 2.
Changes in permeability of FITC-labeled dextran in synovium were assessed using a Transwell® system. TE synovium (A, C) was cultured and tested in situ on the top of a 4.26 mm Transwell insert (arrow). EXP synovium (B, D) was secured to the underside of a 6.5 mm Transwell insert using a rubber gasket for transport measurements (arrow). For both tissues (I), the dextran suspension (II) was placed in the luminal compartment (i.e., the synovial cavity). Phosphate-buffered saline (III) was added to the basolateral compartment (i.e., the fibrous capsule and associated vasculature) and sampled at time points to quantify joint clearance. FITC, fluorescein isothiocyanate; TE, tissue engineered; EXP, explant.