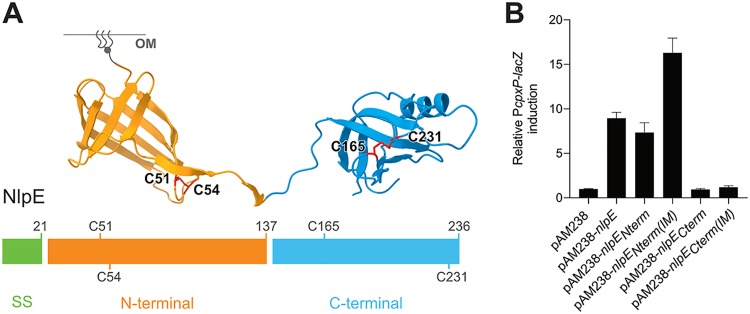
FIG 2.
The N-terminal domain of NlpE is the Cpx-activating domain. (A) NlpE is composed of two structurally distinct domains. The domains were determined empirically using phylogenetic data comparing bacterial species harboring a full-length or N-terminus-only version of NlpE. We define the N-terminal domain as residues 22 to 137 (inclusive) and the C-terminal domain as residues 138 to 236 (inclusive), as indicated. The C-terminal domain was constructed by removing the sequence encoding residues 24 to 137. Disulfide bonds are drawn in red on the structure, and the corresponding cysteine residues are also shown on the one-dimensional schematics of the NlpE sequence. The lipid anchor is schematically represented in gray. SS, signal sequence for secretion. (B) The N-terminal domain of NlpE activates Cpx. β-Galactosidase activity from PcpxP-lacZ was measured in wild-type cells carrying the empty pAM238 plasmid (GL61) or expressing full-length nlpE (GL60), nlpENterm (AD171), nlpECterm (AD172), nlpENterm(IM) (AD165), or nlpECterm(IM) (AD187) from the same plasmid. All values were normalized to the average activity obtained for GL61. Bars represent the averages of normalized values from at least three independent clones. Error bars indicate standard deviations.