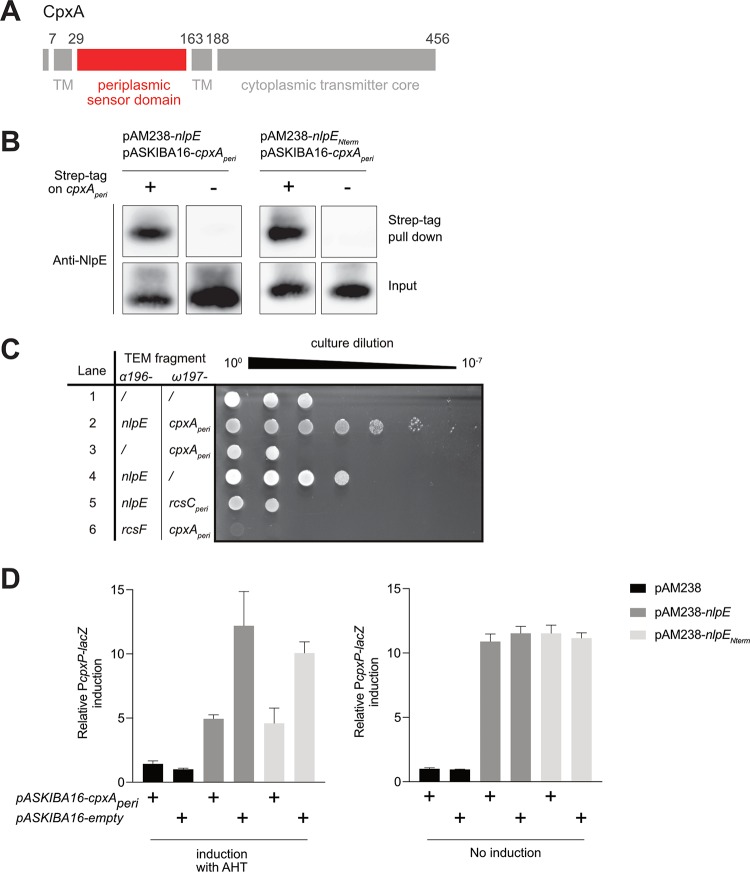
FIG 3.
NlpE physically interacts with CpxA through its N-terminal domain. (A) A representative schematic of CpxA (adapted from reference 49) is shown. CpxA contains a large periplasmic sensor domain (depicted in red and determined according to reference 49 from residue 29 to 163 [inclusive]) and a cytoplasmic transmitter core. TM, transmembrane region. (B) NlpE is pulled down with CpxA. Left, expression of cpxAperi encoding the periplasmic domain of CpxA (as shown in panel A), fused (AD112) or not fused (AD121) to a N-terminal Strep-tag, was induced for 40 min from a pASKIBA-16 plasmid in cells overexpressing nlpE from the pAM238 plasmid. Right, the same procedure was performed but with cells overexpressing nlpENterm from the pAM238 plasmid, instead of the full-length nlpE, along with the expression of cpxAperi tagged with Strep-tag (AD165) or untagged cpxAperi (AD166) from the pASKIBA-16 plasmid. Total cell extracts were then purified on Strep-Tactin–Sepharose resin, and the input and elution (Strep-tag pulldown) fractions were analyzed by Western blotting using an anti-NlpE antibody. (C) A TEM β-lactamase complementation assay confirms in vivo physical interaction. Serial dilution and spotting of cpxR null cells carrying a pCDFDuet plasmid for the expression of the ω197 fragment and the α196 fragment of the TEM β-lactamase, fused or not fused to cpxAperi, nlpE (soluble, without the signal sequence), rcsCperi (periplasmic domain of RcsC, determined according to reference 50), and/or rcsF (soluble, without the signal sequence), as indicated. Lane 1, AD155; lane 2, AD159; lane 3, AD160; lane 4, AD161; lane 5, AD191; lane 6, AD192. Negative controls for interaction are shown in lanes 1, 3, 4, 5, and 6. Shown is a representative image of three biological replicates. We do not know why cells became slightly more resistant in lane 4 or more sensitive in lanes 2, 5, and 6. Expression of both nlpE and cpxAperi constructs (lane 2) increased ampicillin resistance by ≥4 log units, compared to the nlpE fusion alone, indicating physical interaction. (D) Overproduced periplasmic domain of CpxA titrates NlpE or NlpENterm away from the native, full-length CpxA, preventing Cpx activation. β-Galactosidase activity from PcpxP-lacZ was measured with (left) or without (right) AHT induction of cpxAperi expression from the pASKIBA-16 plasmid, in wild-type cells overexpressing nlpE (AD121) or nlpENterm (AD166) or carrying the empty pAM238 vector (AD200). The same activity was measured in cells carrying the empty pASKIBA-16 plasmid (AD162, AD168, and AD201, respectively). All values were normalized to the average activity obtained for strain AD201. Bars represent the averages of normalized values from at least three independent clones. Error bars indicate standard deviations.