Abstract
Extracellular signal-regulated kinases 1 and 2 (ERK1/2) are serine/threonine kinases and function as regulators of cellular proliferation and differentiation. Recently, we demonstrated that inhibition of ERK1/2 alleviates the development and progression of hyperuricemia nephropathy (HN). However, its potential roles in uric acid-induced tubular epithelial-mesenchymal transition (EMT) and tubular epithelial cell injury are unknown. In this study, we showed that hyperuricemic injury induced EMT as characterized by downregulation of E-cadherin and upregulation of vimentin and Snail1 in a rat model of HN. This was coincident with epithelial cells arrested at the G2/M phase of cell cycle, activation of Notch1/Jagged-1 and Wnt/β-catenin signaling pathways, and upregulation of matrix metalloproteinase-2 (MMP-2) and MMP-9. Administration of U0126, a selective inhibitor of ERK1/2, blocked all these responses. U0126 was also effective in inhibiting renal tubular cell injury, as shown by decreased expression of lipocalin-2 and kidney injury molecule-1 and active forms of caspase-3. U0126 or ERK1/2 siRNA can inhibit tubular cell EMT and cell apoptosis as characterized with decreased expression of cleaved caspase-3. Moreover, ERK1/2 inhibition suppressed hyperuricemic injury-induced oxidative stress as indicated by decreased malondialdehyde and increased superoxide dismutase. Collectively, ERK1/2 inhibition-elicited renal protection is associated with inhibition of EMT through inactivation of multiple signaling pathways and matrix metalloproteinases, as well as attenuation of renal tubule injury by enhancing cellular resistance to oxidative stress.
Keywords: cell apoptosis, epithelial-mesenchymal transition, ERK1/2, hyperuricemic nephropathy, oxidative stress
INTRODUCTION
Uric acid is the final product of purine metabolism in humans and is excreted largely by the kidneys. Hyperuricemia occurs as a result of increased uric acid synthesis and decreased renal excretion (20). It leads to hyperuricemic nephropathy (HN) and contributes to the development and progression of chronic kidney disease (CKD) (20). Excessive urate excretion results in urate crystal deposition and tubular obstruction, which can be accompanied by a series of complications such as infection, bleeding, and hydropsy (20, 39). Uric acid also causes glomerular sclerosis and tubulointerstitial fibrosis via urate crystal-independent pathological pathways, including endothelial dysfunction (26), inflammation (24), epithelial-mesenchymal transition (EMT) (40), oxidative stress (52), apoptosis (52), and activation of various signaling pathways such as those involving epidermal growth factor receptor (EGFR) and transforming growth factor-β (TGF-β) (23).
Recently, we found that activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling pathway is closely associated with the development of HN (24). ERK1/2 are serine/threonine kinases belonging to the mitogen-activated protein kinase (MAPK) family. They transmit extracellular signals to the nucleus in response to growth factors, cytokines, and ligands for G protein-coupled receptors (37) and regulate cellular proliferation, differentiation, and apoptosis (46). Recent literature has indicated that ERK1/2 is involved in the pathogenesis of several kidney diseases, including diabetic nephropathy (60), obstructive nephropathy (17), cisplatin-induced acute renal failure (19), chronic allograft nephropathy (55), and kidney carcinoma (58). However, the underlying mechanisms by which ERK1/2 participates in the development of HN are still obscure.
It is well known that in kidneys suffering severe and repeated insults there is increased production and accumulation of extracellular matrix, and repair of the injury can be maladaptive, with many tubular cells arrested in the G2/M phase of the cell cycle (6). This phenomenon is the functional consequence of partial EMT (27). Many signaling pathways such as Notch and β-catenin have been found to facilitate EMT and subsequently tubulointerstitial fibrosis (TIF) (4). Intriguingly, ERK1/2 can modulate Notch signaling by regulating the expression of its ligand Jagged-1 (18, 30, 34) and interconnect with the Wnt/β-catenin axis via multiple coupled feedback loops (44). However, it remains unclear whether ERK1/2 promotes urate-elicited EMT through regulating Notch and Wnt/β-catenin signaling pathways.
Chronic uric acid exposure can cause tubular epithelial cell injury. Tubular injury is accompanied by oxidative stress, which further triggers caspase-dependent apoptosis pathways, leading to the loss of renal epithelial cells (52). It was reported that ERK1/2 can regulate oxidative stress in obstructive nephropathy (38) and mediate renal tubular cell apoptosis in animal models of acute kidney injury induced by diverse insults such as nephrotoxins and ischemia/reperfusion (1, 19). However, whether ERK1/2 activation contributes to renal tubule injury and apoptosis in hyperuricemic nephropathy is still unknown.
In this study, we investigated the effect and mechanisms of ERK1/2 inhibition on urate-induced renal EMT and renal tubule injury in a rat model of HN.
MATERIALS AND METHODS
Antibodies and reagents.
U0126 (HY-12031) was purchased from Med Chem Express (Monmouth Junction, NJ). Antibodies against E-cadherin (no. 14472), cleaved caspase-3 (no. 9664), Snail1 (no. 3879), vimentin (no. 3932), and Notch1 (no. 3608) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to Jagged-1 (SC-135955), collagen I (A2) (SC-28654), and GAPDH (SC-32233) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to lipocalin-2 (Lcn2, BAF1857) and kidney injury molecule 1 (Kim-1, BAF1817) were purchased from R&D Systems (Minneapolis, MN). Antibody to Wnt1 (no. 35866) was purchased from Rockland (Limerick, PA). Antibody to β-catenin (no. 610154) was purchased from BD Biosciences (San Diego, CA). Antibodies to phosphorylated (p-)histone H3 (ab5176), matrix metalloproteinase-2 (MMP-2; ab37150), and MMP-9 (ab38898) were purchased from Abcam (Cambridge, MA). Texas red-labeled or FITC green-labeled secondary antibodies (F-2765) for immunofluorescent staining were purchased from Invitrogen (Grand Island, NY). DAPI staining solution (C1005) for immunofluorescent staining was purchased from Beyotime Biotechnology (Shanghai, China). Malondialdehyde (MDA; A003-1) and superoxide dismutase (SOD; A001-1-1) biochemical reagent kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Antibodies to α-smooth muscle actin (α-SMA; A2547) and β-actin (A1978), anti-mouse secondary antibodies (M8770), and anti-rabbit secondary antibodies (A3687) for Western blot, DMSO, and all other chemicals were from Sigma (St. Louis, MO).
Creation of hyperuricemic nephropathy model in rats and U0126 treatment.
Male Sprague-Dawley rats (6–8 wk old) weighing 200–220 g were purchased from B&K Laboratory Animal (Shanghai, China). Animals were housed in stainless steel cages in a ventilated animal room at the Experimental Animal Center of Tongji University (Shanghai, China). Room temperature was maintained at 20 ± 2°C, relative humidity at 60% ± 10%, and a 12:12-h light-dark cycle. Distilled water and sterilized food for rats were available ad libitum. The rats were acclimated to this environment for 7 days before experiments. Twenty-four male rats were randomly assigned to four groups of six rats: sham, sham treated with U0126 (10 mg/kg), HN, and HN treated with U0126 (10 mg/kg). The HN rat model was established as described in our previous study (23). Briefly, a mixture of adenine (0.1 g/kg) and potassium oxonate (1.5 g/kg) dissolved in distilled water was administered orally once daily for 3 wk. To assess the effect of U0126 on renal protection in HN rats, U0126 at 10 mg/kg in 50 μl of DMSO was administrated via peritoneal injection every other day to the rats 1 h after the mixture of adenine and potassium oxonate exposure was taken by rats. The sham group was injected with DMSO as a control. For the HN-only group, rats were also injected with an equivalent amount of DMSO. After 3 wk, the animals were euthanized, and the kidneys were collected for protein analysis and histological examination. The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Tongji University.
Assay of oxidative stress index.
The concentrations of malondialdehyde (MDA) and superoxide dismutase (SOD) in kidney tissues were detected by commercial kits (A003-1 and A001-1-1, Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions, and the final levels of MDA and SOD were normalized to the protein concentration of kidney tissue homogenate.
Cell culture and treatment.
Human tubular epithelial cells (HK2) were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) and F-12 containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin in an atmosphere of 5% CO2 and 95% air at 37°C. To determine the effect of U0126 in uric acid-induced tubular cell injury, HK2 cells were starved for 24 h with DMEM containing 0.5% FBS and then were pretreated with U0126 (0, 5, 10, and 20 μM) for 1 h. After that, HK2 cells were exposed to uric acid (800 μM) to induce cell injury in the presence of U0126 (0, 5, 10, and 20 μM) for an additional 36 h. The cells were then harvested for immunoblot analysis. All of the in vitro experiments were repeated at least three times.
siRNA transfection.
Small interfering (si) RNA oligonucleotides targeted specially for ERK1/2 were used in this study. ERK1/2 siRNA was obtained from Shanghai GenePharma. HK2 cells were seeded to 30–40% confluence in antibiotic-free medium, grown for 24 h, and then transfected with ERK1/2 siRNA (60 pmol) with Lipofectamine RNAiMAX. In parallel, scrambled siRNA (60 pmol) was used as a control for off-target changes in HK2. At 24 h after transfection, cells were treated with uric acid (800 μM) for an additional 36 h before being harvested for the experiments.
Immunoblot analysis.
Immunoblot analysis for kidney cells and tissue samples were performed according to our previously protocols (22–24). We homogenized the kidney tissue samples with cell lysis buffer (Cell Signaling Technology) and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). After various treatments, the cells were washed once with ice-cold PBS and harvested in a cell lysis buffer mixed with a protease inhibitor cocktail. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% nonfat milk for 1 h at room temperature, the membranes were incubated with a primary antibody overnight at 4°C and then incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Bound antibodies were visualized by chemiluminescence detection. Densitometry analysis of immunoblot results was conducted by using NIH Image software (National Institutes of Health, Bethesda, MD).
Immunohistochemical and immunofluorescent staining.
Formalin-fixed kidneys were embedded in paraffin and prepared in 3-μm-thick sections. Immunohistochemical and immunofluorescent staining were performed according to procedures described in our previous studies (3, 23, 24). Positive areas of vimentin and MMP-2 were quantitatively measured using Image Pro-Plus software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of the positive staining area, and the average ratio to each microscopic field (×200) was calculated and graphed. For immunofluorescent staining, the tissue sections were rehydrated and labeled with primary antibody phospho-histone H3 and then exposed to Texas red-labeled or FITC green-labeled secondary antibodies (Invitrogen). Renal cells arrested at the G2/M phase of the cell cycle were graded by counting the absolute number of phospho-histone H3-positive cells in each field and reported as the mean of 20 random high-power (×200) fields each rat in six rats per group.
Statistical analysis.
All of the experiments were conducted at least three times. Data depicted in graphs represent the means ± SE for each group. Intergroup comparisons were made using one-way ANOVA. Multiple means were compared using Tukey’s test. Differences between two groups were determined by Student’s t-test. Statistically significant differences between mean values are marked in each graph. P < 0.05 was considered significant.
RESULTS
ERK1/2 inhibition attenuates renal EMT in a rat model of HN.
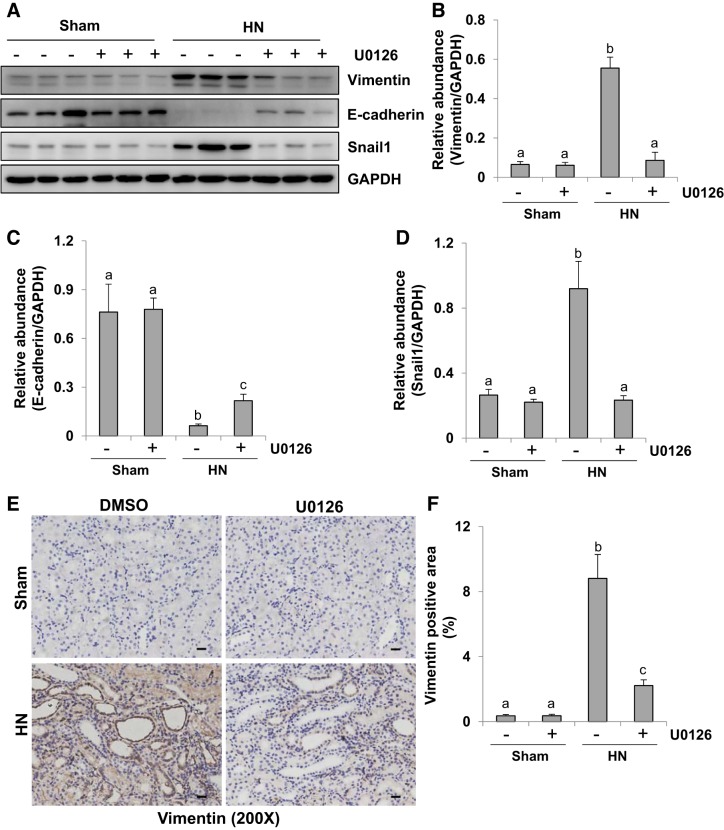
Partial EMT is an important mechanism of renal fibrosis, which is characterized by a decrease of adherens junctions such as E-cadherin, an increase of mesenchymal structural proteins such as vimentin, and upregulation of transcription factors such as Snail1 (7). In our previous study, rats with HN displayed a remarkable upregulation of ERK1/2 phosphorylation (24). Densitometry analysis indicated a 92% reduction of p-ERK1/2 in HN rats treated with U0126, a selective inhibitor of the ERK1/2 pathway, compared with those treated with vehicle (24). In the present study, we elucidated the role of ERK1/2 in tubular EMT by using U0126 on the expression of E-cadherin, vimentin, and Snail1 in the kidney of hyperuricemic rats. As shown in Fig. 1, A–D, immunoblot analysis indicated that kidneys of rats given the mixture of adenine (0.1 g/kg) and potassium oxonate (1.5 g/kg) oral daily for 3 wk displayed reduced expression of E-cadherin and increased the expression of vimentin and Snail1. U0126 treatment significantly reversed those responses. Immunohistochemistry staining also showed a high level of vimentin in the renal tubules after hyperuricemic injury, and administration of U0126 inhibited its expression (Fig. 1, E and F). Taken together, our data suggest that ERK1/2 activation is involved in the development of EMT in the kidney of HN.
Fig. 1.
Administration of U0126, a selective inhibitor of extracellular signal-regulated kinases 1 and 2, attenuates renal epithelial-mesenchymal transition (EMT) in kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against vimentin, E-cadherin, Snail1, and GAPDH. B–D: expression levels of vimentin (B), E-cadherin (C), and Snail1 (D) were quantified by densitometry, and protein levels were normalized to GAPDH. E: photomicrographs (×200) illustrating vimentin immunochemistry staining of kidney tissue. F: vimentin staining graphic presentation of quantitative data. Data are presented as mean ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). All scale bars, 20 μm.
U0126 treatment inhibits uric acid-induced upregulation of α-SMA and collagen I and preserves E-cadherin expression in cultured human tubular epithelial cells.
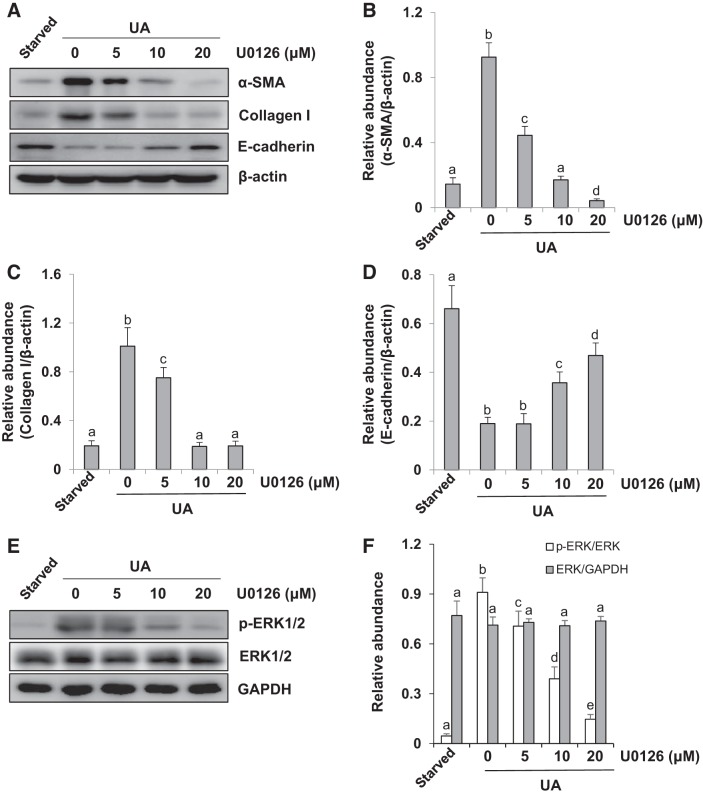
We have demonstrated that activation of ERK1/2 is involved in renal EMT in a rat model of HN. To validate the role of ERK1/2 in uric acid-induced EMT of tubular epithelial cells, we examined the effect of U0126 on the expression of α-SMA, collagen I, and E-cadherin in cultured human tubular epithelial cells (HK2) exposed to uric acid. Serum-starved HK2 cells were pretreated with U0126 (0, 5, 10, and 20 μM) for 1 h and then exposed to uric acid (800 μM) to induce cell injury in the presence of U0126 (0, 5, 10, and 20 μM) for an additional 36 h. Then, cells were harvested for immunoblot analysis. Figure 2, A–F shows that U0126 dose-dependently inhibited uric acid-induced enhancement of α-SMA, collagen I, and p-ERK1/2 and preserved expression of E-cadherin. The expression of total ERK1/2 was not affected by U0126. These data indicate that ERK1/2 plays a vital role in regulating EMT in HK2 cells.
Fig. 2.
ERK1/2 inhibition suppresses expression of α-smooth muscle actin (α-SMA) and collagen I and decreases E-cadherin expression in uric acid (UA)-treated human tubular epithelial (HK2) cells. Serum-starved HK2 cells were pretreated with U0126 (0, 5, 10, and 20 μM) for 1 h and then exposed to UA (800 μM) to induce cell injury in the presence of U0126 (0, 5, 10, and 20 μM) for an additional 36 h. A: cell lysates were subjected to immunoblot analysis with specific antibodies against α-SMA, collagen I, E-cadherin, and β-actin. B–D: expression levels of α-SMA (B), collagen I (C), and E-cadherin (D) were quantitated by densitometry and normalized to β-actin. E: immunoblot analysis was also performed with specific antibodies against phospho (p-)ERK1/2, ERK1/2, and GADPH. F: expression level of p-ERK1/2 was quantitated by densitometry and normalized to total ERK1/Values are means ± SE of at least three independent experiments. Means with different superscript letters are significantly different from one another (P < 0.05).
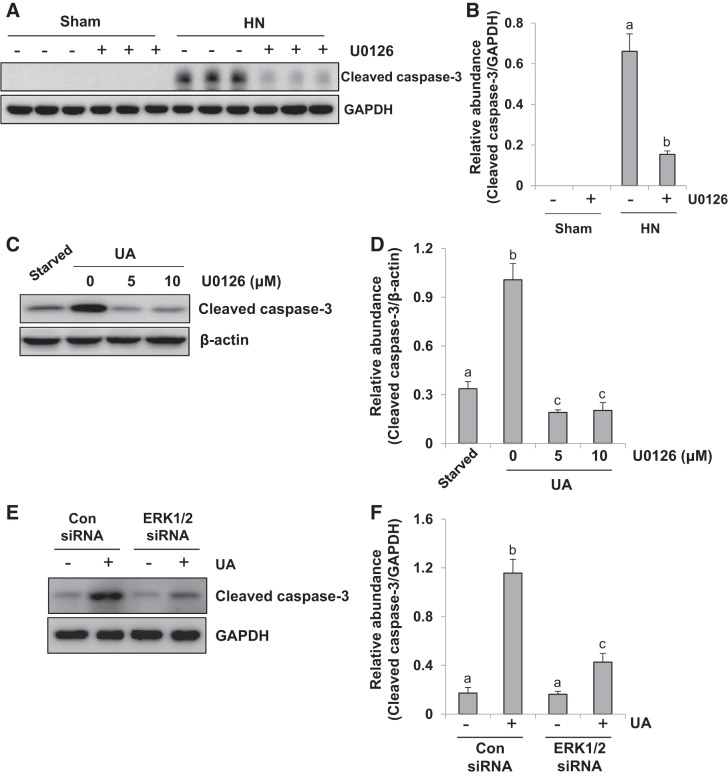
Silencing of ERK1/2 blocks uric acid-induced upregulation of α-SMA and collagen I and preserves E-cadherin expression in cultured human tubular epithelial cells.
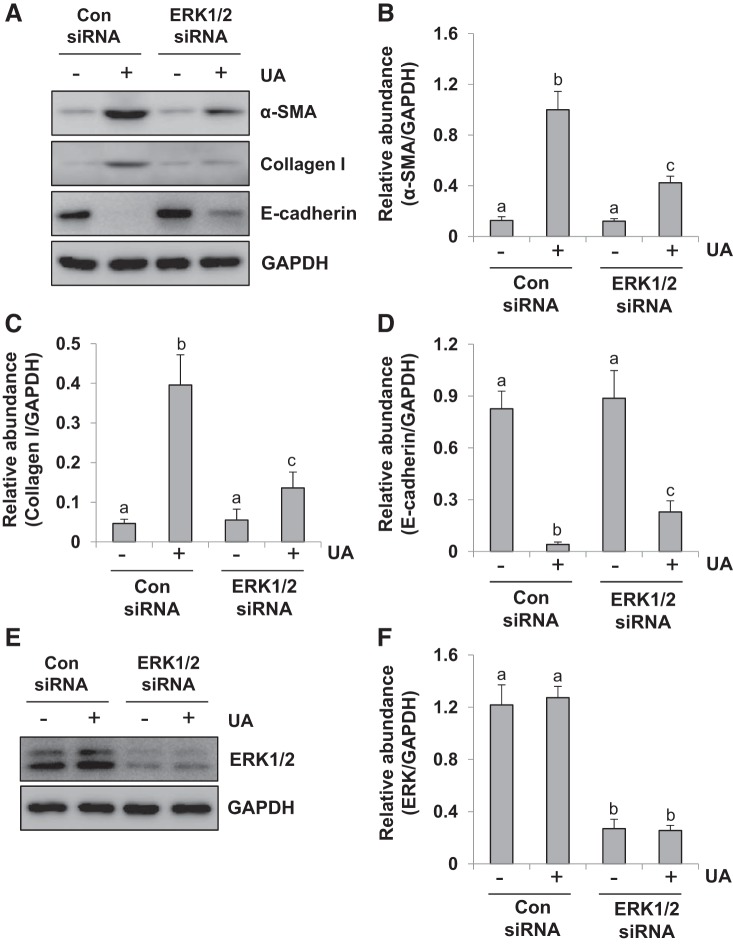
We further validated the effect of siRNA-mediated silencing of ERK1/2 on the expression of α-SMA, collagen I, and E-cadherin in HK2 cells exposed to uric acid. Serum-starved HK2 cells were transfected with siRNA targeting ERK1/2 or scrambled siRNA and exposed to uric acid (800 μM) for 36 h. Then, cells were harvested for immunoblot analysis. Figure 3, A–F shows that transfection of ERK1/2 siRNA reduced ERK1/2 and suppressed enhancement of α-SMA and collagen I and preserved expression of E-cadherin in response to uric acid. These data indicate that specific inhibition of ERK1/2 kinases with siRNA was also effective in reducing the EMT in HK2 cells. The similar inhibitory results with ERK1/2 siRNA and U0126 treatment further elucidate the importance of ERK1/2 in mediating uric acid-induced EMT in human tubular epithelial cells.
Fig. 3.
Silencing of ERK1/2 inhibits upregulation of α-smooth muscle actin (α-SMA) and collagen I and preserves E-cadherin expression in uric acid (UA)-treated human tubular epithelial (HK2) cells. Serum-starved HK2 cells were transfected with siRNA targeting ERK1/2 or scrambled siRNA and exposed to uric acid (800 μM) for 36 h. A: cell lysates were subjected to immunoblot analysis with specific antibodies against α-SMA, collagen I, E-cadherin, and GAPDH. Con, control. B–D: expression levels of α-SMA (B), collagen I (C), and E-cadherin (D) were quantitated by densitometry and normalized to GAPDH. E: immunoblot analysis was also performed with specific antibodies against ERK1/2. F: expression level of ERK1/2 was quantitated by densitometry and normalized to GAPDH. Values are means ± SE of at least three independent experiments. Means with different superscript letters are significantly different from one another (P < 0.05).
ERK1/2 inhibition reduces G2/M phase renal cell cycle arrest in the kidney of hyperuricemic rats.
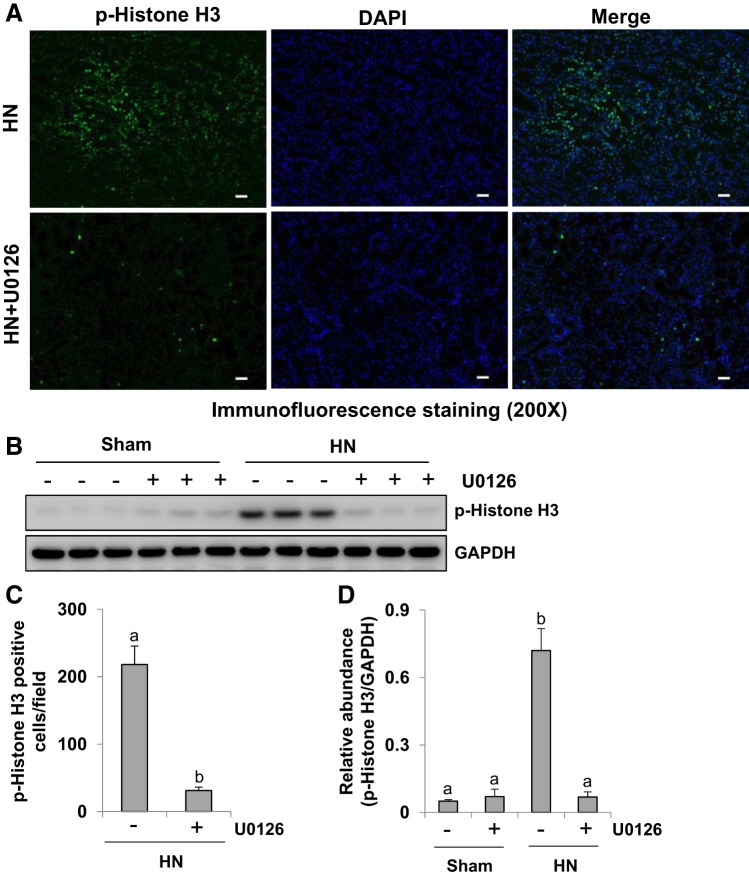
Renal tubular cells undergoing EMT tend to promote maladaptive repair, resulting in proximal tubular cell cycle arrest in the G2/M phase, characterized by high expression of phospho-histone H3 at serine 10, a hallmark of cells in the G2/M phase of the cell cycle (27). To elucidate whether ERK1/2 activation is involved in this process, we examined the effect of U0126 on the expression of phospho-histone H3 in the kidney of HN rats by immunofluorescence staining and immunoblot analysis. As indicated in Fig. 4, A and C, immunofluorescence staining analysis showed that phospho-histone H3 was highly expressed in the nuclei of renal tubular cells in HN rats, and ERK1/2 blockade dramatically reduced the number of phospho-histone H3-positive cells. Immunoblot analysis indicated that expression of phospho-histone H3 was upregulated in the kidney of rats after hyperuricemic injury, and inhibition of ERK1/2 by U0126 suppressed its expression (Fig. 4, B and D). Taken together, our data illustrate that ERK1/2 inhibition suppresses renal tubular cell arrest at the G2/M phase of the cell cycle in kidneys with hyperuricemic injury.
Fig. 4.
Administration of U0126 reduces expression of phospho (p-)histone H3 in the kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: photomicrographs (×200) illustrating p-histone H3 (serine 10) staining of kidney tissues of hyperuricemic rats treated with or without U0126. B: prepared kidney tissue lysates from sham and HN rats treated or untreated with U0126 were subjected to immunoblot analysis with specific antibodies against p-histone H3 (serine 10) and GAPDH. C: positive p-histone H3 (serine 10)-stained cells were counted and expressed as means ± SE. D: expression level of p-histone H3 (serine 10) was quantified by densitometry and normalized to GADPH. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). All scale bars, 20 μm.
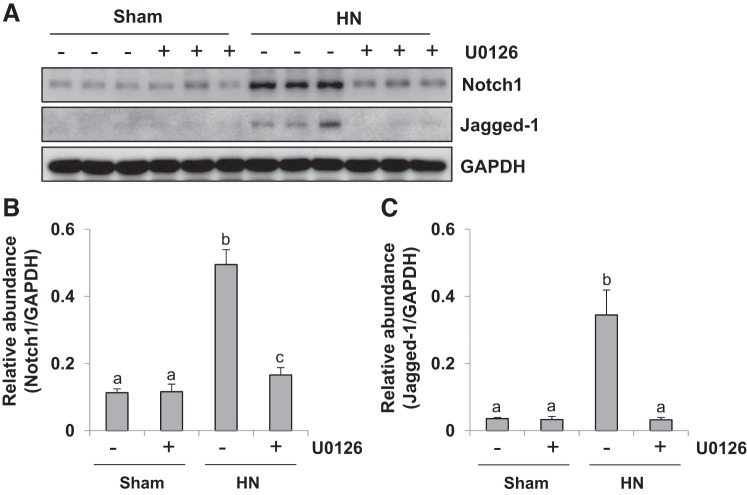
ERK1/2 inhibition blocks Notch/Jagged-1 signaling pathways in the kidney of hyperuricemic rats.
It was reported that activation of the Notch signaling pathway promotes the process of EMT and the progression of renal interstitial fibrosis (4, 48). ERK1/2 can modulate Notch signaling by regulating the expression of its ligand Jagged-1 (18, 30, 34). To further explore the role of ERK1/2 in regulating Notch1/Jagged-1 signaling in the hyperuricemic kidney, we examined the expression of Notch1 and its ligand Jagged-1 after ERK1/2 blockade. As indicated in Fig. 5, A–C, low expression of Notch1 and Jagged-1 was detected in sham kidneys. However, protein levels of both of them were increased significantly in rat kidney after hyperuricemic injury. Treatment with U0126 suppressed uric acid-induced expression of Notch1 and Jagged-1. These data indicate that the Notch signaling pathway is also subject to regulation by ERK1/2 during the process of EMT in hyperuricemic kidney.
Fig. 5.
U0126 treatment suppresses activation of Notch1/Jagged-1 signaling pathway in the kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against Notch1, Jagged-1, and GAPDH. B: expression level of Notch1 was quantified by densitometry and normalized to GAPDH. C: expression level of Jagged-1 was quantified by densitometry and normalized to GAPDH. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
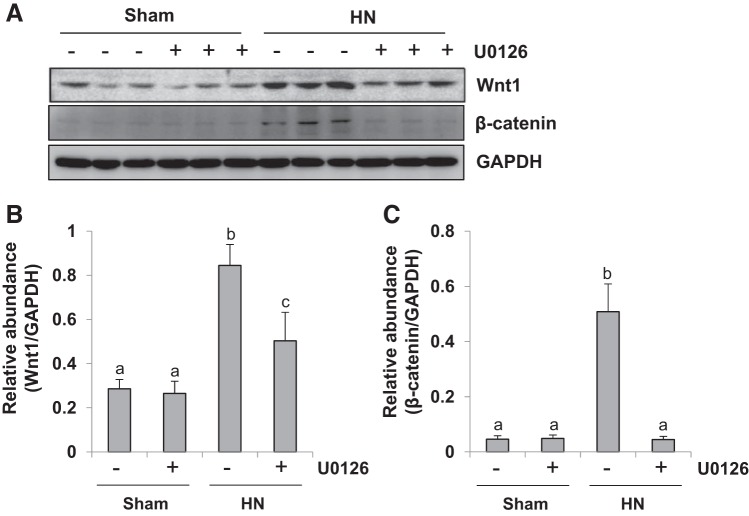
ERK1/2 inhibition abrogates Wnt/β-catenin signaling pathways in the kidney of hyperuricemic rats.
Tubule-derived Wnt plays a vital role in promoting renal tubulointerstitial fibrosis via epithelial-mesenchymal communication (63). To elucidate the mechanisms by which ERK1/2 mediates renal fibrosis, we examined the effect of U0126 on the expression of Wnt1 and β-catenin in the rat model of HN. As indicated in Fig. 6, A–C, there was also enhancement in the expression of Wnt1 and β-catenin after hyperuricemic injury. Administration of U0126 remarkably inhibited the expression of Wnt1 and β-catenin. Thus, ERK1/2 inhibition blocks the Wnt/β-catenin signaling pathway. Given the important role of Wnt in EMT, ERK1/2 inhibition may possibly retard the process of EMT by suppressing the Wnt pathway in the hyperuricemic kidney.
Fig. 6.
U0126 treatment inhibits activation of the Wnt-1/β-catenin signaling pathway in kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against Wnt1, β-catenin, and GAPDH. B: expression level of Wnt1 was quantified by densitometry and normalized to GAPDH. C: expression level of β-catenin was quantified by densitometry and normalized to GAPDH. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
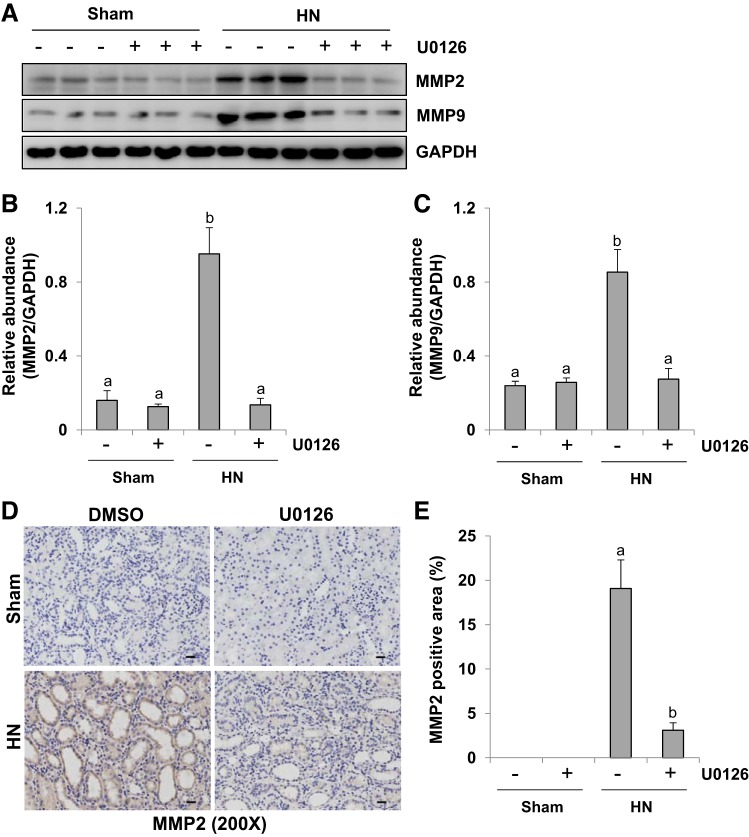
ERK1/2 inhibition suppresses expression of MMP-2 and MMP-9 in the kidney of hyperuricemic rats.
Renal fibrosis occurs as a consequence of tubular cell EMT (50). MMP-2 and MMP-9 have been recognized as promoters of EMT via basement membrane degradation (9, 49). To investigate the mechanism by which ERK1/2 regulates the EMT, we examined the effect of U0126 on the expression of MMP-2 and MMP-9 in the rat model of HN. As shown in immunoblot analysis of whole kidney tissue lysate collected after 3 wk of hyperuricemic stimulus, there was an increase in the expression of MMP-2 and MMP-9. Administration of U0126 significantly reversed these expression levels to base levels (Fig. 7, A–C). Immunohistochemistry staining also showed increased expression of MMP-2 in the kidney of HN, whereas ERK1/2 blockade strongly reduced its expression (Fig. 7, D and E). Collectively, our data suggest that ERK1/2 inactivation inhibits the expression of MMP-2 and MMP-9, which may contribute to attenuation of EMT in the kidney of hyperuricemic rats.
Fig. 7.
Administration of U0126 decreases expression of matrix metalloproteinases MMP-2 and MMP-9 in the kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: kidney tissue lysates from sham or hyperuricemic kidneys of rats treated with or without U0126 were subjected to immunoblot analysis with specific antibodies against MMP-2, MMP-9, and GAPDH. B: expression level of MMP-2 was quantified by densitometry and normalized to GAPDH. C: expression level of MMP-9 was quantified by densitometry and normalized to GAPDH. D: photomicrographs (×200) illustrating MMP-2 immunochemistry staining of kidney tissue. E: MMP-2 staining graphic presentation of quantitative data. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). All scale bars, 20 μm.
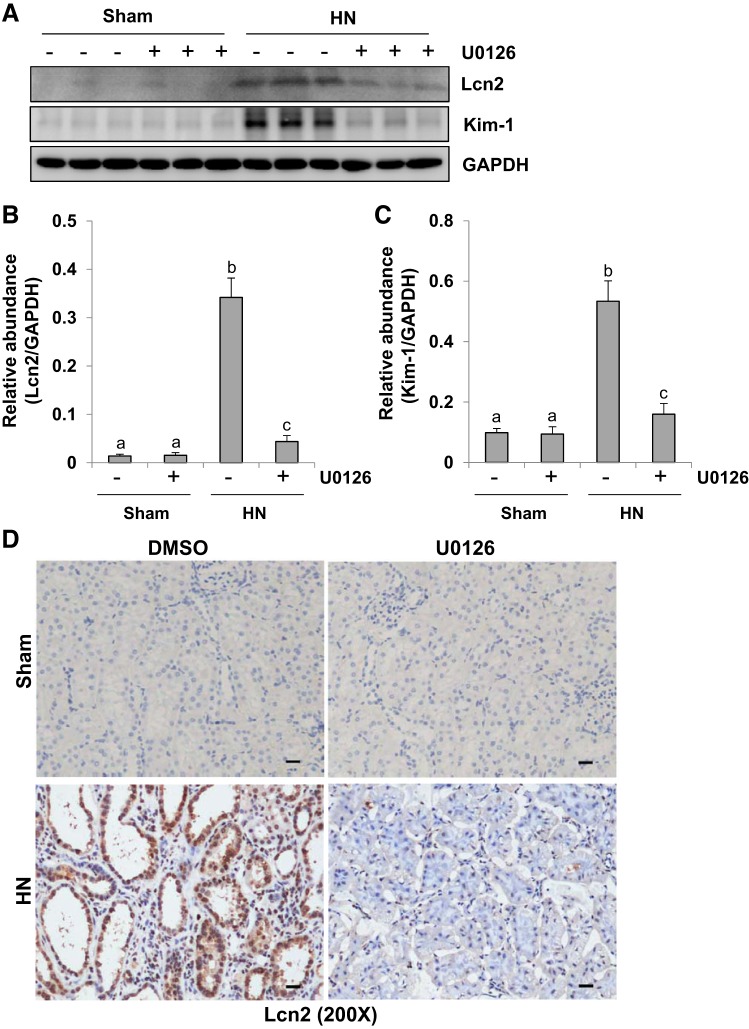
Inhibition of ERK1/2 diminishes hyperuricemia-induced renal tubular injury.
Lipocalin-2 (Lcn2) and kidney injury molecule 1 (Kim-1) are well-known tubular injury biomarkers both in AKI and CKD (15, 16, 32, 36, 53, 57). To assess the effect of ERK1/2 blockade on their expression, we examined the protein levels of Lcn2 and Kim-1 in the kidney of HN treated or untreated with U0126 by immunoblot analysis. As indicated in Fig. 8, A–C, the expression levels of Lcn2 and Kim-1 were barely detected in the kidney of sham kidney with or without administration of U0126, but their expression levels were increased in the kidney of HN. Treatment with U0126 largely suppressed hyperuricemia-induced expression of Lcn2 and Kim-1. These results were confirmed by immunohistochemistry staining of Lcn2. Lcn2 was highly expressed in renal tubules of the kidney with HN. Lcn2-positive staining dots were also detected in the lumen of dilated tubules, which suggested that these debris were from detached tubular cells. Administration of U0126 significantly suppressed this response (Fig. 8D). Collectively, these data suggest that ERK1/2 activation contributes to renal tubular cell injury in HN as well.
Fig. 8.
Administration of U0126 attenuates tubular damage in the kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A: kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against lipocalin-2 (Lcn2), kidney injury molecule 1 (Kim-1), and GAPDH. B: expression level of Lcn2 was quantified by densitometry and normalized to GAPDH. C: expression level of Kim-1 was quantified by densitometry and normalized to GAPDH. D: photomicrographs (×200) illustrating Lcn2 immunochemistry staining of kidney tissue. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). All scale bars, 20 μm.
ERK1/2 blockade reduces renal tubular cell apoptosis in the kidney of hyperuricemic rats and uric acid-induced human tubular epithelial cells.
Activation of apoptotic pathways promotes the loss of renal epithelial cells during acute and chronic kidney diseases (41). Cleaved caspase-3 is a pivotal enzyme responsible for the execution of apoptosis (42). To examine the effect of ERK1/2 inhibition on tubular cell apoptosis, we measured expression of cleaved caspase-3 in the hyperuricemic kidney by immunoblot analysis. As indicated in Fig. 9, A and B, the expression of cleaved caspase-3 was upregulated in the kidney of HN, and U0126 treatment largely diminished this response. Cleaved caspase-3 was not observed in the kidney of sham rats with or without U0126 administration. Therefore, our data illustrate that inhibition of ERK1/2 activity by U0126 reduces renal tubular cell apoptosis in the hyperuricemic kidney.
Fig. 9.
Administration of U0126 decreases renal tubular cell apoptosis in the kidney of hyperuricemic rats and uric acid (UA)-treated human tubular epithelial (HK2) cells. HN, hyperuricemic nephropathy. A: kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against cleaved caspase-3 and GAPDH. B: expression level of cleaved-caspase-3 was quantified by densitometry and normalized to GAPDH. Data are presented as means ± SE (n = 6). Serum-starved HK2 cells were pretreated with U0126 (0, 5, and 10 μM) for 1 h and then exposed to uric acid(800 μM) to induce cell injury in the presence of U0126 (0, 5, and 10 μM) for an additional 36 h. C: cell lysates were subjected to immunoblot analysis with specific antibodies against cleaved caspase-3 and β-actin. D: expression level of cleaved caspase-3 was quantitated by densitometry and normalized to β-actin. In addition, serum-starved HK2 cells were transfected with siRNA targeting ERK1/2 or scrambled siRNA and exposed to UA (800 μM) for 36 h. E: cell lysates were subjected to immunoblot analysis with specific antibodies against cleaved caspase-3 and GAPDH. F: expression level of cleaved caspase-3 was quantitated by densitometry and normalized to GAPDH. Values are means ± SE of at least three independent experiments. Means with different superscript letters are significantly different from one another (P < 0.05).
To further elucidate the role of ERK1/2 in uric acid-induced tubular cell apoptosis, we examined the effect of U0126 on uric acid-induced cleavage of caspase-3 in cultured human tubular epithelial cells. Fig. 9, C and D, showed that the basal level of cleaved caspase-3 was detectable by immunoblot analysis in HK2 cells starved for 36 h, and exposure to uric acid increased expression of the active form of this enzyme, whereas treatment with U0126 reduced cleaved caspase-3 to a level lower than its basal expression. In addition, Fig. 9, E and F, showed that transfection of ERK1/2 siRNA also inhibited the expression of cleaved caspase-3 in response to uric acid. These data confirm the importance of ERK1/2 in mediating renal tubular cell apoptosis and activation of the external apoptotic pathway in human tubular epithelial cells.
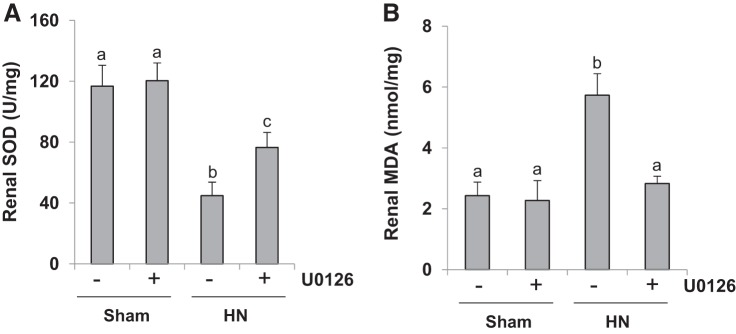
ERK1/2 inhibition reduces oxidative stress in the kidney of hyperuricemic rats.
Oxidative stress is one of important mechanisms involved in the pathogenesis of kidney diseases (47). To further explore the role of ERK1/2 in HN, we measured the content of SOD and MDA, two sensitive biomarkers of oxidative stress, in the kidney (47). As shown in Fig. 10, U0126 significantly reduced the MDA level and preserved expression of SOD in the kidneys of HN rats. Therefore, ERK1/2 inhibition may also alleviate hyperuricemic nephropathy by suppressing oxidative stress.
Fig. 10.
Treatment with U0126 reduces oxidative stress in the kidney of hyperuricemic rats. HN, hyperuricemic nephropathy. A and B: superoxide dismutase (SOD; A) and malondialdehyde (MDA; B) in kidney tissues were measured according to the protocol described in materials and methods. Data are presented as means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
DISCUSSION
Although accumulating evidence has suggested that hyperuricemia facilitates development and progression of kidney fibrosis and contributes to CKD (20, 23), the underlying mechanisms are largely unknown. Although our previous studies have illustrated that ERK1/2 mediates hyperuricemic nephropathy (HN) in a rat model and that this process is involved in the activation of TGF-β signaling, increased inflammation responses, and reduced uric acid excretion in the kidney (24), the regulatory mechanism responsible for tubular epithelial-mesenchymal transition (EMT) and tubular cell injury in the hyperuricemic kidney remains obscure. Our present study further investigated the role of ERK1/2 in HN and found that administration of ERK1/2 inhibitor U0126 protected the kidney from EMT through inactivation of multiple signaling pathways and downregulation of matrix metalloproteinase expression. In addition, inactivation of ERK1/2 attenuated uric acid-induced tubule injury by enhancing cellular resistance to oxidative stress and apoptosis. Thus, we identify ERK1/2 as an important regulator of renal EMT and tubular cell injury and suggest that it could be a novel target for treatment of HN.
EMT involves up-expression of vimentin, and downregulation of E-cadherin, a major component of adherens junctions (35). Snail transcription factor has been recognized as a strong repressor of E-cadherin in epithelial cell lines (35). It was reported that ERK1/2 can directly regulate expression of Snail at a transcriptional level through direct binding to the Snail promoter region (31, 44). As such, we examined the effect of ERK1/2 inhibition with U0126 on the development of renal EMT in the rat model of HN. Our results showed that the ERK1/2 inhibitor significantly suppresses Snail1 expression and reverses hyperuricemic-induced decreased expression of E-cadherin. Thus, ERK1/2 plays an important role in uric acid-induced renal EMT.
Notch activation in renal tubular epithelial cells induces the expression of mesenchymal genes and loss of differentiated epithelial markers, leading to EMT and subsequently to renal fibrogenesis (4). Intriguingly, ERK1/2 can modulate Notch signaling by increasing the expression of its ligand Jagged-1 (18, 30, 34). In the current study, we found that inhibition of ERK1/2 with U0126 abolished uric acid-elicited upregulation of Jagged-1 and Notch in the kidney of HN rats. Thus, we suggest that ERK1/2 blockade may also suppress EMT and renal fibrosis by suppressing the Jagged/Notch signaling pathway.
β-Catenin functions as a component of adherens junctions and links E-cadherin to the cytoskeleton (25, 54). Given the complex structure of the cytoplasmic domain of E-cadherin and β-catenin, proteolytic shedding of the E-cadherin ectodomain causes the nuclear translocation of β-catenin (62). The translocation of β-catenin from the plasma membrane to the nucleus induces transcription repressors, which further facilitates the loss of E-cadherin (62). This Wnt-independent β-catenin transactivation is observed to be due to the loss of E-cadherin and consequent release of free β-catenin, mimicking Wnt signaling (28). In addition, multiple feedback loops in ERK1/2 and the Wnt-dependent β-catenin signaling pathway also account for the activation of the transcriptional repressor Snail1, resulting in the development of EMT and fibrosis (44). Our data indicate that ERK1/2 activity is necessary for activation of the β-catenin pathway in HN rats. On this basis, we speculate that the ERK1/2 blockade-induced EMT attenuation effect is also associated with suppression of the Wnt-independent β-catenin pathway.
It is well known that integrity of the underlying basal lamina is required for the maintenance of a polarized epithelial phenotype (59). The enzymatic degradation of basement membrane components laminin and type IV collagen by specific MMPs such as MMP-2 and MMP-9, disrupts cell-cell or cell-matrix attachment and facilitates the epithelial-mesenchymal transformation (9, 49). It was reported that phosphorylated ERK can bind to the promoter of MMP-2 and MMP-9 and facilitate their transcription (8, 14, 43, 56). Here, we have further demonstrated that ERK1/2 inhibition reduces expression levels of these two proteinases. This suggests that suppression of MMP-2 and MMP-9 expression are also important steps for ERK1/2 inhibition-elicited attenuation of renal EMT.
The functional consequence of renal EMT after chronic injury is the development of proximal tubular cells arrested at the G2/M stage of the cell cycle (27). Tubular epithelial cells under this type of maladaptive repair represent a profibrotic phenotype and produce numerous growth factors and cytokines such as TGF-β1, interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α). All of those cytokines are associated with fibroblast activation and extracellular matrix deposition, as indicated in previous studies (23, 24). In accordance with this concept, we found that ERK1/2 inhibition by U0126 effectively inhibits the arrest of tubular cells at the G2/M phase in the kidney of HN.
ERK1/2 signaling is generally pro-survival. Numerous oncology studies have demonstrated the pro-survival role of ERK1/2 signaling, mainly by regulating the activity of the Bcl-2 (B cell lymphoma 2) family of proteins (12, 13, 61). However, depending on the different cell types and stimuli, ERK1/2 activity can also play an antiproliferative role (29, 64). ERK1/2 activation can promote the extrinsic pathway of apoptosis by activating the TNF receptor family that promotes the recruitment and activation of initiator caspase-8 (10, 45). ERK1/2 activation is also involved in the activation of the intrinsic apoptotic pathway by mediating Bcl-2 family proteins and increasing the death-promoting factors released from the mitochondria, subsequently activing initiator caspase-9, which in turn activates executioner caspases such as caspase-3 or -7 (5). This ERK1/2 activation-induced intrinsic apoptotic pathway may relate to tissue injury-elicited oxidative stress. It is often triggered by DNA-damaging agents (21), particularly implicated in cisplatin-mediated renal cell apoptosis (2, 19, 33, 51). Consistently, our results demonstrated ERK1/2 activation facilitates oxidative stress and apoptosis in uric acid-induced nephropathy. Since oxidative stress is an upstream activator of apoptosis (11), the caspase-3-dependent apoptosis pathway is triggered in response to cell microenvironment fluctuation (41, 42). Therefore, we speculate that ERK1/2 inhibition-mediated suppression of oxidative stress may inhibit renal tubular cell apoptosis and attenuate tubular cell injury.
In summary, our study demonstrates that ERK1/2 is a pivotal regulator of renal EMT and tubular cell injury in the hyperuricemic kidney. The anti-EMT actions of ERK1/2 blockade are involved in the preservation of E-cadherin expression, inhibition of transcription factor activation, inactivation of the Jagged-1/Notch and Wnt/β-catenin signaling pathways, and decreases of MMP-2 and MMP-9. ERK1/2 blockade also rescues renal cells from G2/M arrest, thus avoiding maladaptive repair. The anti-renal injury actions of ERK1/2 inhibition are associated with the resistance of renal tubular cells to oxidative stress and subsequent apoptosis. Thus, pharmacological inhibition of ERK1/2 could have therapeutic potential for treatment of hyperuricemic nephropathy.
GRANTS
This study was supported by the National Nature Science Foundation of China Grants 81670690, 81470991, and 81200492 (to N. Liu), Grants 81270778, 81470920, 81670623, and 81830021 (to S. Zhuang), and Grant 81500059 (to L. Tang); Key Discipline Construction Project of Pudong Health Bureau of Shanghai Grant PWZxk2017-05 (to N. Liu); Jiangxi Province Municipal Health Commission Science Technology Grant 20184077 (to L. Fang); Ministry of Science and Technology Branch National Key Grant 2018YFA0108802 (to S. Zhuang); Shanghai Scientific Committee of China Grant 13PJ1406900 (to N. Liu); and National Institute of Diabetes and Digestive and Kidney Diseases Grant 2R01 DK-08506505A1 (to S. Zhuang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.L. conceived and designed research; M.T., Y.S., L.T., Y.W., L.F., W.J., T.L., and N.L. performed experiments; M.T., Y.S., and N.L. analyzed data; M.T., Y.S., and N.L. prepared figures; M.T. drafted manuscript; Y.S., L.T., L.F., A.Q., S.Z., and N.L. edited and revised manuscript; M.T., Y.S., L.T., Y.W., L.F., W.J., T.L., A.Q., S.Z., and N.L. approved final version of manuscript.
REFERENCES
- 1.Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, van de Water B. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol 171: 452–462, 2007. doi: 10.2353/ajpath.2007.060805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol 287: F543–F549, 2004. doi: 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bao J, Shi Y, Tao M, Liu N, Zhuang S, Yuan W. Pharmacological inhibition of autophagy by 3-MA attenuates hyperuricemic nephropathy. Clin Sci (Lond) 132: 2299–2322, 2018. doi: 10.1042/CS20180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040–4054, 2010. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J 277: 2–21, 2010. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 6.Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 30: 575–583, 2015. doi: 10.1093/ndt/gfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res 347: 103–116, 2012. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen CM, Hsieh SC, Lin CL, Lin YS, Tsai JP, Hsieh YH. Alpha-mangostin suppresses the metastasis of human renal carcinoma cells by targeting MEK/ERK expression and MMP-9 transcription activity. Cell Physiol Biochem 44: 1460–1470, 2017. doi: 10.1159/000485582. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 162: 1937–1949, 2003. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Qiu F, Tashiro S, Onodera S, Ikejima T. ERK and JNK mediate TNFalpha-induced p53 activation in apoptotic and autophagic L929 cell death. Biochem Biophys Res Commun 376: 483–488, 2008. doi: 10.1016/j.bbrc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Choe JY, Park KY, Kim SK. Oxidative stress by monosodium urate crystals promotes renal cell apoptosis through mitochondrial caspase-dependent pathway in human embryonic kidney 293 cells: mechanism for urate-induced nephropathy. Apoptosis 20: 38–49, 2015. doi: 10.1007/s10495-014-1057-1. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, Greninger P, Brown RD, Godfrey JT, Cohoon TJ, Song Y, Lifshits E, Hung KE, Shioda T, Dias-Santagata D, Singh A, Settleman J, Benes CH, Mino-Kenudson M, Wong KK, Engelman JA. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 23: 121–128, 2013. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 118: 3651–3659, 2008. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong QZ, Wang Y, Tang ZP, Fu L, Li QC, Wang ED, Wang EH. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol 182: 954–964, 2013. doi: 10.1016/j.ajpath.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner L, Akintola A, Chen G, Catania JM, Vaidya V, Burghardt RC, Bonventre JV, Trzeciakowski J, Parrish AR. Structural equation modeling highlights the potential of Kim-1 as a biomarker for chronic kidney disease. Am J Nephrol 35: 152–163, 2012. doi: 10.1159/000335579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang M, Zhang J, Xu H, Ding T, Tang D, Yuan Q, Tao L, Ye Z. The TGFβ-ERK pathway contributes to Notch3 upregulation in the renal tubular epithelial cells of patients with obstructive nephropathy. Cell Signal 51: 139–151, 2018. doi: 10.1016/j.cellsig.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFβ pathways as fundamental Notch regulators in breast cancer. Proc Natl Acad Sci USA 110: 1714–1719, 2013. doi: 10.1073/pnas.1214014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int 67: 458–466, 2005. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 71: 851–865, 2018. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta 1763: 958–968, 2006. doi: 10.1016/j.bbamcr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, Bao W, Chin YE, Cheng SB, Yan H, Qiu A, Zhuang S. EGF receptor inhibition alleviates hyperuricemic nephropathy. J Am Soc Nephrol 26: 2716–2729, 2015. doi: 10.1681/ASN.2014080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu N, Xu L, Shi Y, Fang L, Gu H, Wang H, Ding X, Zhuang S. Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats. Oncotarget 8: 33807–33826, 2017. doi: 10.18632/oncotarget.16995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long CL, Qin XC, Pan ZY, Chen K, Zhang YF, Cui WY, Liu GS, Wang H. Activation of ATP-sensitive potassium channels protects vascular endothelial cells from hypertension and renal injury induced by hyperuricemia. J Hypertens 26: 2326–2338, 2008. doi: 10.1097/HJH.0b013e328312c8c1. [DOI] [PubMed] [Google Scholar]
- 27.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21: 998–1009, 2015. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle 3: 569–573, 2004. doi: 10.4161/cc.3.5.885. [DOI] [PubMed] [Google Scholar]
- 29.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31: 268–275, 2006. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Neradugomma NK, Subramaniam D, Tawfik OW, Goffin V, Kumar TR, Jensen RA, Anant S. Prolactin signaling enhances colon cancer stemness by modulating Notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis 35: 795–806, 2014. doi: 10.1093/carcin/bgt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo HK, Lee HG, Piao JY, Zhong X, Lee HN, Han HJ, Kim W, Kim DH, Cha YN, Na HK, Surh YJ. Helicobacter pylori induces Snail expression through ROS-mediated activation of Erk and inactivation of GSK-3β in human gastric cancer cells. Mol Carcinog 55: 2236–2246, 2016. doi: 10.1002/mc.22464. [DOI] [PubMed] [Google Scholar]
- 32.Nickolas TL, Forster CS, Sise ME, Barasch N, Solá-Del Valle D, Viltard M, Buchen C, Kupferman S, Carnevali ML, Bennett M, Mattei S, Bovino A, Argentiero L, Magnano A, Devarajan P, Mori K, Erdjument-Bromage H, Tempst P, Allegri L, Barasch J. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int 82: 718–722, 2012. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem 277: 43377–43388, 2002. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyhan KC, Faherty N, Murray G, Cooey LB, Godson C, Crean JK, Brazil DP. Jagged/Notch signalling is required for a subset of TGFβ1 responses in human kidney epithelial cells. Biochim Biophys Acta 1803: 1386–1395, 2010. doi: 10.1016/j.bbamcr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem 278: 21113–21123, 2003. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 36.Peters HP, Waanders F, Meijer E, van den Brand J, Steenbergen EJ, van Goor H, Wetzels JF. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant 26: 3581–3588, 2011. doi: 10.1093/ndt/gfr135. [DOI] [PubMed] [Google Scholar]
- 37.Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol 64: 755–763, 2002. doi: 10.1016/S0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 38.Qin J, Mei WJ, Xie YY, Huang L, Yuan QJ, Hu GY, Tao LJ, Peng ZZ. Fluorofenidone attenuates oxidative stress and renal fibrosis in obstructive nephropathy via blocking NOX2 (gp91phox) expression and inhibiting ERK/MAPK signaling pathway. Kidney Blood Press Res 40: 89–99, 2015. doi: 10.1159/000368485. [DOI] [PubMed] [Google Scholar]
- 39.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9: 13–23, 2013. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol 304: F471–F480, 2013. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 41.Sanz AB, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol 19: 1634–1642, 2008. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 42.Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochemistry (Mosc) 80: 1393–1405, 2015. doi: 10.1134/S0006297915110012. [DOI] [PubMed] [Google Scholar]
- 43.Shi MD, Shih YW, Lee YS, Cheng YF, Tsai LY. Suppression of 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 breast adenocarcinoma cells invasion/migration by α-tomatine through activating PKCα/ERK/NF-κB-dependent MMP-2/MMP-9 expressions. Cell Biochem Biophys 66: 161–174, 2013. doi: 10.1007/s12013-012-9465-8. [DOI] [PubMed] [Google Scholar]
- 44.Shin SY, Rath O, Zebisch A, Choo SM, Kolch W, Cho KH. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res 70: 6715–6724, 2010. doi: 10.1158/0008-5472.CAN-10-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivaprasad U, Basu A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med 12: 1265–1271, 2008. doi: 10.1111/j.1582-4934.2008.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35: 600–604, 2015. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 47.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 4: 208–214, 2015. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweetwyne MT, Tao J, Susztak K. Kick it up a notch: Notch signaling and kidney fibrosis. Kidney Int Suppl (2011) 4: 91–96, 2014. doi: 10.1038/kisup.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan TK, Zheng G, Hsu TT, Wang Y, Lee VW, Tian X, Wang Y, Cao Q, Wang Y, Harris DC. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol 176: 1256–1270, 2010. doi: 10.2353/ajpath.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int 76: 1248–1257, 2009. doi: 10.1038/ki.2009.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang J, Shi Y, Liu N, Xu L, Zang X, Li P, Zhang J, Zheng X, Qiu A, Zhuang S. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 132: 339–359, 2018. doi: 10.1042/CS20171417. [DOI] [PubMed] [Google Scholar]
- 52.Verzola D, Ratto E, Villaggio B, Parodi EL, Pontremoli R, Garibotto G, Viazzi F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One 9: e115210, 2014. doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 9: E171, 2017. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Jiang J, Guan Q, Wang H, Nguan CY, Jevnikar AM, Du C. Reduction of chronic allograft nephropathy by inhibition of extracellular signal-regulated kinase 1 and 2 signaling. Am J Physiol Renal Physiol 295: F672–F679, 2008. doi: 10.1152/ajprenal.90285.2008. [DOI] [PubMed] [Google Scholar]
- 56.Wu Z, Wang T, Fang M, Huang W, Sun Z, Xiao J, Yan W. MFAP5 promotes tumor progression and bone metastasis by regulating ERK/MMP signaling pathways in breast cancer. Biochem Biophys Res Commun 498: 495–501, 2018. doi: 10.1016/j.bbrc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Yang HT, Yim H, Cho YS, Kym D, Hur J, Kim JH, Chun W, Kim HS. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit Care 18: R151, 2014. doi: 10.1186/cc13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Zhang N, Gao R, Zhu Y, Zhang Z, Xu X, Wang J, Li Z, Liu X, Li Z, Li J, Bi J, Kong C. TGF-β1 induced fascin1 expression facilitates the migration and invasion of kidney carcinoma cells through ERK and JNK signaling pathways. Biochem Biophys Res Commun 501: 913–919, 2018. doi: 10.1016/j.bbrc.2018.05.081. [DOI] [PubMed] [Google Scholar]
- 59.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Müller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 159: 1313–1321, 2001. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Zhang J, Liu X, Liu S, Tian J. Tribbles 3 regulates the fibrosis cytokine TGF-β1 through ERK1/2-MAPK signaling pathway in diabetic nephropathy. J Immunol Res 2014: 1–11, 2014. doi: 10.1155/2014/240396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, McCubrey J, Cortes J, Andreeff M. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 22: 808–818, 2008. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 62.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, Alexander SI, Harris DC. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol 175: 580–591, 2009. doi: 10.2353/ajpath.2009.080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou D, Fu H, Zhang L, Zhang K, Min Y, Xiao L, Lin L, Bastacky SI, Liu Y. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol 28: 2322–2336, 2017. doi: 10.1681/ASN.2016080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther 319: 991–997, 2006. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]