ABSTRACT
Background
Gout is a frequently occurring, complex rheumatologic form of inflammatory arthritis caused by the accumulation of serum uric acid (sUA) and deposition of uric acid crystals in the joints and tissues of the body. Hyperuricemia is also a significant independent risk factor for all-cause and cardiovascular morbidity and mortality and is associated with hypertension, diabetes, obesity, and osteoarthritis. However, patient adherence to prescribed urate-lowering therapies ranges from 20% to 70%, suggesting that other additional strategies, such as dietary intervention with specific, efficacious foods or beverages, may be necessary to mitigate the risk of arthritis, as well as other comorbidities. Tart cherry juice (TCJ) has been used for decades by some for gout based largely on anecdotal evidence of its efficacy and its antioxidant and anti-inflammatory properties.
Objectives
We designed this study to test the effect of TCJ on uricemia, lipidemia, glycemia, and inflammation in at-risk overweight and obese humans with a specific hypothesis that TCJ consumption would reduce sUA concentrations.
Methods
In this randomized, placebo-controlled crossover study, we recruited overweight and obese participants with body mass index (BMI) >25.0 kg/m2 (n = 26, 18 women/8 men, 41 ±11 y; BMI 31.3 ± 6.0; 12 obese, 14 overweight) to consume 240 mL/d (8 oz/d) of either TCJ or placebo beverage, for 4 wk each with a 4-wk intervening washout period followed by 4 wk of the alternate beverage.
Results
TCJ significantly reduced sUA concentration by 19.2% (P < 0.05) and reduced by 19.4% (P = 0.09) and 6.3% (P = 0.08) proinflammatory high-sensitivity C-reactive protein and monocyte chemoattractant protein-1, respectively. The participants in this study displayed risk ratios indicating increased cardiovascular disease risk and insulin resistance but no differences in the pre- and postintervention groups of either placebo or TCJ groups.
Conclusion
Collectively, the data suggest that 100% TCJ reduces sUA concentrations, mitigating hyperuricemia associated with gouty arthritis. This trial was registered at clinicaltrials.gov as NCT03636529.
Keywords: tart cherry juice, metabolic syndrome, serum urate, overweight/obese, hsCRP, MCP-1
Introduction
Gout is a frequently occurring, complex rheumatologic form of inflammatory arthritis caused by the accumulation of serum uric acid (sUA) and deposition of uric acid crystals in the joints and tissues of the body (1). This deposition leads to heat, redness, and swelling with considerable accompanying tenderness and intense pain. Gout affects 8.3 million people in the United States and occurs in ∼4% of the population, with the risk increasing to ∼10% with age (>80 y) (2). Normally, uric acid produced via the metabolism of purines is solubilized in blood until renal filtration and excretion via the urine. However, excessive production of uric acid due to high dietary intake of purines or decreased renal elimination, or a combination of the two, results in accumulation of sUA, a condition known as hyperuricemia, and in some individuals ultimately precipitation of sharp, needlelike monosodium urate crystals and chronic tophi formation.
Fortunately, efficacious pharmacologic treatments are available for those with recurring gout (3–5). However, patient adherence to prescribed urate-lowering therapies (ULTs) ranges from 20% to 70%, suggesting that other additional strategies such as dietary intervention with specific, efficacious foods or beverages, e.g., tart cherry juice (TCJ) may be necessary to mitigate the risk of arthritis, as well as other comorbidities (1).
Hyperuricemia is a significant independent risk factor for all-cause and cardiovascular morbidity and mortality and is associated with hypertension, diabetes, obesity, and osteoarthritis (2). In 2 studies, the risk of diabetes among women and men in a population-based, BMI-matched cohort study found gout to be independently associated with an increased risk of diabetes, with the risk greater for women (6, 7). In veterans (n = 1923), hyperuricemia was significantly associated with diabetes risk and ∼1 in 11 (9%) of the new cases were strongly and statistically attributed to hyperuricemia (8). The current epidemiology of gouty arthritis indicates a rising prevalence worldwide, closely related with the epidemics of obesity and metabolic syndrome (MetS). MetS includes the occurrence of ≥3 of the following 5 risk factors: 1) waist circumference (WC) >0.89 m (>35 in) for women and >1.02 m (>40 in) for men; 2) hypertriglyceridemia [>1.69 mM (>150 mg/dL)]; 3) low HDL cholesterol [<1.29 mM (<50 mg/dL) for women, <1.03 mM (<40 mg/dL) for men]; 4) hypertension (blood pressure >130/85 mm Hg); and 5) hyperglycemia [>5.6 mM (>100 mg/dL)] (9).
Since the 1950 article by Blau (10), there has been considerable, growing interest in the use of anthocyanin-rich tart cherries as a potentially effective option in the prevention and management of hyperuricemia and resultant gout for those susceptible to crystal deposition. This is particularly timely given the relatively low adherence by patients to ULTs and the increasing occurrence of gout worldwide. In a few published, small studies in healthy human subjects and animals, investigators have demonstrated that cherry consumption can significantly lower sUA concentrations (11, 12). We have also reported reductions in sUA, although not statistically significant, in a small (n = 10) study of overweight and obese adults (13). Others have reported that cherry product–derived, polyphenolic anthocyanins possess and exert anti-inflammatory and antioxidant activities, which are critical functions for interrupting cellular and tissue damage (14–18). In human studies, consumption of cherries (227 g/d, 3 mo) significantly reduced the occurrence of proinflammatory arthritis and in fasted healthy women reduced, within hours, circulating proinflammatory markers including high-sensitivity C-reactive protein (hsCRP) and NO (10, 19). We have also noted in a small, preliminary pilot study (n = 10) of overweight and obese adults (registered as NCT036038362), the reduction, after TCJ consumption for 4 wk, of the proinflammatory chemokine monocyte chemoattractant protein-1 (MCP-1), TNF-α, and a significant difference in erythrocyte sedimentation rate (ESR), a marker of chronic inflammation, compared with the placebo arm as well as a reduction in plasma TGs, VLDL, and TG/HDL cholesterol (13, 18, 20). TCJ has also been shown to decrease oxidative stress and inflammation induced by exercise in older individuals and marathon runners as well as in younger college students postexercise (21, 22). Collectively, data support the capacity of cherries, both sweet and tart, to reduce oxidative stress and inflammation associated frequently with the onset and elaboration of inflammation although additional studies are needed, particularly in at-risk human populations.
Our initial results from the small pilot study (n = 10) of overweight and obese adults discussed above provided the foundation for a larger (n = 26), second study (registered as NCT03636529) further exploring the effects of TCJ on lipidemia, glycemia, inflammation, and sUA. Moreover, the pilot study provided information regarding experimental design that permitted appropriate, necessary, modifications—e.g., new placebo formulation, extended washout period (4 wk compared with 2 wk)—to illuminate and corroborate the beneficial effects of TCJ. Thus, in this second study we recruited at-risk individuals who were overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥30.0) and likely to exhibit ≥1 conditions associated with MetS. In this 12-wk placebo-controlled 2 × 2 crossover dietary intervention, we randomly assigned participants to consume 240 mL (8 oz) daily of either placebo (artificial cherry-flavored, anthocyanin-free beverage) or TCJ (equivalent to ∼50 tart cherries) for 4 wk, followed by a 4-wk washout period, then consumption of the alternate beverage for 4 wk. We subsequently determined the effect of TCJ in at-risk participants on markers of lipidemia, glycemia, inflammation, and uricemia.
Methods
Participants, study design, and intervention
This study was a 12-wk 2 × 2 crossover, randomized, placebo-controlled dietary intervention in overweight and obese participants (BMI ≥25.0). Individuals were recruited from the Phoenix, AZ, metropolitan area through the use of handbills, word-of-mouth notification, and poster displays as reviewed and approved by the Institutional Review Board at Arizona State University. Respondents were screened by telephone and onsite interviews at the metabolic kitchen at the Polytechnic campus at Arizona State University (Mesa, AZ) to assess general health; height and weight were measured to determine BMI. Participants were aged ≥18 y, not pregnant, not diabetic, with no unresolved infections or diseases (diabetes, cardiovascular disease, inflammatory bowel disease, cancer, or liver disease), and nonsmokers. Histories of medication and dietary supplement use were collected and those taking anti-inflammatory or lipid-lowering medications were excluded. This study was approved by the Institutional Review Board at Arizona State University and informed consent was obtained from each respondent prior to entering the study. This trial was registered at clinicaltrials.gov as NCT03636529.
After enrollment, subjects were randomly assigned to 1 of 2 arms by an automated online randomization program (http://www.graphpad.com/quickcalcs/index.cfm) to consume daily either 240 mL (8 oz) of TCJ diluted (1:6 v/v) from concentrate (Coloma Frozen Foods) or a placebo beverage for 4 wk. The placebo was prepared by combining 48.3 g each of dextrose and fructose (Batory Foods), food-grade red and blue dyes (2.0 and 0.1 mL, respectively; McCormick & Company, Inc.), lemon powder drink mix (0.8 g; True Citrus), powdered black cherry drink mix (4.0 g; Kraft Foods Group, Inc.), and filtered, bottled water (local supermarket) to produce 1 L of masked (color), carbohydrate- and calorie-matched placebo beverage. Beverages were distributed weekly in plastic (polyethylene terephthalate) bottles and labeled only as tart cherry juice study. After a 4-wk washout period, subjects were switched to the alternate beverage for an additional 4 wk. At each visit, participants returned all beverage bottles for inspection and count, as well as diet records, to ensure compliance. Participants were instructed to refrain from consuming any other anthocyanin-containing fruits and juices during the study period, including cherries, red grapes, pomegranates, berries, and their juices, red wine, or dark chocolate. Respondents were provided with oral or written instructions, or both, to assist entry of accurate, relevant details for all foods and beverages consumed, including brand name, preparation method, and location consumed. Portion size was either estimated through the use of food models, pictures, or other visual aids, or measured with a portable digital scale or graduated container. Dietary, medical, and physical activity questionnaires and records were collected and analyzed with Food Processor Nutrition and Fitness Software version 8.5 (ESHA). Physical activity was calculated as metabolic equivalent of task (MET)-min/wk.
Analysis of total polyphenols
Total polyphenols were quantified through the use of the Folin-Ciocalteu method as described previously (23). Briefly, water (0.3 mL), Folin-Ciocalteu reagent (0.2 mL), and 0.2 mL of diluted sample or external standard (gallic acid) were added to test tubes and mixed (3 s; low speed). Tubes were incubated at 25°C for 10 min followed by addition of 0.6 mL of 20% (w/v) sodium carbonate. Tubes were then incubated in a water bath at 40°C for 20 min, cooled for 30 min to 25°C, and analyzed at 755 nm with purified gallic acid serving as the external standard.
Biomedical analysis and anthropometric measurement
After a ≥12-h fast, fasting blood samples were drawn by standard venipuncture protocols into evacuated vacutainer tubes (Fisher Scientific). Plasma was separated by centrifugation at 1100 × g at 4°C for 20 min and archived in 0.5-mL aliquots at −80°C until analysis. ESRs were determined immediately following each blood draw. Anthropometric measurements, including weight, height, and body composition (body fat percentage, fat mass, fat-free mass, total body water, and basal metabolic rate), were measured at each visit by bioelectrical impedance (TBF 300A Tanita Body Composition Analyzer). Dietary records and physical activity questionnaires were collected and reviewed at each visit. Blood pressure and pulse rate were determined with an automated IntelliSense blood pressure monitor (Omron Healthcare, Inc.).
Total cholesterol, TGs, LDL cholesterol, HDL cholesterol, fasting glucose, and hsCRP were measured directly from plasma samples with the use of a COBAS C111 blood chemistry analyzer (Roche Diagnostics) and VLDL cholesterol was calculated as TG/5. Plasma insulin was analyzed with an Immulite 1000 automated chemiluminescent blood chemistry analyzer (Diagnostic Products Corp., Siemens). MCP-1 was analyzed by standard sandwich enzyme-linked immunosorbent assay performed with single-analyte ELISArray kits (SABiosciences Corp.).
Analysis and calculation of insulin sensitivity and resistance
Insulin sensitivity and resistance were calculated through the use of a panel of indirect indices including the following: 1) the HOMA index, calculated as glucose (mg/dL) × insulin (μU/mL)/22.5; 2) the quantitative insulin-sensitivity check index (QUICKI), calculated as 1/[log(insulin in mU/L) + log(glucose in mg/dL)]; and 3) the McAuley index, calculated as exp[2.63 – 0.28 ln(insulin in mU/L) – 0.31 ln(TGs in mmol/L)]. The glucose/insulin ratio and 1/insulin reciprocal were also calculated.
Measurement of serum uric acid
Archived plasma samples were thawed, warmed to 25°C, and aliquots combined with assay buffer, uric acid probe, and enzymatic reaction mix as part of a Uric Acid Assay kit and as directed by the manufacturer (Sigma-Aldrich). Each master reaction mix was added to the wells of a 96-well microplate and incubated for 30 min at 37°C in the dark. Samples were then analyzed at 570 nm and quantified, with purified uric acid serving as an external standard. The CV was <6.6%.
Statistical analyses
The sample size for this study was based on a 15% reduction of serum uric acid as shown in previous studies in healthy adults consuming TCJ and similar experimental designs (24–26). Based on the SD of the difference in sUA pre- and postintervention with TCJ, it was calculated that with a sample of n = 25, a 15% reduction in sUA could be detected with an α = 0.05 and power = 0.80.
Statistical analysis was conducted with SPSS version 18.0 (2010). All values are expressed as means ± SDs. Differences were considered significant at P < 0.05 and as trends (to significance) at P = 0.05–0.10. A histogram and Shapiro-Wilk test were used to test variables for normality, and data were logarithmically transformed to achieve a normal distribution. Nonparametric data for BMI were inversely transformed to achieve a normal distribution. Anthropometric, inflammatory, and plasma lipid values were subsequently analyzed with 2-factor repeated-measures ANOVA as well as by group (treatment) and by time (pre/post) to determine interaction effects. Dietary and physical activity data from individual treatment groups were compared with respective controls through the use of paired t tests for the 2 experimental arms and data that were nonparametric were analyzed via the Wilcoxon Signed Rank test.
Results
Determination of total polyphenols and anthocyanins in TCJ
Authentic TCJ concentrate (68 brix) was diluted 1:6 (v/v) with bottled water by the investigators to obtain single-strength juice (11 brix; industry range 11–16 brix; pH 3.2–3.6). Both beverages contained 96 g carbohydrate/L providing 23 g per 240 mL (8 oz), the volume of the daily treatments. TCJ contained 65 mg anthocyanins/L (15.6 mg/240 mL) and 33.6 g total phenolics/L (993.6 mg/240 mL); no concentrations in placebo beverage were detected. The CV for the Folin-Ciocalteu method for total polyphenols was <9.8% for interassay analysis.
Baseline characteristics, dietary intake, and physical activity
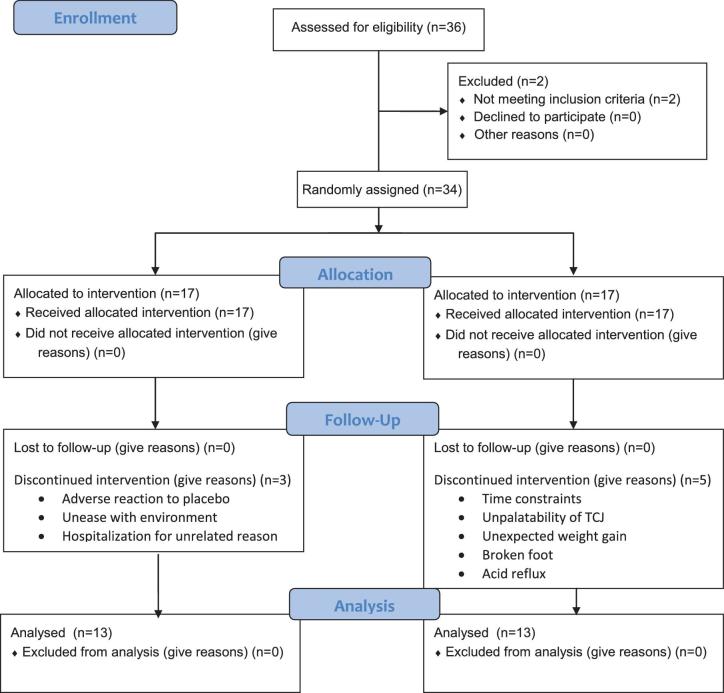
In this study, 34 participants were enrolled from 36 initial respondents with 26 completing the study as shown in Figure 1 (27). The appropriate reference ranges for gender and age were applied to determine if clinical values were considered normal or abnormal (high or low) (28). Anthropometric measurements were recorded at baseline and following weeks 4, 8, and 12. Mean age of participants (18 women and 8 men) was 41 ± 11 y (range 22–61 y) and mean BMI was 31.3 ± 6.3 (range 25.1–51.3) (Table 1). The percentage body fat was 37.1 ± 10.0%, exceeding the healthy ranges for men (<25%) and women (<32%), and WC was 1.0 ± 0.1 m (39.1 ± 5.5 in), indicating adiposity with 12 obese and 14 overweight participants. As a cohort, the collective group of participants displayed normal concentrations of total cholesterol (TC), VLDL cholesterol, HDL cholesterol, and TGs. However, LDL cholesterol was elevated, as were the lipid risk factor ratios TG/HDL cholesterol and TC/HDL cholesterol. The CV for analysis of TC, HDL cholesterol, LDL cholesterol, and TGs was 1.1–3.5%. Fasting glucose and insulin were normal although the QUICKI, HOMA, and McAuley indices approached values indicative of insulin resistance (29, 30). The CVs for insulin and glucose analyses were <6.9% and <1.8%, respectively. Regarding inflammation, hsCRP was elevated to 35.7 ± 38.0 nM (3.7 ± 4.0 mg/L), indicating increased inflammation, but the ESR was normal. We also determined the occurrence and frequency of abnormal risk factors for MetS for each participant. Five participants displayed no MetS risk factors (BMI = 27.2 ± 1.5) although were overweight. For others, the numbers of risk factors and participants displaying each were: 1 (n = 6), 2 (n = 5), 3 (n = 9), 4 (n = 0), and 1 individual displayed abnormalities in all 5 risk factors (BMI = 40.3). There was considerable variability and inconsistency in the occurrence and magnitude of risk factors with increasing BMI.
FIGURE 1.
Description of recruitment, enrollment, and completion of the tart cherry juice study.
TABLE 1.
Baseline characteristics of study participants1
| Characteristics | Mean | Reference range |
|---|---|---|
| Sex (M, F) | 8, 18 | |
| Age, y | 41 ± 11 | |
| BMI, kg/m2 | 31.3 ± 6.0 | <25.0 |
| Body fat, % | 37.1 ± 10.0 | <25, <322 |
| TC, mM | 4.95 ± 1.04 | <5.17 |
| LDL cholesterol, mM | 2.79 ± 0.77 | <2.59 |
| VLDL cholesterol, mM | 0.32 ± 0.31 | <0.34 |
| HDL cholesterol, mM | 1.09 ± 0.31 | >1.03, > 1.293 |
| TG, mM | 1.62 ± 1.55 | <1.69 |
| TG/HDL risk ratio | 4.25 ± 6.23 | <3 |
| TC/HDL risk ratio | 4.97 ± 2.21 | <4 |
| LDL/HDL risk ratio | 2.76 ± 1.1 | <3 |
| Glucose, mM | 5.2 ± 0.4 | <5.6 |
| Insulin, µU/mL | 12.95 ± 6.98 | 5.0–20.0 |
| QUICKI | 0.336 ± 0.040 | >0.312 |
| HOMA | 2.99 ± 1.65 | <4 |
| hsCRP, nM | 35.7 ± 38.0 | <28.6 |
| ESR, mm/h | 18 ± 11 | 0–304 |
| MCP-1, pg/mL | 243 ± 161 | 163–438 |
Values are means ± SDs. ESR, erythrocyte sedimentation rate; HOMA, homeostatic model assessment; hsCRP, high-sensitivity C-reactive protein; MCP-1, monocyte chemoattractant protein-1; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol.
Men: 18–25%; women: 25–31% (acceptable ranges).
Men: >1.03 mM; women: >1.29 mM.
Men: <50 y, 0–15 mm/h; >50 y, 0–20 mm/h; women: <50 y, 0–20 mm/h; >50 y, 0–30 mm/h.
Dietary records were collected and analyzed at each visit and, upon analysis, indicated no significant differences in dietary intake patterns or intakes of macronutrients, energy, or fatty acids, e.g., ω-3 (n–3), between arms (Table 2). Data expressed as a percentage of total caloric intake (energy) further indicate that participants were consuming an average 51.3%, 16.5%, and 32.2% of kcal from carbohydrate, protein, and fat, respectively, which were within the current acceptable macronutrient distribution ranges of 45–65%, 10–35%, and 20–35%, respectively (31, 32). The amount and intensity of physical activity were not different between placebo and TCJ arms with calculated values of 2193 ± 2024 MET-min/wk for placebo and 2256 ± 2497 MET-min/wk for the TCJ arm.
TABLE 2.
Dietary intake during placebo and TCJ intervention1
| Variable | Placebo | TCJ |
|---|---|---|
| Energy, kJ/d | 8439 ± 1385 | 8585 ± 2305 |
| Protein, g/d | 83 ± 35 | 9 ± 40 |
| Carbohydrate, g/d | 258 ± 68 | 259 ± 69 |
| Sugar, g/d | 114 ± 51 | 107 ± 55 |
| Total fat, g/d | 72 ± 30 | 74 ± 24 |
| MUFA, g/d | 15.5 ± 7.4 | 17.9 ± 7.4 |
| PUFA, g/d | 10.6 ± 5.7 | 9.8 ± 6.5 |
| Saturated, g/d | 21.9 ± 12.3 | 24.7 ± 10.1 |
| Trans, g/d | 0.22 ± 0.38 | 0.47 ± 0.61 |
| Total fiber, g/d | 19.1 ± 7.5 | 19.4 ± 6.1 |
| Soluble fiber, g/d | 1.49 ± 0.84 | 1.69 ± 0.62 |
| n–3 fatty acids, g/d | 0.96 ± 0.67 | 0.88 ± 0.69 |
Data were analyzed with paired t tests. Values are means ± SDs, n = 26. Data were not statistically significant between placebo and TCJ arms. TCJ, tart cherry juice.
Anthropometric measures
Over the course of the study, we noted no significant changes between pre- and postintervention measures of anthropometric endpoints or parameters of total daily energy expenditure (basal metabolic rate and physical activity), fat and lean body mass, blood pressure, heart rate, or percentage body fat in the placebo and TCJ arms (Table 3). We observed a percentage fat mass and WC of 37.1 ± 10.0% and 0.99 ± 0.14 m (39.1 ± 5.5 in), respectively, for the cohort. When we stratified by gender, women (n = 18) exhibited 42.4 ± 5.1% body fat (reference range 25–31%) and men exhibited 26.8 ± 10.0% body fat (reference range 18–25%). WCs were 0.98 ± 0.13 m (38.5 ± 5.3 in) and 1.01 ± 0.16 m (39.8 ± 6.3 in) for women and men, respectively. These data indicate, that as a group, men were borderline overweight and obese with mean BMI = 29.9 ± 6.01 (range 25.3–43.1).
TABLE 3.
Anthropometric parameters of participants in the TCJ study1
| Placebo | TCJ | |||
|---|---|---|---|---|
| Variable2 | Pre | Post | Pre | Post |
| Age, y | 43.8 ± 11.3 | 41.9 ± 11.1 | 42.2 ± 12.1 | 40.4 ± 11.7 |
| BMI, kg/m2 | 32.1 ± 6.7 | 31.5 ± 6.5 | 29.7 ± 4.5 | 29.6 ± 3.8 |
| Body fat, % | 37.1 ± 9.7 | 37.6 ± 9.3 | 35.6 ± 10.9 | 35.9 ± 10.2 |
| BMR, J/d | 7184 ± 1006 | 7214 ± 1048 | 7042 ± 750 | 7146 ± 797 |
| Fat mass, kg | 34.6 ± 14.9 | 34.9 ± 13.8 | 31.7 ± 12.9 | 317 ± 11.4 |
| FFM, kg | 56.4 ± 9.2 | 55.8 ± 9.5 | 54.9 ± 8.4 | 55.6 ± 8.7 |
| TBW, kg | 40.4 ± 6.7 | 40.9 ± 6.9 | 40.3 ± 6.2 | 40.6 ± 6.5 |
| WC, m | 1.02 ± 0.12 | 0.99 ± 0.14 | 0.95 ± 0.10 | 0.96 ± 0.11 |
| WC, in | 40.1 ± 4.7 | 39 ± 5.5 | 37.5 ± 4.1 | 37.6 ± 4.1 |
| BP systolic, mm Hg | 127.6 ± 10.9 | 123.9 ± 10.1 | 123.4 ± 10.1 | 120.6 ± 9.9 |
| BP diastolic, mm Hg | 83.9 ± 9.8 | 79.6 ± 7.8 | 76.8 ± 5.7 | 77.6 ± 6.6 |
| HR, bpm | 71.1 ± 10.9 | 73.2 ± 14.2 | 73.6 ± 10.4 | 74.9 ± 10.2 |
| ESR, mm/h | 18.6 ± 12.3 | 21.9 ± 13.5 | 18.4 ± 11.2 | 22.4 ± 14.8 |
Data were analyzed with 2-factor repeated-measures ANOVA. Values are means ± SDs, n = 26. No significant difference for interaction (time and treatment) or main effect of treatment. BP, blood pressure; BMR, basal metabolic rate; ESR, erythrocyte sedimentation rate; FFM, fat-free mass; HR, heart rate; TBW, total body water; TCJ, tart cherry juice; WC, waist circumference.
Data were inversely transformed (1/variable) to achieve normality; values reported untransformed.
Lipids
There were no significant differences between pre- and postintervention values for either placebo or TCJ arms for plasma lipids. There also were no differences between cardiovascular disease risk ratios (Table 4). However, risk ratios, including TG/HDL cholesterol, TC/HDL cholesterol, and LDL cholesterol/HDL cholesterol, were elevated above the normal range, indicating increased relative risk for cardiovascular and coronary heart diseases (33).
TABLE 4.
Effect of placebo and TCJ on blood lipids and CVD risk ratios1
| Placebo | TCJ | |||
|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post |
| TC | 192 ± 44 | 196 ± 43 | 187 ± 33 | 196 ± 35 |
| LDL cholesterol | 111 ± 32 | 113 ± 31 | 112 ± 30 | 120 ± 31 |
| VLDL cholesterol | 28 ± 28 | 32 ± 43 | 26 ± 14 | 26 ± 15 |
| HDL cholesterol | 42 ± 10 | 42 ± 10 | 42 ± 8 | 43 ± 7 |
| TG | 140 ± 137 | 161 ± 216 | 129 ± 68 | 128 ± 74 |
| TG/HDL | 4.1 ± 6.3 | 5.1 ± 1.0 | 3.3 ± 2.2 | 3.2 ± 2.5 |
| TC/HDL | 4.8 ± 2.2 | 4.9 ± 2.4 | 4.5 ± 1.4 | 4.6 ± 1.4 |
| LDL/HDL | 2.7 ± 1.1 | 2.8 ± 1.1 | 2.7 ± 1.1 | 2.9 ± 1.2 |
Data were analyzed with 2-factor-repeated measures ANOVA. Values are means ± SDs; n = 26. No significant difference for interaction (time and treatment) or main effect of treatment. CVD, cardiovascular disease; TC, total cholesterol; TCJ, tart cherry juice.
Measures of glycemia, insulin resistance, and inflammation
We noted no significant differences between fasting glucose or fasting insulin concentrations between pre- and postintervention samples from either the placebo or TCJ arms (Table 5). We also did not note any effect (increase or decrease) of TCJ on parameters of insulin resistance and sensitivity. However, we noted decreases of 19.2% and 6.3% between placebo and TCJ arms for proinflammatory hsCRP and MCP-1, respectively. CVs for the colorimetric methods for hsCRP and MCP-1 were <10% and <6.7%, respectively.
TABLE 5.
Effect of placebo and TCJ on direct and indirect markers of glycemia and inflammation1
| Placebo | TCJ | Reference range | |||
|---|---|---|---|---|---|
| Glucose, mM | 5.2 ± 0.4 | 5.4 ± 0.6 | 5.1 ± 0.4 | 5.3 ± 0.6 | <5.6 |
| Insulin, pM | 92.0 ± 67.7 | 108.6 ± 69.2 | 118.5 ± 87.6 | 136.9 ± 137.2 | <173.6 |
| HOMA | 3.13 ± 2.44 | 3.91 ± 2.81 | 3.92 ± 2.76 | 4.96 ± 6.05 | <2.5 |
| QUICKI | 0.34 ± 0.03 | 0.33 ± 0.04 | 0.33 ± 0.04 | 0.32 ± 0.03 | ≥0.38 |
| McAuley | 6.84 ± 0.39 | 6.81 ± 0.42 | 0.33 ± 0.04 | 6.49 ± 0.35 | <5.8 |
| 1/insulin | 0.08 ± 0.10 | 0.06 ± 0.11 | 0.06 ± 0.34 | 0.05 ± 0.05 | 0.05 = IR |
| Glucose/insulin | 7.06 ± 0.74 | 6.23 ± 1.08 | 6.55 ± 0.08 | 4.87 ± 0.56 | >4.5 |
| hsCRP, nM | 38.5 ± 40.9 | 36.5 ± 37.1 | 46.9 ± 67.6 | 37.8 ± 38.8 | <28.6 |
| MCP-1, pg/mL | 250 ± 164 | 276 ± 131 | 287 ± 216 | 269 ± 146 | |
Data were analyzed with 2-factor repeated-measures ANOVA. Values are means ± SDs, n = 26. No significant differences for the interaction (time and treatment) or main effects of time and treatment. HOMA, homeostatic model assessment; hsCRP, high-sensitivity C-reactive protein; MCP-1, monocyte chemoattractant protein-1; QUICKI, quantitative insulin sensitivity check index; TCJ, tart cherry juice.
Data were logarithmically transformed (log variable) to achieve normality; values reported untransformed.
Plasma urate
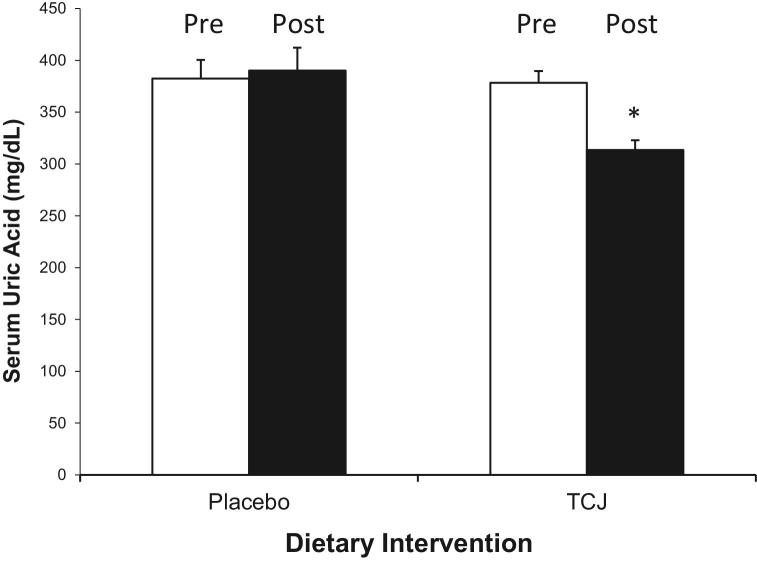
Plasma urate decreased significantly after TCJ consumption for 4 wk. Plasma urate decreased from 378.3 ± 11.3 to 320.8 ± 9.5 µM, a 19.2% reduction (P < 0.05), compared with in increase from 382.05 ± 17.8 to 390.2 ± 22.0 µM for pre- and postintervention values for the placebo arm (Figure 2). In this cohort, hyperuricemia occurred in 46.2% of participants, with 37.5% (3/8) in men and 50.0% (9/18) in women, with elevated sUA defined as >475.8 µM (>8.0 mg/dL) for men and >362.8 µM (>6.1 mg/dL) for women (28). As a collective cohort, the mean sUA concentrations were 382.5 µM (6.4 mg/dL) and 375.9 µM (6.3 mg/dL) for placebo and TCJ arms, respectively. Four men exceeded the normal reference range of 202.2–416.4 µM (3.4–7.0 mg/dL) with the remaining 4 in the upper range of normal. Nine women exceeded the reference range of 142.8–356.9 µM (2.4–6.0 mg/dL) and 9 were within range. Based on these criteria, 12 of the 26 (46%) participants displayed hyperuricemia.
FIGURE 2.
Effect of TCJ intervention on sUA concentrations compared with a placebo arm. Participants (n = 26) were randomly assigned to either the TCJ or placebo arm and consumed 240 mL/d of test beverage for 4 wk. After a 4-wk washout, participants consumed the alternate beverage for an additional 4 wk. Plasma samples were analyzed to determine percentage change from start and end of each intervention arm through the use of a paired repeated-measures t test. The asterisk indicates a significant difference (P < 0.05) between TCJ and placebo arms. sUA, serum uric acid; TCJ, tart cherry juice.
Discussion
In this 12-wk placebo-controlled crossover study, we have shown in at-risk overweight and obese participants that daily consumption of TCJ for 4 wk significantly reduced sUA concentrations by 19.2%. We also observed 19.4% (P = 0.09) and 6.3% (P = 0.08) reductions in proinflammatory hsCRP and MCP-1, respectively. The data indicate that TCJ consumption can reduce sUA, i.e., hyperuricemia, which may be applicable to those with gouty arthritis, as well as other chronic inflammatory conditions, and can suppress proinflammatory markers in at-risk humans.
In this study, 46% of the participants were hyperuricemic although reporting no occurrences of gouty arthritis or other inflammatory conditions. This is not surprising because only ∼5% of hyperuricemic individuals will ultimately develop gout (34). To that end, in this study 9 out of 19 women and 3 out of 8 men were hyperuricemic (12 total), and hence 5% incidence would equate to 0.6 participants (<1 person). The occurrence of hyperuricemia, however, has substantially increased over the past several decades and is calculated from NHANES data to be 15–23% up to age 60 y and up to 37% for individuals after age 80 y (35). Given the disparity between our data and the aforementioned, we collected dietary data and found no significant differences in amounts or intake patterns, e.g., purine-rich foods and beverages, or dietary intake of fructose, which can also increase uric acid. Moreover, the carbohydrate and fructose concentrations of the placebo and test beverages were matched to account for this possibility. It is, however, possible that genetic, environmental, dietary, or pathophysiologic influences, or combinations thereof, may have contributed because 90% of gout cases are caused by renal underexcretion and the remaining 10% by increased production of uric acid (36). In men, sUA concentrations are typically higher than in premenopausal women, but in postmenopausal women, concentrations are similar to values for men (2). In this study, there were 18 women, with 6 (33%) within the average age range or above for onset of menopause (48–55 y) but only 2 of these 6 were hyperuricemic.
Consumption of cherries has been shown to exert anti-inflammatory activities that can alleviate gout. In 1950, Blau demonstrated that fresh and canned cherries prevented arthritic attacks and reduced sUA concentrations in patients (n = 12) with gout (10). Moreover, 33% of the patients experienced a greater range of motion in their extremities. In a case-crossover study, consumption of fresh cherries or cherry extract (n = 633) over a 2-d period also significantly decreased by 35% the risk of gout attacks compared with no intake (37). Moreover, when combining cherry intake with commonly prescribed allopurinol (purine analog and xanthine oxidase inhibitor), the risk for gout was reduced by 75% compared with periods without either exposure, although sUA concentrations were not reported. In healthy women (n = 10), who consumed 2 servings (280 g) of cherries after an overnight fast, sUA was significantly reduced at 5 h postconsumption by 14% from 214.1 μM (3.6 mg/dL) to 178.5 μM (3.0 mg/dL). There was also a trend toward decreased inflammatory indices, namely, hsCRP and NO (11). In the current study (n = 26), we observed similar results with a significant 19.4% reduction in sUA and a 19.2% reduction, although nonsignificant, in hsCRP, a well-recognized indicator of inflammation. In our previous pilot study (registered as NCT03638362), we noted no significant difference in hsCRP between the TCJ and placebo arms in 10 overweight and obese adults (18). We attribute the latter to sample size and variation in hsCRP concentrations between participants observed in this study. Interestingly, TCJ treatment significantly reduced sUA concentrations of hyperuricemic, but not normouricemic, rodents in a time-dependent manner (12). Collectively, evidence is accumulating indicating the efficacy of tart cherry products to reduce sUA and inhibit inflammatory processes via, in part, inhibition of enzymatic activities.
In a previous study with 10 overweight and obese participants, we did note a significant difference in ESR, a marker of inflammation, between placebo and TCJ intervention groups, which appeared driven, in large part, by a placebo-induced increase in ESR and a marginal 6% reduction in the TCJ group (manuscript under review). We suggested that this may have been due to the fructose (monosaccharide) concentrations in the artificially flavored fruit drink with added sugars purchased from a local supermarket, a strategy used often in placebo-controlled beverage studies. To circumvent this potential occurrence in this study, we prepared the placebo in our metabolic kitchen and controlled for carbohydrate and total fructose concentrations, color, and acidity. We noted no significant differences in ESR between arms in this study, and thus we assume that our initial supposition was valid. It is possible, however, that other factors could have contributed to this result, including the TCJ formulation with lower total phenolics, which was different than in our previous studies, the nature of the cohort (incidence and severity of pathophysiology), or the experimental design with longer washout period (4 compared with 2 wk), or a combination of these factors. We can, however, glean from these data that when given a dietary choice of beverages, i.e., artificially flavored with added sugars or 100% authentic fruit juice, our results may be a consideration in this choice.
As an acute-phase reactant, hsCRP is released during inflammation and thus is a potential target for anti-inflammatory, bioactive agents or mixtures such as tart cherries. In this study, the overall baseline concentration of C-reactive protein in participants was elevated at 36.2 ± 38.1 nM (3.8 ± 4.0 mg/L) [range 12.4–225.7 nM (1.3–23.7 mg/L)]. The range for average risk is 9.5–28.6 nM (1.0–3.0 mg/L). Two participants exhibited consistent values of ∼95.2 nM (>10 mg/L), suggesting severe chronic metabolic inflammation compared with other participants, which is typical with some mild-to-moderate stressors where C-reactive protein concentrations can increase to 476–952 nM (50–100 mg/L) within a few hours. Our initial telephone interviews, screening, and observation during laboratory visits indicated no overt proinflammatory source, e.g., disease or anxiety, other than BMI >25.0 (overweight/obese), upon entry and participation in the study. Overall, TCJ consumption reduced hsCRP concentrations by 19.4% (P = 0.09) compared with the placebo arm, which is consistent with the results of several studies. For example, in 1 study, men and women (n = 18) with BMI 20–30 consumed pitted sweet cherries (280 g for 4 wk), which significantly decreased numerous proinflammatory and associated biomarkers, including hsCRP, by 20.1% (19). Others have also shown significant reductions in serum hsCRP after TCJ consumption in 16 trained cyclists postexercise and in 12 healthy men and women 3 h postconsumption (38, 39). Collectively, there are numerous studies supporting marked reductions in hsCRP after consumption of cherries (tart and sweet) but the data are inconsistent, with some results displaying statistical significance whereas other results do not, even when the magnitudes of change are similar. This suggests that more attention should be given to specific inclusion and exclusion criteria, or other aspects of the experimental study design, to ensure consistency of results and minimize variability. This is a limitation of the current study because participants displayed considerable variability in the presence and combinations, or lack thereof, of risk factors for MetS, as well as in the magnitudes of many criteria although meeting the BMI inclusion criterion. This limitation could be overcome by selection of specific combinations of risk factors for MetS (3 of 5), identification of a desired analyte range, i.e., hsCRP 1–3, >3, >10 mg/L, etc., limiting the window for BMI for participants, i.e., 30–35, 40–50, or ensuring the absence, or presence, of inflammation, e.g., hsCRP, for all participants prior to dietary intervention. Providing more specific, pertinent, and responsive endpoints, or combinations thereof, could potentially improve the consistency of results between studies.
In conclusion, in this study we have shown in at-risk overweight and obese participants that daily consumption of TCJ for 4 wk significantly reduced sUA concentrations. We also observed reductions in proinflammatory hsCRP and MCP-1, respectively; although not statistically significant, these reductions are potentially biologically relevant. The data indicate that TCJ consumption can reduce sUA, i.e., hyperuricemia, and may be useful in those individuals prone to gouty arthritis or other proinflammatory conditions. Given the increasing prevalence of hyperuricemia associated with the obesity and MetS epidemics and the low adherence to ULTs, consumption of authentic TCJ may be an efficacious dietary approach in mitigating the occurrence and pathology of inflammatory conditions with particular application to gouty arthritis.
Acknowledgments
We thank Michael Stroup and Ginger Hook, RN, for assistance with phlebotomy and analysis of samples.
The authors’ responsibilities were as follows—KRM: initiated and designed the study; KC: recruited, screened, and provided informed consent to respondents under the supervision of KRM, as well as collected, processed, and analyzed, in part, data, samples, and questionnaires; KRM: interpreted the data and prepared the manuscript; KC: analyzed data and performed statistics; and both authors: critically reviewed and approved the manuscript. The corresponding author is responsible for all aspects of the paper.
Notes
This work was supported by a grant from the Cherry Research Committee of the Cherry Marketing Institute (Dewitt, MI). The funding source provided the tart cherry juice concentrate and placebo formulation but had no further role in any aspect of the study design, data collection or analysis, writing of the manuscript, or decision to publish.
Author disclosures: KRM and KMC, no conflicts of interest.
Abbreviations used: ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein; MCP-1, monocyte chemoattractant protein-1; MET, metabolic equivalent of task; MetS, metabolic syndrome; QUICKI, quantitative insulin sensitivity check index; sUA, serum uric acid; TCJ, tart cherry juice; TC, total cholesterol; ULT, urate-lowering therapy; WC, waist circumference
References
- 1. Perez-Ruiz F, Desideri G. Improving adherence to gout therapy: an expert review. Ther Clin Risk Manag 2018;14:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective – a review. J Adv Res 2017;8:495–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sattui SE, Gaffo AL. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musc Dis 2016;8:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keenan R, Schlesinger N. New and pipeline drugs for gout. Curr Rheumatol Rep 2016;18:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Soskind R, Abazia DT, Bridgeman MB. Updates on the treatment of gout, including a review of updated treatment guidelines and use of small molecule therapies for difficult-to-treat gout and gout flares. Exp Opin Pharmacotherapy 2017;18:1115–25. [DOI] [PubMed] [Google Scholar]
- 6. Rho YH, Lu N, Peloquin CE, Man A, Zhu Y, Zhang Y, Choi HK. Independent impact of gout on the risk of diabetes mellitus among women and men: a population-based, BMI-matched cohort study. Ann Rheum Dis 2016;75:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collier A, Stirling A, Cameron L, Hair M, Crosbie D. Gout and diabetes: a common combination. Postgrad Med J 2016;92:372–8. [DOI] [PubMed] [Google Scholar]
- 8. Krishnan E, Akhras KS, Sharma H, Marynchenko M, Wu EQ, Tawk R, Liu J, Shi L. Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. QJM 2013;106:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang PL. A comprehensive definition for metabolic syndrome. Dis Models Mech 2009;2:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blau LW. Cherry diet control for gout and arthritis. Tex Rep Biol Med 1950;8:309–11. [PubMed] [Google Scholar]
- 11. Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. J Nutr 2003;133:1826–9. [DOI] [PubMed] [Google Scholar]
- 12. Haidari F Jr., Mohammad Shahi M, Keshavarz SA, Rashidi MR. Inhibitory effects of tart cherry (Prunuscerasus) juice on xanthine oxidoreductase activity and its hypouricemic and antioxidant effects on rats. Malays J Nutr 2009;15:53–64. [PubMed] [Google Scholar]
- 13. Martin KR, Bopp J, Burrell L, Hook G. The effect of 100% tart cherry juice on serum uric acid levels, biomarkers of inflammation and cardiovascular disease risk factors. FASEB J 2011;25. [Google Scholar]
- 14. Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomed 2001;8:362–9. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, DeWitt DL. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod 1999;62:294–6. [DOI] [PubMed] [Google Scholar]
- 16. Kirakosyan A, Gutierrez E, Ramos Solano B, Seymour EM, Bolling SF. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem 2018;252:142–6. [DOI] [PubMed] [Google Scholar]
- 17. He Y, Hu Y, Jiang X, Chen T, Ma Y, Wu S, Sun J, Jiao R, Li X, Deng L, Bai W. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. J Photochem Photobiol B Biol 2017;177:24–31. [DOI] [PubMed] [Google Scholar]
- 18. Martin KR, Burrell L, Bopp J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Function 2018;9:5290–300. [DOI] [PubMed] [Google Scholar]
- 19. Kelley DS, Adkins Y, Reddy A, Woodhouse LR, Mackey BE, Erickson KL. Sweet bing cherries lower circulating concentrations of markers for chronic inflammatory diseases in healthy humans. The J Nutr 2013;143:340–4. [DOI] [PubMed] [Google Scholar]
- 20. Martin KR, Bopp J, Neupane S, Vega-Lopez S. 100% Tart cherry juice reduces plasma triglycerides and CVD risk in overweight and obese subjects. FASEB J 2010;24:1.20047894 [Google Scholar]
- 21. Dimitriou L, Hill JA, Jehnali A, Dunbar J, Brouner J, McHugh MP, Howatson G. Influence of a Montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running–a pilot investigation. J Int Soc Sports Nutr 2015;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garrido M, Gonzalez-Gomez D, Lozano M, Barriga C, Paredes SD, Rodriguez AB. Characterization and trials of a Jerte Valley cherry product as a natural antioxidant-enriched supplement. Ital J Food Sci 2013;25:90–7. [Google Scholar]
- 23. Martin KR, Wooden A. Tart cherry juice induces differential dose-dependent effects on apoptosis, but not cellular proliferation, in MCF-7 human breast cancer cells. J Med Food 2012;15:945–54. [DOI] [PubMed] [Google Scholar]
- 24. Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. J Nutr 2003;133:1826–9. [DOI] [PubMed] [Google Scholar]
- 25. Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients 2018;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehmood A, Zhao L, Wang C, Nadeem M, Raza A, Ali N, Shah AA. Management of hyperuricemia through dietary polyphenols as a natural medicament: a comprehensive review. Crit Rev Food Sci Nutr 2017 Dec 26:1–23. doi: 10.1080/10408398.2017.1412939. [DOI] [PubMed] [Google Scholar]
- 27. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pagana TN, Pagana KD, Pagana TJ. Mosby's diagnostic and laboratory test reference. Mosby; 2016. [Google Scholar]
- 29. Quon MJ. Limitations of the fasting glucose to insulin ratio as an index of insulin sensitivity. J Clin Endocrin Metab 2001;86:4615–7. [DOI] [PubMed] [Google Scholar]
- 30. Patarrão RS, Wayne Lautt W, Paula Macedo M. Assessment of methods and indexes of insulin sensitivity. Rev Port Endocrin Diabetes Metab 2014;9:65–73. [Google Scholar]
- 31. Manore MM. Exercise and the Institute of Medicine recommendations for nutrition. Curr Sports Med Rep 2005;4:193–8. [DOI] [PubMed] [Google Scholar]
- 32. McAuley K, Mann J. Thematic review series: patient-oriented research. Nutritional determinants of insulin resistance. J Lipid Res 2006;47:1668. [DOI] [PubMed] [Google Scholar]
- 33. Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, Masana L, Mangas A, Hernández-Mijares A, González-Santos P, Ascaso JF, Pedro-Botet J.. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 34. Benn CL, Dua P, Gurrell R, Loudon P, Pike A, Storer RI, Vangjeli C. Physiology of hyperuricemia and urate-lowering treatments. Front Med 2018;5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63:3136–41. [DOI] [PubMed] [Google Scholar]
- 36. Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Ann Rev Physiol 2015;77:323–45. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum 2012;64:4004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bell P, Gaze D, Davison G, George T, Scotter MJ, Howatson G. Montmorency tart cherry (Prunuscerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J Funct Food 2014;11:82–90. [Google Scholar]
- 39. Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014;6:829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]