Figure 2. Roles of the TRPP2 Residues W201 (Pre-S1 Domain) and K688 (TRP-like Domain) in the Channel Function and N-C Binding.
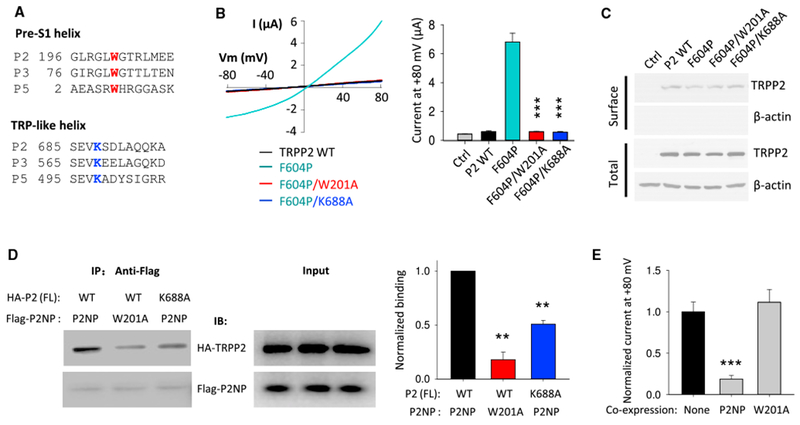
(A) Sequence alignment of pre-S1 and TRP-like helices among human TRPP channels.
(B) Left panel: representative current-voltage (I-V) curves obtained from oocytes expressing human WT or a mutant TRPP2, as indicated, in the presence of a Na+-containing, divalent-free solution (in mM): 100 NaCl; 2 KCl; and 10 HEPES (pH 7.5). Right panel: averaged currents at +80 mV are shown. Ctrl, H2O-injected oocytes. Currents were averaged from 13–17 oocytes of three batches. ***p < 0.001.
(C) Representative immunoblots of surface biotinylated and total proteins of TRPP2 WT and mutants.
(D) Left panel: representative co-IP data showing the effects of TRPP2 W201 and K688 on the N-C interaction using oocytes co-expressing HA-tagged human FL TRPP2 and Flag-tagged TRPP2 N-terminal peptide (Flag-P2NP; G161-S215). Right panel: data from experiments in left panel were quantified, averaged, and normalized. **p < 0.01; n = 3.
(E) Blocking effects of Flag-P2NP and Flag-P2NP-W201A on channel function of FL TRPP2 mutant F604P by use of co-expression. None, no P2NP was co-expressed with FL F604P. Normalized currents at +80 mV were obtained and averaged from three independent experiments. ***p < 0.001.
Data are presented as mean ± SEM. See also Figures S2 and S6.
