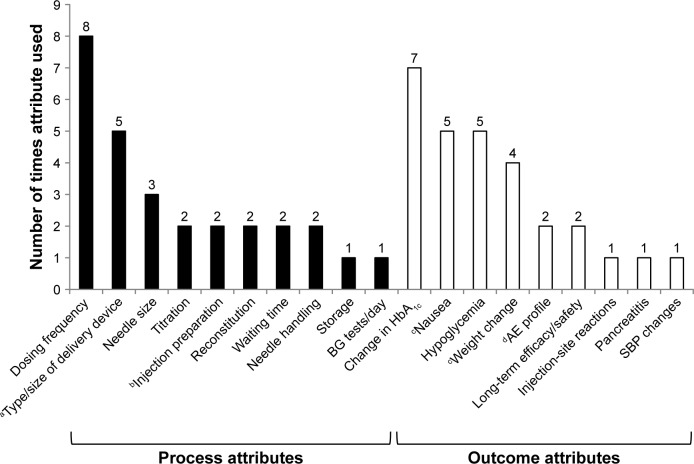
Figure 2.
Frequency of individual treatment-attribute evaluation across ten patient-preference studies identified by literature review.
Notes: aVariously across studies: MUP, SUP, vial and syringe, or autoinjector. bInjection preparation associated with vial and syringe, SUP, MUP, and autoinjector (Qin et al).15,23 cIn one study, nausea was described as “frequency of GI AEs,” but described by levels of nausea incidence only (Poon et al).17 dCommon AEs are a combination of nausea, vomiting, diarrhea, and injection-site nodules (Qin et al).15,23
Abbreviations: AE, adverse event; BG, blood glucose; GI, gastrointestinal; HbAlc, glycated hemoglobin; MUP, multiuse pen; SBP, systolic blood pressure; SUP, single-use pen.