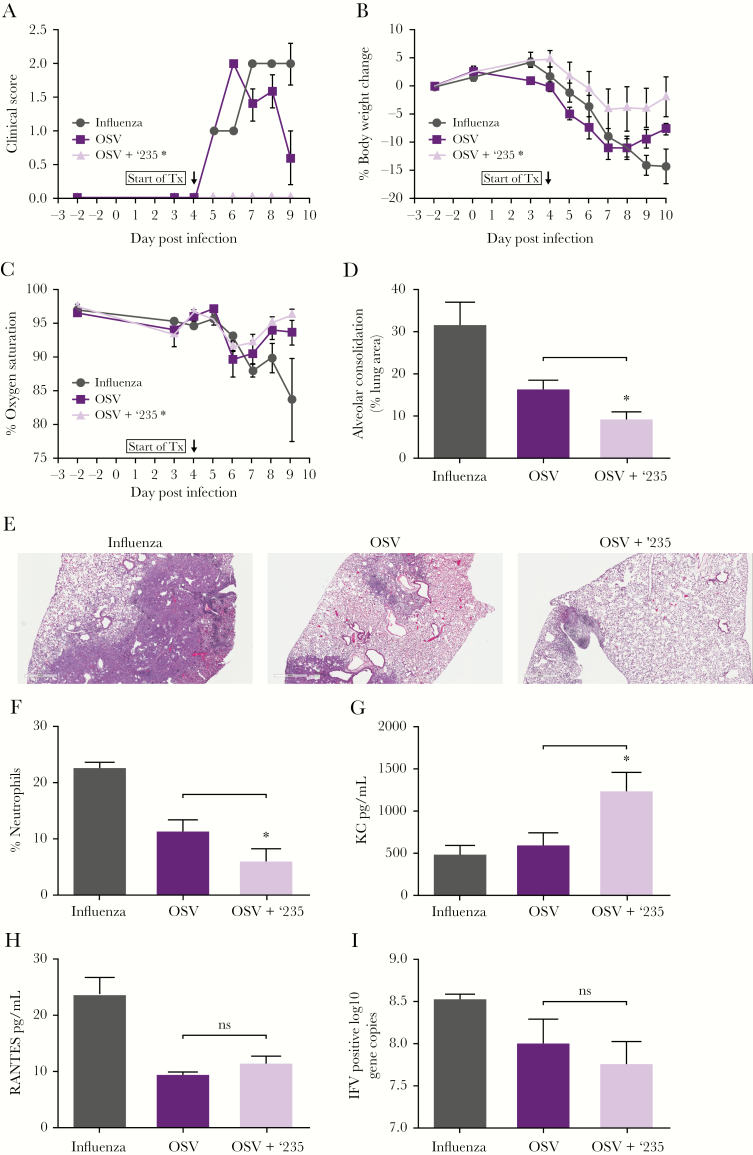
Figure 1.
Improvement in clinical symptoms and lung function: mice were infected with H1N1 influenza (A/PR/8/34), and starting on Day 4 postinfection they were treated with placebo, oseltamivir phosphate (OSV), or the combination of the CXCR2 antagonist SB-332235Z (‘235) plus OSV. Mice were evaluated starting 2 days before infection for (A) clinical scores on a 0–3 severity scale (based on appearance, grooming, posture, and activity) through Day 9 postinfection (p.i.) (*average clinical score OSV vs OSV+ ‘235, P = .0012); (B) body weight changes through Day 10 p.i. (*area under the curve [AUC] body weight change OSV vs OSV+ ‘235 P = .0176); and (C) oxygen saturation through Day 9 p.i. Improvement in lung consolidation: on Day 14 p.i., lungs were evaluated for (D) histopathologic changes by quantifying areas of alveolar consolidation (*, P = .0225) and (E) a representative pictures of hematoxylin and eosin stain (H&E)-stained lungs are depicted. Pathway engagement: bronchoalveolar lavage fluid (BALF) was collected on Day 7. (F) Neutrophils were evaluated by flow cytometry (*, P = .028) and protein concentrations of chemokines (G) KC (*, P = .0067) and (H) RANTES were measured. Viral load: (I) viral ribonucleic acid levels in the lungs of mice treated with the combination of OSV + ‘235 were similar to the OSV-treated group on Day 7 postinfection. Error bars represent standard error of the mean; 5–6 animals were used per group.