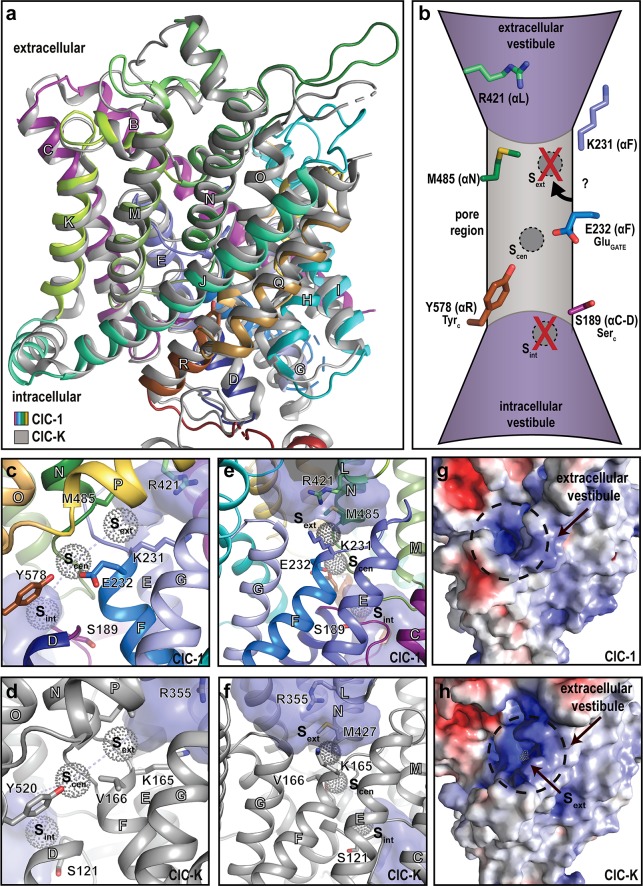
Fig 2. The ion-conducting pathway.
Ion transport in CLC proteins depends on extra- and intracellular vestibules and a connecting pore. In CLC transporters, the pore is marked by chloride ion binding sites (sext, scen, sint; not directly observed in this work) as well as specific glutamate (GluGATE, or E232; ClC-1 numbering throughout), tyrosine (TyrC, Y578), and serine (SerC, S189) residues. Chloride conductance in voltage-dependent CLC channels such as ClC-1 may involve shuttling (i) to protonated E232-Y578 (scen) from the vestibules - directly (or through a weak sint) from the intracellular side and (ii) through K231/R421 to overcome the hydrophobic barrier (including M485) from the extracellular side. (a) Comparison of the transmembrane domains of ClC-1 (colored as in Fig 1D) and ClC-K (gray), respectively. Helices are labelled with white letters throughout. (b) Schematic overview of the chloride permeation pathway with key residues pinpointed. Labels in the parentheses refer to the corresponding helices and the αC–D loop, respectively. (c–f) Side views of the pore region of ClC-1 (panels c and e; colored as in Fig 1D) with equivalent views of ClC-K (panels d and f, shown in gray) [9]. The chloride binding sites are positioned based on the Escherichia coli and Cyanidioschyzon merolae transporter structures (and are not located in ClC-1 or ClC-K) [8, 14]. The vestibules were calculated using HOLLOW [23] with a probe radius of 1.7 Å and are shown in purple surface. (g–h) Surface electrostatics from the extracellular side of ClC-1 (panel g) and ClC-K (panel h). Red and blue colors represent electronegative and electropositive surfaces, respectively. The chloride binding sites (in CLC transporters) are positioned as in panels c–f. The aperture of the vestibule is narrower in ClC-1 (without visible chloride binding site).