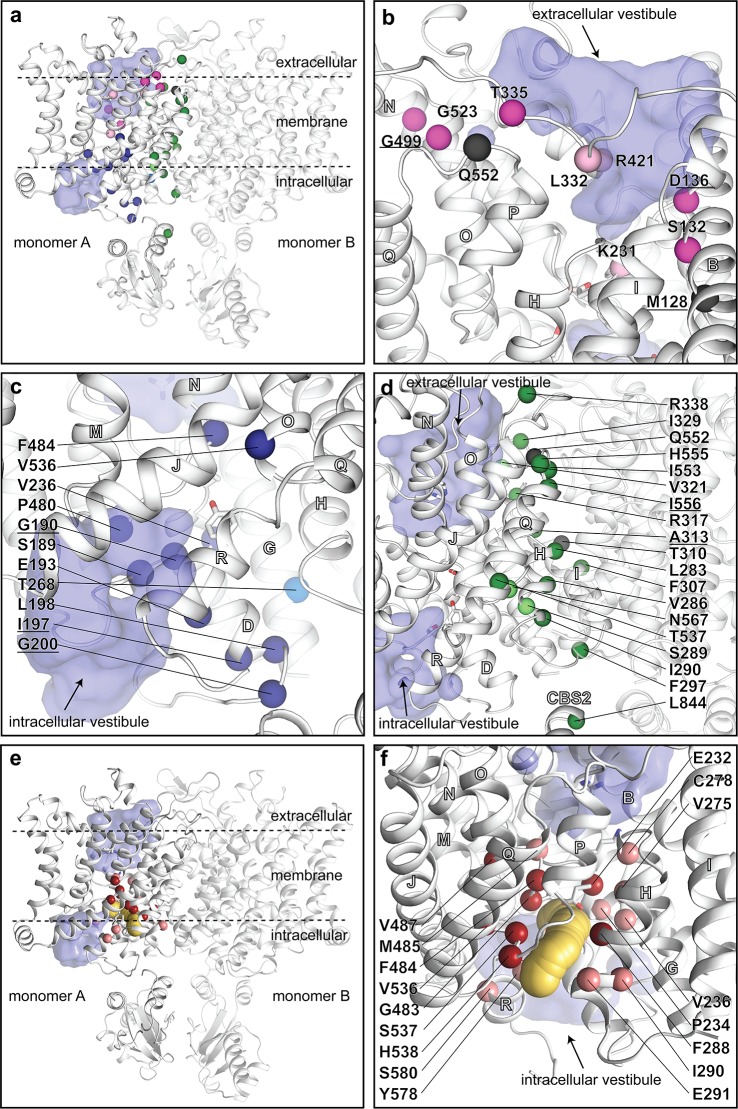
Fig 4. Myotonia-causing mutations and the putative binding pocket of the 9-AC inhibitor.
(a–d) Disease-causing and experimental missense mutations in ClC-1. Substitutions that invert (from depolarization to hyperpolarization activated) or shift the voltage dependence are shown in pink (located to the extracellular side) and blue (intracellular vestibule) or green (subunit interface), respectively. Bright colors represent disease-causing (recessive, with a stronger phenotype, but not dominant mutations are underscored), whereas experimental mutations are shown in pale colors. ClC-1 is shown in white and the Cl− vestibules in purple (calculated using HOLLOW as for Fig 2). (a) Overall view with all known disease and selected experimental mutations. We note that mutations of 5 residues that cause recessive myotonia and inward rectification are facing the extracellular vestibule; 3 located in a row on the same face of on helix B (M128, S132, D136) and 2 being the pore-constricting residues (K231, R421). Therefore, the phenotype may reflect a decreased chloride affinity of an extracellularly accessible site. (b) Close view of mutations that invert voltage dependence. Helices are labelled with white letters throughout. (c) Close view of mutations that shift voltage dependence (located at the intracellular vestibule). (d) Close view of mutations that shift voltage dependence (located at the monomer:monomer interface). (e−f) A putative binding pocket of 9-AC. Residues known to affect binding of 9-AC are highlighted as spheres (red for strong effect and pink for minor) and overlay a CAVER calculated pathway (shown in yellow) that stretches from the intracellular membrane interface to GluGATE. The clustering hints at a suitable target point for future rational drug-design efforts (see also alternative view in S11 Fig). CBS, cystathionine-β-synthase; 9-AC, 9-anthracene-carboxylic acid.