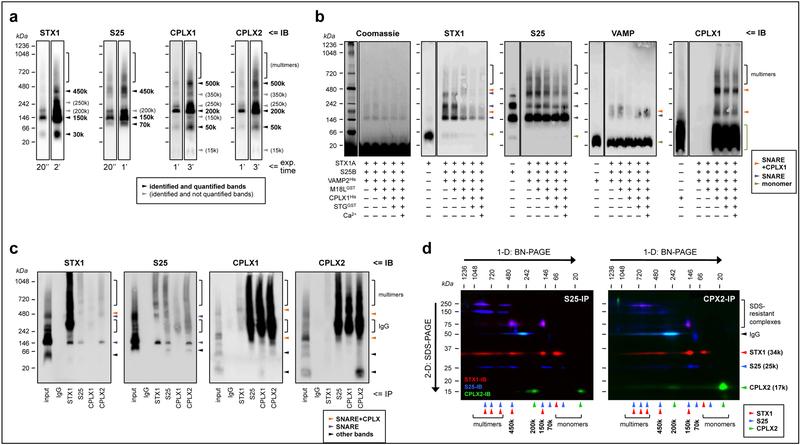
Fig. 1.
Characterization of the presynaptic complexes targeted in the present study. (a) Solubilized brain protein complexes from human inferior temporal cortex (IT) were resolved by blue-native (BN)-PAGE and immunoblotted (IB) with specific antibodies against syntaxin-1 (STX1), SNAP25 (S25) and complexins I (CPLX1) and II (CPLX2) (see Supplementary Table S1). The same representative case is shown in all individual immunoblots, with two exposure (exp.) times (ranging 20 s to 3 min; indicated at the bottom) per probing antibody. Arrowheads point to the identified and/or quantified complexes for each antibody. (b) Reconstitution assays were achieved by sequentially adding, from top to bottom, 1 μm of the recombinant proteins indicated (+) beneath the immunoblots. Abbreviations of the recombinant protein names and tags, and other key information of these constructs, are indicated in Supplementary Table S2. After incubation at 37°C for 30 min, the resulting protein complexes were resolved by BN-PAGE followed by Coomassie de-staining (following manufacturer’s instructions), or immunolabeled as above. Antibodies against munc18–1 (long variant; M18L) and synaptotagmin (STG) were unable to react against their corresponding recombinant constructs under the present experimental conditions (not shown). Arrowheads point to the identified SNARE (purple) or SNARE+complexin (tangerine) heteromers, or the monomeric species (green) for each immunoblot. Of note, recombinant SNAP25 alone, but not syntaxin-1 or VAMP, mimicked all of the above bands, suggesting that SNAP25 has the ability to self-assemble into aggregates with similar stoichiometries as those in SNARE heteromers in vitro and ex vivo. While this observation represents a novel finding with potential implications for the biochemistry of SNARE dynamics, it is also beyond the aim and scope of the present work, and future studies should determine the physiological relevance (if any) of SNAP25 aggregates. (c) Human IT solubilized complexes were immunoprecipitated (IP) with anti-mouse IgG (negative control), anti-STX1, anti-SNAP25, anti-CPLX1, or anti-CPLX2 specific antibodies, and the resulting IP products resolved, along the IP input sample, by BN-PAGE followed by immunoblotting standard procedures. (d) Anti-SNAP25 (left panel) and anti-CPLX2 (right panel) IP products were resolved by one- (1-D) followed by two-dimensional (2-D) BN+SDS-PAGE. Proteins were transferred to PVDF membranes and sequentially immunoblotted with anti-STX1 (red), anti-SNAP25 (blue), and anti-CPLX2 (green) specific antibodies. Top and left arrows indicate the directions of BN- and SDS-PAGE, respectively. Note the presence of SDS-resistant STX1/SNAP25 complexes/aggregates at 75–250 kDa, as well as the immunostaining for the primary antibodies used in IP at 50 kDa. For unknown reasons, the antibody-antigen reaction between the anti-SNAP25 antibody (i.e. SP12) used in the co-IP reactions and the anti-mouse IgG1 secondary antibody and was faint in 1-D BN-PAGE (c), while strong upon 2-D SDS-PAGE separation (d). Note, however, that the same secondary antibody reacted as expected against anti-complexin-I/II antibodies (i.e. SP33 and LP27, both IgG1), but not against anti-syntaxin-1 (i.e. SP7 IgG2a), in both 1-D and 2-D BN/SDS-PAGE. (a–d) Molecular masses (in kDa) of native and SDS-PAGE prestained standards are shown on the left and above.