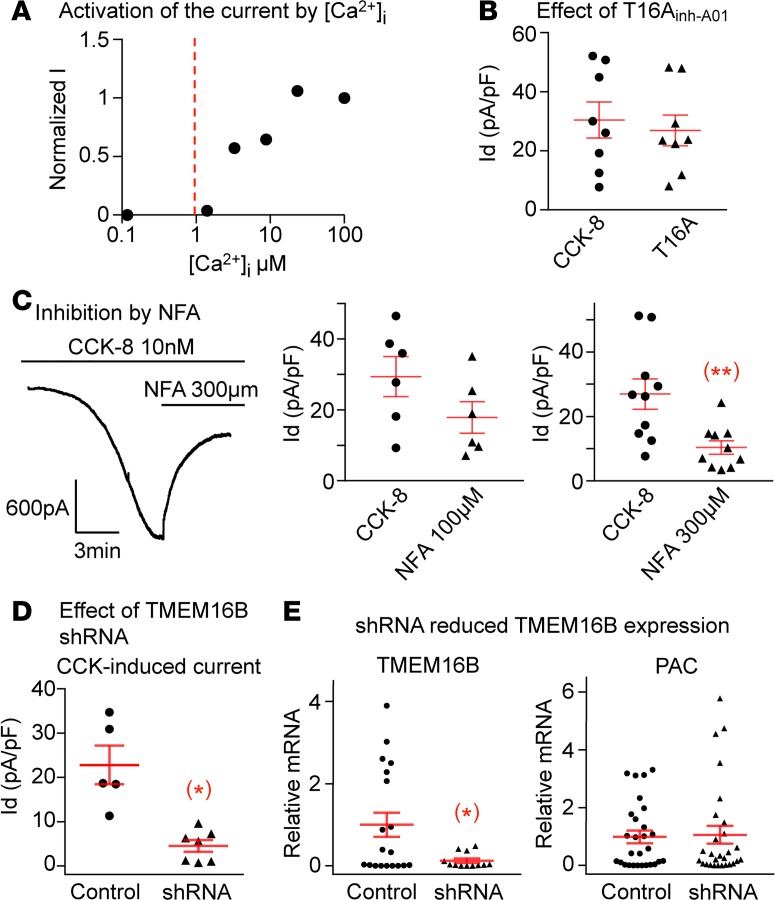
Figure 2. The CCK-8 induced Ca2+-activated Cl– current is dependent on Ano2/TMEM16B subunit.
(A) The dose response obtained using 1 excised inside-out patch showed that the Cl– channel is only activated by Ca2+ concentrations higher than 1 μM. (B) TMEM16A specific inhibitor T16Ainh-A01 does not suppress the CCK-8–induced current (n = 8 neurons from 4 ganglia of 2 mice, P > 0.05). (C) The CCK-8–induced current is reduced from 29.4 ± 5.6 to 17.9 ± 4.4 pA/pF by 100 μM of NFA (n = 6 neurons from 4 ganglia of 2 mice, P > 0.05) but is significantly inhibited from 26.9 ± 4.7 to 10.3 ± 2.1 pA/pF (n = 10 neurons from 6 ganglia of 3 mice, **P < 0.01) by 300 μM of NFA. (D) ShRNA against Ano2/TMEM16B greatly reduced the CCK-8–induced current to 4.6 ± 1.3 from 22.8 ± 4.3 pA/pF in control neurons transduced with scrambled sequence (n = 5 and 7 neurons from 4 ganglia of 2 mice, *P < 0.05). (E) The shRNA reduced the relative mRNA expression of TMEM16B from 1.00 ± 0.30 in control to 0.13 ± 0.01 in shRNA transduced neurons (n = 19 and 12 neurons from 4 ganglia of 2 mice in each group, *P < 0.05). The expression of puromycin acetyltransferase (PAC) as an indicator of transduction was similar in both groups (n = 28 neurons from 4 ganglia of 2 mice in each group, P > 0.05). Data are presented as means ± SEM, paired (B and C) or unpaired (D and E) 2-tailed Student’s t test; each data point in the panels represents 1 individual nodose neuron.