Abstract
Background
Gestational diabetes mellitus (GDM) is associated with adverse health outcomes for mothers and their infants both perinatally and long term. Women with a history of GDM are at risk of recurrence in subsequent pregnancies and may benefit from intervention in the interconception period to improve maternal and infant health outcomes.
Objectives
To assess the effects of interconception care for women with a history of GDM on maternal and infant health outcomes.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (7 April 2017) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials, including quasi‐randomised controlled trials and cluster‐randomised trials evaluating any protocol of interconception care with standard care or other forms of interconception care for women with a history of GDM on maternal and infant health outcomes.
Data collection and analysis
Two review authors independently assessed study eligibility. In future updates of this review, at least two review authors will extract data and assess the risk of bias of included studies; the quality of the evidence will be assessed using the GRADE approach.
Main results
No eligible published trials were identified. We identified a completed randomised controlled trial that was designed to evaluate the effects of a diet and exercise intervention compared with standard care in women with a history of GDM, however to date, it has only published results on women who were pregnant at randomisation (and not women in the interconception period). We also identified an ongoing trial, in obese women with a history of GDM planning a subsequent pregnancy, which is assessing the effects of an intensive lifestyle intervention, supported with liraglutide treatment, compared with usual care. We also identified a trial that was designed to evaluate the effects of a weight loss and exercise intervention compared with lifestyle education also in obese women with a history of GDM planning a subsequent pregnancy, however it has not yet been published. These trials will be re‐considered for inclusion in the next review update.
Authors' conclusions
The role of interconception care for women with a history of GDM remains unclear. Randomised controlled trials are required evaluating different forms and protocols of interconception care for these women on perinatal and long‐term maternal and infant health outcomes, acceptability of such interventions and cost‐effectiveness.
Plain language summary
Care prior to the next pregnancy for women diagnosed with gestational diabetes
What is the issue?
The aim of this Cochrane review was to look at the effects of specialised, targeted care given to mothers who have had a least one pregnancy affected by gestational diabetes. Does this sort of care improve the health of the mother and her baby, during and after her next pregnancy? We collected and analysed all relevant studies to answer this question (date of search: April 2017).
Why is this important?
Gestational diabetes (GD), also called gestational diabetes mellitus (GDM), is glucose intolerance arising during pregnancy. GDM can lead to health complications for the mother. These complications might include high blood pressure during pregnancy and at the birth, pre‐eclampsia (high blood pressure plus protein in the urine), and the development of type 2 diabetes in the future. The birth is more likely to be induced. The babies of mothers with GDM are more likely to be born by caesarean section, and to develop diabetes as children or young adults. Women who experience GDM are at risk of developing it again in a subsequent pregnancy.
If targeted care between the birth of one child and the next pregnancy – known as interconception care – reduces the incidence of GDM, then perhaps these health risks can be reduced, too.
Interconception care may include education, dietary and lifestyle advice, intervention with medication and careful monitoring of the mother’s health, focusing on testing for glucose tolerance.
What evidence did we find?
We searched for trials which looked at the health outcomes for women and babies after specific interconception care, and compared the outcomes for standard care (with no interconception care of this type). Our search identified one trial which has yet to issue a full set of results, plus two further trials; one of these is still underway and the other has yet to be published.
What does this mean?
Because there are no studies currently available, there is not enough evidence at present to say if interconception care for women with a history of GDM can help to improve the health of mothers and their infants. More high‐quality studies are needed, which assess both short‐ and long‐term health outcomes for women and their babies, as well as evaluating the impact on the health services.
Background
Description of the condition
Gestational diabetes mellitus (GDM) is defined as 'carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy' (WHO 1999). This definition includes women who first present with type 1 or type 2 diabetes during pregnancy, or where diabetes was previously undetected. Although GDM typically resolves following birth, it is associated with adverse outcomes for both mother and infant, both in the perinatal period and in the long term.
In subsequent pregnancies of women with a history of GDM, one of the main issues is recurrence of GDM and the associated outcomes. Irrespective of subsequent pregnancies, other long‐term considerations for these women include the development of type 2 diabetes, metabolic syndrome and the risk of cardiovascular disease.
Epidemiology
The reported incidence of GDM varies between different populations and the method and criteria by which the diagnosis is made, with some studies estimating that between 1% and 28% of pregnancies are affected by GDM (Jiwani 2012). Despite variation in diagnostic criteria, there is widespread agreement that the prevalence of diabetes, including GDM, is increasing across the world, in line with the escalating prevalence of obesity. In women with a history of GDM, recurrence occurs in 30% to 84% of subsequent pregnancies (Kim 2007).
A number of risk factors have been linked to GDM, including a history of GDM or glucose intolerance (Kim 2007), family history of first‐degree relatives with GDM or type 2 diabetes, ethnicity (e.g. African, Hispanic, South or East Asian, Native American and Pacific Islander), advanced maternal age, maternal high or low birthweight, high parity, a past history of a macrosomic (large) baby or a stillbirth (Petry 2010), polycystic ovarian syndrome (Toulis 2009), and maternal overweight or obesity (body mass index (BMI) equal to or greater than 25 kg/m² or 30 kg/m², respectively) (Torloni 2009).
Many of these risk factors are unmodifiable background characteristics of the women. It is therefore unsurprising that women with a history of GDM are at an increased risk of recurrent GDM. In addition to a history of GDM in a previous pregnancy, other risk factors for recurrence include ethnicity, maternal age, prepregnancy obesity, weight gain between pregnancies, a short interpregnancy interval and the number of previous pregnancies affected by GDM (Gaudier 1992; Getahun 2010; Kwak 2008; Major 1998). Certain characteristics of the pregnancy affected by GDM have also been reported to increase the risk of recurrence, specifically, earlier diagnosis of GDM, insulin requirement for blood glucose control and higher infant birthweight (Gaudier 1992;Kwak 2008; MacNeill 2001; Major 1998).
Screening and diagnosis of gestational diabetes
There is little consensus on the most appropriate methods by which to screen and diagnose GDM. Screening methods include selective‐ (risk factor‐) based screening, or universal screening, commonly performed between 24 and 28 weeks' gestation, with the use of random or fasting blood glucose concentrations and a 50 g oral glucose challenge test. Diagnostic testing commonly involves either a 75 g or 100 g oral glucose tolerance test, with various diagnostic cut‐offs used. These are addressed in the Cochrane reviews 'Screening and subsequent management for gestational diabetes for improving maternal and infant health' (Tieu 2014) and 'Different strategies for diagnosing gestational diabetes to improve maternal and infant health' (Farrar 2015).
Women with a history of GDM are acknowledged as being at high risk for both GDM recurrence and type 2 diabetes, and it is suggested that women with a history of GDM may require greater monitoring for glucose intolerance during subsequent pregnancies, as such through early self‐monitoring of blood glucose, or an early oral glucose tolerance test (NICE 2015).
Clinical features
Maternal
GDM is usually diagnosed before women experience symptoms, such as polyuria, polydipsia or fatigue. GDM is associated with increased rates of caesarean birth and pre‐eclampsia (Dodd 2007). As mentioned above, women who develop GDM represent a subset of the population prone to developing subsequent type 2 diabetes, in addition to recurrent GDM in future pregnancies. Within 10 years of women developing GDM, approximately half develop type 2 diabetes (Kim 2002). Furthermore, there is increasing evidence that women with a history of GDM may also be at increased risk of cardiovascular disease and metabolic syndrome (Reece 2009; Reece 2010; Vohr 2008).
Infant
Excess insulin due to maternal hyperglycaemia acts in two ways on the fetus. Firstly, insulin promotes fat deposition due to the state of nutrient excess (Pedersen 1954; Whitelaw 1977). Insulin also acts as a growth factor, stimulating further growth of the infant in utero (Hunt 2007). Thus, fetal hyperinsulinaemia results in excessive growth of the fetus, leading to one of the major perinatal concerns in GDM, macrosomia (birthweight greater than 4000 g). Macrosomia may lead to birth trauma including shoulder dystocia, nerve palsies and fractures (Reece 2009; Reece 2010). GDM is associated with respiratory distress syndrome, neonatal hypoglycaemia (low blood glucose), hyperbilirubinaemia (high bilirubin levels), polycythaemia (excess red blood cells), and hypocalcaemia (low calcium) (Reece 2009; Reece 2010). In utero exposure to hyperglycaemia has long‐lasting effects on the infant, increasing their risk of future obesity and type 2 diabetes (Reece 2009; Reece 2010).
While there is relatively little reported on the effects of recurrent GDM on infants, infants born to mothers with recurrent GDM are likely to be larger, as measured by birthweight, incidence of large‐for‐gestational age, or macrosomia compared with infants born to mothers without recurrent GDM in a subsequent pregnancy (Spong 1998).
Management of GDM
The importance of management for women with GDM has been recognised (Crowther 2005; Landon 2009), and several Cochrane reviews have (or plan to) assess alternative management strategies for GDM (Alwan 2009), including lifestyle interventions (Brown 2017a), insulin (Brown 2016a), oral anti‐diabetic pharmacological therapies (Brown 2017b), exercise (Brown 2017c), dietary supplementation with myo‐inositol (Brown 2016b), and different intensities of glycaemic control (Martis 2016).
Description of the intervention
Interconception care may encompass a variety of interventions, including education, dietary and lifestyle advice, pharmacological intervention and active surveillance for illness and complications. It includes care between the birth of one child to the next pregnancy.
Internationally, clinical practice guidelines and consensus statements generally recommend postpartum assessment for continuing glucose intolerance after six to 12 weeks, by oral glucose tolerance testing to detect type 2 diabetes, and on a regular basis thereafter (i.e. every one to three years thereafter depending on other risk factors) (ACOG 2013; ADA 2017; CDA 2013; Metzger 2007; Nankervis 2014; NICE 2015). Despite such recommendations, a high proportion of women with previous GDM do not have testing for diabetes in the postpartum period (Blatt 2011). A Cochrane review evaluating 'Reminder systems for women with previous GDM to increase uptake of testing for type 2 diabetes or impaired glucose tolerance', showed low‐quality evidence supporting an increase in the uptake of testing for type 2 diabetes in women with previous GDM following the issue of postal reminders (Middleton 2014).
While often recommended, there is little evidence on what care women with a history of GDM should receive prior to a subsequent pregnancy. Clinical guidelines recommend that women be assessed preconceptually for a medical review and/or an oral glucose tolerance test, with early evaluation for glucose intolerance during pregnancy (ACOG 2013; NICE 2015). In addition to earlier identification and management of diabetes, interconception care after the postpartum period would ideally aim to target the modifiable risk factors for GDM, thus improving women's metabolic profiles.
How the intervention might work
In a survey of women with a history of GDM within the last five years, while 90% understood that previous GDM placed women at high risk of type 2 diabetes, only 16% believed that they themselves were at high risk of developing diabetes (Kim 2007b). This was partially explained by women planning to improve their behaviour in the future. When women considered the risks if they continued their current lifestyle, this risk perception rose to 39%. Importantly 85% of these women had plans for risk‐reducing behaviour (Kim 2007b). Another survey comparing women with children and a history of GDM with women with children without a history of GDM, found that those with a history of GDM were more likely to smoke and less likely to meet fruit and vegetable consumption recommendations (Kieffer 2006).
The interconception period provides an opportunity to provide advice on potential risks and possible interventions to improve health. Moreover, it provides the opportunity to identify undiagnosed pre‐existing diabetes. Since women with a history of GDM are at increased risk of diabetes, and pre‐existing diabetes in pregnancy is linked to poor maternal and infant health outcomes, it is important to identify and manage accordingly in the interconception period.
Risk factor reduction is a potential area of focus for these women, targeting modifiable risk factors such as maternal obesity where dietary and lifestyle interventions could be implemented and potentially benefit women with a history of GDM. Reducing maternal obesity itself may also lead to better maternal and infant health outcomes outside of its potential effect in the prevention of GDM. Regardless of whether women subsequently become pregnant, such interventions may improve the health of these women.
There is little information on the value of pharmacological agents such as oral anti‐diabetics for women with a history of GDM in the preconceptual period; assessed in the Cochrane review, 'Oral anti‐diabetic agents for women with pre‐existing diabetes mellitus/impaired glucose tolerance or previous gestational diabetes mellitus' (Tieu 2010b). The use of oral anti‐diabetic agents has been predominantly in the setting of polycystic ovarian syndrome or for prevention of type 2 diabetes in women with a history of GDM. 'Interventions for the prevention of type 2 diabetes in women with previous gestational diabetes' will be the focus of a planned Cochrane review (Li 2017).
Why it is important to do this review
Women with a history of GDM are recognised to be at high risk for recurrence in subsequent pregnancies, type 2 diabetes and cardiovascular disease, and therefore, for adverse maternal and infant health outcomes. While management of GDM is worthwhile, interconception care for these women also has the potential to improve maternal and infant health. Interconception care may also allow for detection and appropriate management of asymptomatic pre‐existing diabetes and provide an opportunity for risk factor reduction and, potentially, prevention of recurrent GDM and its sequelae.
Routine pre‐pregnancy care and preconception care for women with known diabetes mellitus are reviewed by the Cochrane reviews 'Routine pre‐pregnancy health promotion for improving pregnancy outcomes' (Whitworth 2009) and 'Preconception care for diabetic women for improving maternal and infant health' (Tieu 2010).
Objectives
To assess the effects of interconception care for women with a history of gestational diabetes mellitus on maternal and infant health outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including quasi‐RCTs and cluster‐RCTs. We plan to exclude cross‐over trials. We plan to exclude trials presented only as abstracts where information on risk of bias and primary or secondary outcomes cannot be obtained; we plan to reconsider these trials for inclusion once the full publication is available.
Types of participants
Women who have been diagnosed with gestational diabetes mellitus (GDM) in a previous pregnancy. Diagnosis of GDM made according to individual study criteria.
Types of interventions
Any protocol of care compared with no care and other forms of interconception care. Interventions may continue during pregnancy.
Types of outcome measures
For this update, we used the core outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus (GDM) and pre‐existing diabetes, which we adapted, as appropriate for this review question.
Primary outcomes
For women
GDM (diagnostic criteria as defined in individual trials)
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Caesarean section
For children
Large‐for‐gestational age
Perinatal mortality (stillbirth or neonatal death)
Mortality or morbidity composite (e.g. death, shoulder dystocia, bone fracture or nerve palsy)
Secondary outcomes
For women
All women (interconception, and if pregnant, antenatal and postnatal)
Adherence to the intervention
Behaviour changes associated with the intervention
Sense of well‐being and quality of life
Views of the intervention
Glycaemic control during/at the end of the intervention (e.g. HbA1c, blood glucose)
Pregnancy
Weight gain
Body mass index (BMI)
Pregnant women
Spontaneous abortion/miscarriage/therapeutic abortion
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Breastfeeding
Postnatal depression
Pregnant women with GDM
Use of additional pharmacotherapy
Hypoglycaemia
Mortality
Longer term
GDM in a subsequent pregnancy
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Cardiovascular health (e.g. blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
For children
Fetuses/neonates
Stillbirth
Neonatal death
Gestational age at birth
Preterm birth (before 37 weeks' gestation; before 34 weeks' gestation)
Apgar score less than seven at five minutes
Macrosomia
Small‐for‐gestational age
Birthweight and z score
Head circumference and z score
Length and z score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia
Hyperbilirubinaemia
Children/adults
Weight and z scores
Height and z scores
Head circumference and z scores
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (e.g. blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
Education, employment and social status/achievement
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Neurosensory disability
Employment, education and social status/achievement
For the use of health services
Number of hospital or health professional visits
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the intervention
Costs associated with the intervention
Cost of maternal care
Cost of infant care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (7 April 2017).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts;
scoping search of clinical trials registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP)).
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Studies awaiting classification; Ongoing studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeTieu 2013.
Two review authors independently assessed for inclusion the reports identified as a result of the search strategy. No studies were eligible for inclusion. We would have resolved any disagreement through discussion with a third person.
Full methods of data collection and analysis to be used in future updates of this review, if eligible studies are identified, are given in Appendix 1.
Results
Description of studies
The updated search of Cochrane Pregnancy and Childbirth's register identified nine reports, relating to one trial (Koivusalo 2016), which we have listed as awaiting further classification, and one report relating to one trial (ISRCTN76189107) which we have listed as ongoing.
Koivusalo 2016 recruited 788 women including 235 women who were not pregnant during their first study visit (women with a previous history of gestational diabetes mellitus (GDM) (187) or a pre‐pregnancy BMI ≥ 30 kg/m2 (48)), and assessed a diet and exercise intervention compared with standard care. To date, however, results have only been reported for the subset of women who were pregnant during the first study visit. See Characteristics of studies awaiting classification.
We identified one new ongoing trial (ISRCTN76189107), which plans to include 50 obese women with a previous history of GDM pre‐pregnancy, and assess the effects of an intensive lifestyle intervention supported with liraglutide treatment compared with standard care. In the previous version of this review we identified one additional trial (NCT00924599), which to date, has not been published (and is thus listed as ongoing). This trial planned to include 12 obese women with a history of GDM and to assess the effects of a pre‐pregnancy weight loss and exercise intervention compared with lifestyle education. See Characteristics of ongoing studies.
See: Figure 1.
1.
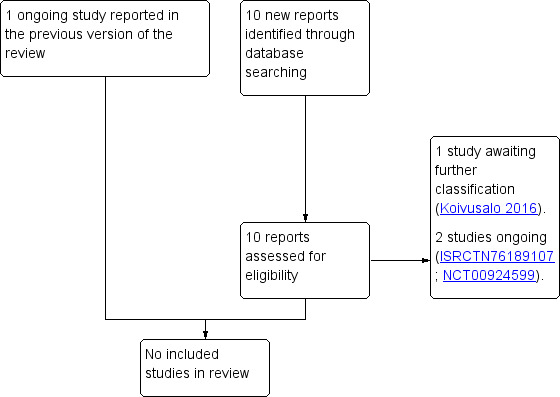
Study flow diagram.
Risk of bias in included studies
No randomised controlled trials were included in the review.
Effects of interventions
No randomised controlled trials were included in the review.
Discussion
Summary of main results
One randomised controlled trial (Koivusalo 2016) was identified that was designed to assess the effects of interconception care on maternal and infant outcomes in women with a history of gestational diabetes mellitus (GDM), however to date, has only published results on women who were pregnant at randomisation, and not non‐pregnant women. A further two trials (ISRCTN76189107; NCT00924599), have been designed to assess the effects of lifestyle interventions for obese women with a history of GDM planning a subsequent pregnancy, however to date have not been published, or are ongoing.
Women with a history of GDM are at increased risk of recurrence of GDM in subsequent pregnancies, future impaired glucose tolerance and diabetes, cardiovascular disease and their sequelae. Given the potential poor outcomes identified in these women, they represent a group who could potentially benefit from intervention(s) aiming to prevent these outcomes. A number of interventions studied in women with a history of GDM after birth, most notably to increase follow‐up testing for impaired glucose tolerance or diabetes (Clark 2009), and interventions such as metformin and lifestyle modification to prevent type 2 diabetes (Ratner 2008) have demonstrated benefit. It remains uncertain how this may translate to interventions in the interconception period for the prevention of GDM recurrence.
In theory, the interconception period in these women represents a time in a high‐risk person's life for: identification and management of undiagnosed impaired glucose tolerance and type 2 diabetes; to initiate lifestyle interventions to potentially improve maternal and infant health outcomes; and to reinforce dietary and lifestyle behaviours for prevention of long‐term adverse health outcomes. No results from randomised controlled trials have been published relating to the effects of interventions in this interconception period for women with a history of GDM for improving health outcomes for women and their children. Given the potential benefits, it remains important to evaluate the effects of such interventions and identify the ideal form of intervention for these women.
Authors' conclusions
Implications for practice.
The role of interconception care for women with a history of gestational diabetes mellitus (GDM) on maternal and infant health outcomes remains unclear.
Implications for research.
Research should be conducted to investigate the effects of interconception care for women with a history of GDM on health outcomes for mothers and their infants. Although such trials are faced with difficulties in identifying women in this time period between pregnancies, women with a history of GDM do represent a population at risk for potentially reversible poor health outcomes.
Trials should consider the role of different forms of intervention including dietary, lifestyle and pharmacological therapies, in addition to the duration of such interventions. Such trials should not only evaluate the effects on maternal and infant health outcomes, but also the acceptability and cost‐effectiveness, to enable translation to clinical practice. Furthermore, future research should focus on long‐term follow‐up, evaluating the effects of such interventions on the long‐term health outcomes associated with GDM for both mothers and their infants.
What's new
| Date | Event | Description |
|---|---|---|
| 7 April 2017 | New search has been performed | Search updated and one new trial listed as 'awaiting classification', and one new trial listed as 'ongoing'. Planned methods updated. |
| 7 April 2017 | New citation required but conclusions have not changed | No change to conclusions. |
Acknowledgements
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool, and the Australia and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by the Australian National Health and Medical Research Council (NHMRC)).
We thank Therese Dowswell from Cochrane Pregnancy and Childbirth who provided support for this update. Therese Dowswell's contribution to this project was supported by the National Institute for Health Research (NIHR), via Cochrane programme grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. Methods of data collection and analysis to be used in future updates of this review
Data collection and analysis
The following methods of data collection and analysis are based on Cochrane Pregnancy and Childbirth standard methods text template.
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third author.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult the third author. We will enter data into Review Manager software (RevMan 2014) and check the data for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We will assess the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison. We will use the Cochrane Pregnancy and Childbirth GRADE core outcome set for reviews of prevention and treatment of gestational diabetes mellitus (GDM) and pre‐existing diabetes in pregnancy, adapted for this review question.
For women
Gestational diabetes mellitus (GDM) (diagnostic criteria as defined in individual trials)
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Caesarean section
Perineal trauma
Weight gain
Postnatal depression
Type 2 diabetes mellitus
For children
Fetuses/neonates
Large‐for‐gestational age
Perinatal mortality (stillbirth or neonatal death)
Mortality or morbidity composite (e.g. death, shoulder dystocia, bone fracture or nerve palsy)
Hypoglycaemia
Children/adults
Adiposity (e.g. as measured by body mass index (BMI), skinfold thickness)
Type 2 diabetes mellitus
Neurosensory disability
We will use the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we will use the mean difference (MD) if outcomes are measured in the same way between trials. We will use the standardised mean difference (SMD) to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Cochrane Handbook using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from another source (Higgins 2011). If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Cross‐over trials
We will exclude cross‐over trials from this review.
Multi‐armed trials
Where a multi‐armed trial is included, we will record and include all outcome data in the review as two‐arm comparisons. We will include the data for the different arms in independent two‐arm comparisons in separate meta‐analyses. In instances where we cannot include the data in separate comparisons, we will combine it to create a single pair‐wise comparison (Higgins 2011). If the control group is shared by two or more study arms, we will divide the control group between relevant subgroup categories to avoid double‐counting the participants (for dichotomous data we will divide the events and the total population, while for continuous data we will assume the same mean and standard deviation (SD) but will divide the total population). We will describe the details in the 'Characteristics of included studies' tables.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
We will carry out all outcome analyses, as far as possible, on an intention‐to‐treat basis i.e. we will attempt to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, we will present the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Periconceptual BMI (e.g. underweight versus normal range versus overweight versus obese versus morbidly obese).
Polycystic ovarian syndrome (yes versus no).
Diagnostic criteria for GDM in previous pregnancy (e.g. International Association of Diabetes and Pregnancy Study Groups criteria (IADPSG 2010) versus other).
Management of GDM in previous pregnancy (e.g. non‐pharmacological measures (dietary and lifestyle advice) versus oral anti‐diabetic agents versus insulin).
Ethnicity (e.g. high risk versus low risk).
Pregnancy interval (e.g. estimated date of delivery less than one year from last birth versus one year to five years from last birth versus more than five years from last birth).
We will restrict subgroup analyses to the review's primary outcomes.
We will assess differences between subgroups by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We plan to carry out sensitivity analysis on primary outcomes to explore the effect of trial quality where there is an overall high risk of bias associated with included trials or where quasi‐randomised or cluster‐randomised trials are included in the review. We will consider a study to be at overall high risk of bias if both concealment of allocation and attrition rates are assessed as being at high risk of bias. We plan to exclude studies of poor quality from the analysis (those rating as high risk in total overall risk of bias) or quasi‐randomised or cluster‐randomised trials in order to assess for any substantive difference in the overall result.
Characteristics of studies
Characteristics of studies awaiting assessment [ordered by study ID]
Koivusalo 2016.
| Methods | Randomised controlled trial. |
| Participants |
Inclusion criteria: women with a previous history of GDM or a pre‐pregnancy BMI ≥ 30 kg/m², either planning pregnancy or pregnant at < 20 + 0 weeks’ gestation. Exclusion criteria: age < 18 years; diabetes diagnosed before pregnancy; medications that influence glucose metabolism (e.g. oral corticosteroids and metformin); multiple pregnancies; physical disability; current substance abuse; severe psychiatric disorders and significant difficulties to co‐operate (e.g. inadequate Finnish language skills). |
| Interventions | Women randomised to structured counselling on diet and exercise or standard care. Diet and exercise intervention Women visited the study nurse every 3 months before and during pregnancy, and at 6 weeks, 6 and 12 months postpartum. Trained study nurses and nutritionists provided counselling, as below, and weight targets were set: 5% to 10% weight loss before pregnancy for women with pre‐pregnancy BMI ≥ 25 kg/m²; no weight gain during the first 2 trimesters for women with pre‐pregnancy BMI ≥ 30 kg/m². Dietary counselling was based on national Finnish nutritional guidelines. The 'plate model' was used during the counselling sessions (filling half a plate with raw/cooked vegetables, one quarter with starchy carbohydrates, and one quarter with meat, fish, beans, eggs or other proteins). The aim was to achieve a total energy intake of 1600‐1800 kcal/day; 40% to 50% from carbohydrates, 30% to 40% from fats, and 20% to 25% from protein. Women were encouraged to increase intake of vegetables, legumes, fruits and berries; wholegrain and fibre; low‐fat dairy and vegetable fats. In the postpartum period, women received breastfeeding and infant nutrition counselling based on national recommendations. Every 3 months women filled in 3‐day food diaries. In addition to regular visits to the study nurse, women took part in structured group visits to a nutritionist, at enrolment, during the first trimester and at 6 and 12 month postpartum; with additional visits arranged if needed. The aim of the physical activity counselling was to achieve a minimum of 30 minutes of moderate intensity exercise (exercise during which the woman becomes at least slightly out of breath and perspires but is still able to talk) 5 times/week or 50 minutes 3 times/week, and to adopt an overall active lifestyle. An individual exercise program was planned for each woman during the counselling visits, and modified as needed. Women also received pedometers, with a recommendation of at least 10,000 steps/day. Women had the option of attending guided exercise groups, or got tickets (e.g. to public swimming pooled once a week. Physical activity logbooks were used. Standard care Women received basic dietary and exercise information leaflets similar to those provided at primary health care centres at the time of enrolment. During pregnancy, they received usual health education provided at their local antenatal clinic. |
| Outcomes | Primary outcome: GDM. |
| Notes |
Funding: "This study was funded by the Ahokas Foundation, the Finnish Foundation for Cardiovascular Disease, Special State Subsidy for Health Science Research of Helsinki University Central Hospital, Samfundet Folkhälsan, The Finnish Diabetes Research Foundation, the State Provincial Office of Southern Finland, and The Social Insurance Institution of Finland. The funders have not had any role in designing or conducting the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; and in the decision to submit the manuscript for publication". Declarations of interest: "No potential conflicts of interest relevant to this article were reported". Between February 2008 and November 2011, 788 women were recruited into the study. 235 were non‐pregnant and 493 pregnant during the first study visit. In the non‐pregnant and pregnant groups, 79.6% (women = 187) and 40.4% (women = 199), respectively, had a history of previous GDM. To date, results have only been published for the women who were pregnant during the first study visit. Last correspondence with Saila Koivusalo 01/12/2016 indicated manuscript relating specifically to non‐pregnant women with a history of GDM has not yet been published. |
BMI: body mass index GDM: gestational diabetes mellitus
Characteristics of ongoing studies [ordered by study ID]
ISRCTN76189107.
| Trial name or title | An open‐label randomized trial of an intensive lifestyle package supported with Liraglutide treatment in obese, non‐pregnant women with previous history of Gestational Diabetes Mellitus. |
| Methods | Randomised controlled trial. |
| Participants |
Inclusion criteria: severe obesity (BMI ≥ 35 kg/m²) without type 2 and with previous GDM (with or without insulin); willing to give written informed consent and to comply with the requirements of this study protocol; aged ≥ 18 years at baseline; planning a pregnancy within the next 1 to 2 years; negative pregnancy test; contraception during the study period. Exclusion criteria: allergy/sensitivity to study medication; pregnant or breast feeding or considering becoming pregnant during the study period; medical disorder requiring medication other than stable hypertension, hypothyroidism, polycystic ovarian syndrome; ongoing abuse of alcohol or narcotics; family or personal history of multiple endocrine neoplasia type 2 or familial medullary thyroid carcinoma; personal history of non‐familial medullary thyroid carcinoma; history of acute or chronic pancreatitis; obesity induced by drug treatment; use of approved weight lowering pharmacotherapy; previous surgical treatment of obesity; history of major depressive disorder or suicide attempt; uncontrolled hypertension; unable to provide written informed consent. |
| Interventions |
Intervention: a treatment package of an intensive lifestyle approach (including diet and physical activity advice) supported by a daily treatment of liraglutide for a period of 6 months. Control: usual care. |
| Outcomes |
Primary outcomes: proportion of eligible women who would agree to participate in the study; acceptability of women of taking daily liraglutide injections; proportion of women that complied with the study protocol and completed the study intervention. Secondary outcomes: fasting glucose; glucose homeostasis (OGTT, HbA1c, HOMA‐IR); inflammatory markers (C‐reactive protein); weight loss; GDM and/or impaired glucose tolerance in a subsequent pregnancy. |
| Starting date | Overall: September 2016; recruitment: April 2017; planned end date: December 2020. |
| Contact information | Professor Fionnuala McAuliffe, UCD Perinatal Research Centre, Obstetrics and Gynaecology, School of Medicine, University College Dublin, National Maternity Hospital, Dublin, Ireland Phone: +353 1 6373216 Email: fionnuala.mcauliffe@ucd.ie |
| Notes |
Target recruitment: 50. Sponsor: Clinical Research Centre, University College Dublin. |
NCT00924599.
| Trial name or title | A pilot study using weight loss and exercise to prevent recurring gestational diabetes in obese women. |
| Methods | Randomised controlled trial. |
| Participants |
Inclusion criteria: 18 to 40 year old women; English or Spanish speaking; GDM in last pregnancy; BMI 30 to 40 kg/m²; 1 to 5 years since last pregnancy; non‐smoking; planning to have a baby but willing to use birth control during a 3‐month weight loss program. Exclusion criteria: 3 or more miscarriages; history of infertility; type 1 or type 2 diabetes; any weight loss since last pregnancy (based on last pre‐pregnancy weight); history of major psychiatric illness, drug abuse, or unsafe dieting practices; history of bariatric surgery, major medical conditions that prohibit physical activity or dietary intervention. |
| Interventions |
Weight loss and exercise: women attended sessions focused on healthy weight loss, healthy eating and exercise; weekly sessions for 12 weeks followed by monthly group meetings until conception; aimed for loss of 7% of body weight and increased physical activity to 2.5 hours per week. Lifestyle education: women received education focusing on learning about healthy eating and healthy activity, stress reduction techniques, ways of increasing activity; once a month for 3 months, then once a month until conception. |
| Outcomes | Primary outcome: GDM not present in pregnancy. |
| Starting date | Start date: June 2009; study completion: June 2014. No manuscript identified as yet. |
| Contact information | Associate Professor, Suzanne Phelan, California Polytechnic State University‐San Luis Obispo. |
| Notes |
Enrolment: 12 women. Sponsor: California Polytechnic State University‐San Luis Obispo. |
BMI: body mass index GDM: gestational diabetes mellitus HbA1c: glycated haemoglobin HOMA‐IR: homeostatic model assessment ‐ insulin resistance OGTT: oral glucose tolerance test
Differences between protocol and review
In this update of the review:
we updated the outcomes, using the standard outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus and pre‐existing diabetes (which we adapted, as appropriate for this review question);
we updated the planned methods to be in line with those in the standard template used by Cochrane Pregnancy and Childbirth (including use of the GRADE approach to assess the quality of the body of evidence and the use of ’Summary of findings’ tables).
Contributions of authors
Emily Shepherd wrote the update of this review with regular input and feedback from Joanna Tieu, Philippa Middleton and Caroline Crowther.
Sources of support
Internal sources
ARCH: Australian Research Centre for Health of Women and Babies, Robinson Research Institute, The University of Adelaide, Australia.
External sources
Department of Health and Ageing, Australia.
-
NHMRC: National Health and Medical Research Council, Australia.
Funding for the Pregnancy and Childbirth Australian and New Zealand Satellite
-
NIHR: National Institute for Health Research, UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Joanna Tieu: is supported by an NHMRC postgraduate scholarship and Arthritis Australia Ken Muirden fellowship (jointly funded by the Australian Rheumatology Association and Roche).
Emily Shepherd: none known.
Philippa Middleton: none known.
Caroline A Crowther: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies awaiting assessment
Koivusalo 2016 {published data only}
- Grotenfelt NE, Wasenius NS, Rono K, Laivuori H, Stach‐Lempinen B, Orho‐Melander M, et al. Interaction between rs10830963 polymorphism in MTNR1b and lifestyle intervention on occurrence of gestational diabetes. Diabetologia 2016;59(8):1655‐8. [DOI] [PubMed] [Google Scholar]
- Huvinen E, Grotenfelt NE, Eriksson JG, Rono K, Klemetti MM, Roine R, et al. Heterogeneity of maternal characteristics and impact on gestational diabetes (GDM) risk ‐ Implications for universal GDM screening?. Annals of Medicine 2016;48(1‐2):52‐8. [DOI] [PubMed] [Google Scholar]
- Huvinen H, Koivusalo S, StachLempinen B, Kautiainen H, Eriksson J. Effects of a lifestyle intervention during pregnancy and 1‐year postpartum ‐ results from the RADIEL study. Gynecological Endocrinology 2016;32:161. [Google Scholar]
- Koivusalo SB, Rono K, Klemetti MM, Roine RP, Lindstrom J, Erkkola M, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: The Finnish gestational diabetes prevention study (RADIEL): A randomized controlled trial. Diabetes Care 2016;39(1):24‐30. [DOI] [PubMed] [Google Scholar]
- Meinila J, Valkama A, Koivusalo SB, Rono K, Kautiainen H, Lindstrom J, et al. Association between diet quality measured by the healthy food intake index and later risk of gestational diabetes ‐ a secondary analysis of the RADIEL trial. European Journal of Clinical Nutrition 2017;71(4):555‐7. [DOI] [PubMed] [Google Scholar]
- NCT01698385. Prevention of gestational diabetes through lifestyle modification (RADIEL). clinicaltrials.gov/ct2/show/NCT01698385 Date first received: 11 September 2012.
- Rono K, Stach‐Lempinen B, Klemetti MM, Kaaja RJ, Poyhonen‐Alho M, Eriksson JG, et al. Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy and Childbirth 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama A, Koivusalo S, Lindstrom J, Meinila J, Kautiainen H, Stach‐Lempinen B, et al. The effect of dietary counselling on diet in pregnant women at risk for gestational diabetes. Annals of Nutrition and Metabolism 2015;67(Suppl 1):138. [Google Scholar]
- Valkama A, Koivusalo S, Lindstrom J, Meinila J, Kautiainen H, Stach‐Lempinen B, et al. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: a secondary analysis of a randomised controlled trial RADIEL. European Journal of Clinical Nutrition 2016;70(8):912‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN76189107 {published data only}
- ISRCTN76189107. A pre‐pregnancy study examining the effects of an intensive lifestyle package supported with Liraglutide treatment, a medication equivalent to a natural hormone produced in the stomach, in obese women with previous history of pregnancy diabetes. isrctn.com/ISRCTN76189107 Date first received: 21 December 2016.
NCT00924599 {published data only}
- NCT00924599. Prevention of gestational diabetes pilot study. clinicaltrials.gov/show/NCT00924599 Date first received: 4 May 2009.
Additional references
ACOG 2013
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 137: Gestational diabetes mellitus. Obstetrics and Gynecology 2013;122(2 Pt 1):406‐16. [DOI] [PubMed] [Google Scholar]
ADA 2017
- American Diabetes Association. Management of diabetes in pregnancy. Diabetes Care 2017;40(Suppl 1):S114‐9. [Google Scholar]
Alwan 2009
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blatt 2011
- Blatt AJ, Nakamoto JM, Kaufman HW. Gaps in diabetes screening during pregnancy and postpartum. Obstetrics and Gynecology 2011;117:61‐8. [DOI] [PubMed] [Google Scholar]
Brown 2016a
- Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016b
- Brown J, Crawford TJ, Alsweiler J, Crowther CA. Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012048.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017a
- Brown J, Alwan NA, West J, Brown S, McKinlay CJD, Farrar D, Crowther CA. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011970.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017b
- Brown J, Martis R, Hughes B, Rowan J, Crowther CA. Oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD011967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017c
- Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012202.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDA 2013
- Canadian Diabetes Association. 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: diabetes and pregnancy. Canadian Journal of Diabetes 2013;37(Suppl 1):S168–183. [DOI] [PubMed] [Google Scholar]
Clark 2009
- Clark HD, Graham ID, Karovitch A, Keely EJ. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2009;200:634.e1‐7. [DOI] [PubMed] [Google Scholar]
Crowther 2005
- Crowther C, Hiller J, Moss J, McPhee A, Jeffries W, Robinson J, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352:2477‐86. [DOI] [PubMed] [Google Scholar]
Dodd 2007
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(4):307‐12. [DOI] [PubMed] [Google Scholar]
Farrar 2015
- Farrar D, Duley L, Medley N, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD007122.pub3] [DOI] [PubMed] [Google Scholar]
Gaudier 1992
- Gaudier FL, Hauth JC, Poist M, Corbet D, Cliver SP. Recurrence of gestational diabetes mellitus. Obstetrics and Gynecology 1992;80(5):755‐8. [PubMed] [Google Scholar]
Getahun 2010
- Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: risk of recurrence in subsequent pregnancies. American Journal of Obstetrics and Gynecology 2010;203(5):467. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 2007
- Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstetrics and Gynecology Clinics of North America 2007;34(2):173‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
IADPSG 2010
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiwani 2012
- Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational diabetes mellitus: results from a survey of country prevalence and practices. Journal of Maternal Fetal and Neonatal Medicine 2012;25(6):600‐10. [DOI] [PubMed] [Google Scholar]
Kieffer 2006
- Kieffer EC, Sinco B, Kim C. Health behaviours among women of reproductive age with and without a history of gestational diabetes mellitus. Diabetes Care 2006;29(8):1788‐93. [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862‐8. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care 2007;30(5):1314‐9. [DOI] [PubMed] [Google Scholar]
Kim 2007b
- Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 2007;30(9):2281‐6. [DOI] [PubMed] [Google Scholar]
Kwak 2008
- Kwak SH, Kim HS, Choi SH, Lim S, Cho YM, Park KS, et al. Subsequent pregnancy after gestational diabetes mellitus: frequency and risk factors for recurrence in Korean women. Diabetes Care 2008;31(9):1867‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thorn E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017
- Li J, Shepherd E, Hague W, Crowther CA, Middleton P. Interventions for preventing type 2 diabetes in women with previous gestational diabetes. Personal correspondence 2017.
MacNeill 2001
- MacNeill S, Dodds L, Hamilton DC, Armson BA, VandenHof M. Rates and risk factors for recurrence of gestational diabetes. Diabetes Care 2001;24(4):659‐62. [DOI] [PubMed] [Google Scholar]
Major 1998
- Major CA, deVeciana M, Weeks J, Morgan MA. Recurrence of gestational diabetes: who is at risk?. American Journal of Obstetrics and Gynecology 1998;179(4):1038‐42. [DOI] [PubMed] [Google Scholar]
Martis 2016
- Martis R, Brown J, Alsweiler J, Crawford TJ, Crowther CA. Different intensities of glycaemic control for women with gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011624.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 2007
- Metzger BE, Buchanan TA, Coustan DR, Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop‐conference on gestational diabetes mellitus. Diabetes Care 2007;30(Suppl 2):S251‐60. [DOI] [PubMed] [Google Scholar]
Middleton 2014
- Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD009578.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nankervis 2014
- Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. http://adips.org/ (accessed 17 February 2017).
NICE 2015
- National Institute for Health and Clinical Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. https://www.nice.org.uk/guidance/ng3 (accessed 17 February 2017). [PubMed]
Petry 2010
- Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition 2010;104(6):775‐87. [DOI] [PubMed] [Google Scholar]
Ratner 2008
- Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi‐Sunyer X, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reece 2009
- Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373(9677):1789‐97. [DOI] [PubMed] [Google Scholar]
Reece 2010
- Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(3):199‐203. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Spong 1998
- Spong CY, Guillermo MD, Kuboshige J, Cabalum T. Recurrence of gestational diabetes mellitus: identification of risk factors. American Journal of Perinatology 1998;15(1):29‐33. [DOI] [PubMed] [Google Scholar]
Tieu 2010
- Tieu J, Middleton P, Crowther C. Preconception care for diabetic women for improving maternal and infant health. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007776.pub2] [DOI] [PubMed] [Google Scholar]
Tieu 2010b
- Tieu J, Coat S, Hague W, Middleton P. Oral anti‐diabetic agents for women with pre‐existing diabetes mellitus/impaired glucose tolerance or previous gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007724.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieu 2014
- Tieu J, Middleton P, McPhee AJ, Crowther CA. Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007222.pub3] [DOI] [PubMed] [Google Scholar]
Torloni 2009
- Torloni M R, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta‐analysis. Obesity Reviews 2009;10(2):194‐203. [DOI] [PubMed] [Google Scholar]
Toulis 2009
- Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I, et al. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta‐analysis. Fertility and Sterility 2009;92(2):667‐77. [DOI] [PubMed] [Google Scholar]
Vohr 2008
- Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome?. Journal of Maternal, Fetal and Neonatal Medicine 2008;21(3):149‐57. [DOI] [PubMed] [Google Scholar]
Whitworth 2009
- Whitworth M, Dowswell T. Routine pre‐pregnancy health promotion for improving pregnancy outcomes. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007536.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus ‐ report of a WHO/IDF consultation. www.who.int/diabetes/publications/diagnosis_diabetes1999/en/index.html (accessed 25 July 2012).
References to other published versions of this review
Tieu 2012
- Tieu J, Middleton P, Crowther CA 10.1002/14651858.CD010211. Interconception care for women with a history of gestational diabetes for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010211] [DOI] [PubMed] [Google Scholar]
Tieu 2013
- Tieu J, Bain E, Middleton P, Crowther CA. Interconception care for women with a history of gestational diabetes for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010211.pub2] [DOI] [PubMed] [Google Scholar]


