Abstract
Background
Calcineurin inhibitors (CNI) can reduce acute transplant rejection and immediate graft loss but are associated with significant adverse effects such as hypertension and nephrotoxicity which may contribute to chronic rejection. CNI toxicity has led to numerous studies investigating CNI withdrawal and tapering strategies. Despite this, uncertainty remains about minimisation or withdrawal of CNI.
Objectives
This review aimed to look at the benefits and harms of CNI tapering or withdrawal in terms of graft function and loss, incidence of acute rejection episodes, treatment‐related side effects (hypertension, hyperlipidaemia) and death.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 11 October 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs) where drug regimens containing CNI were compared to alternative drug regimens (CNI withdrawal, tapering or low dose) in the post‐transplant period were included, without age or dosage restriction.
Data collection and analysis
Two authors independently assessed studies for eligibility, risk of bias, and extracted data. Results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
We included 83 studies that involved 16,156 participants. Most were open‐label studies; less than 30% of studies reported randomisation method and allocation concealment. Studies were analysed as intent‐to‐treat in 60% and all pre‐specified outcomes were reported in 54 studies. The attrition and reporting bias were unclear in the remainder of the studies as factors used to judge bias were reported inconsistently. We also noted that 50% (47 studies) of studies were funded by the pharmaceutical industry.
We classified studies into four groups: CNI withdrawal or avoidance with or without substitution with mammalian target of rapamycin inhibitors (mTOR‐I); and low dose CNI with or without mTOR‐I. The withdrawal groups were further stratified as avoidance and withdrawal subgroups for major outcomes.
CNI withdrawal may lead to rejection (RR 2.54, 95% CI 1.56 to 4.12; moderate certainty evidence), may make little or no difference to death (RR 1.09, 95% CI 0.96 to 1.24; moderate certainty), and probably slightly reduces graft loss (RR 0.85, 95% CI 0.74 to 0.98; low quality evidence). Hypertension was probably reduced in the CNI withdrawal group (RR 0.82, 95% CI 0.71 to 0.95; low certainty), while CNI withdrawal may make little or no difference to malignancy (RR 1.10, 95% CI 0.93 to 1.30; low certainty), and probably makes little or no difference to cytomegalovirus (CMV) (RR 0.87, 95% CI 0.52 to 1.45; low certainty)
CNI avoidance may result in increased acute rejection (RR 2.16, 95% CI 0.85 to 5.49; low certainty) but little or no difference in graft loss (RR 0.96, 95% CI 0.79 to 1.16; low certainty). Late CNI withdrawal increased acute rejection (RR 3.21, 95% CI 1.59 to 6.48; moderate certainty) but probably reduced graft loss (RR 0.84, 95% CI 0.72 to 0.97, low certainty).
Results were similar when CNI avoidance or withdrawal was combined with the introduction of mTOR‐I; acute rejection was probably increased (RR 1.43; 95% CI 1.15 to 1.78; moderate certainty) and there was probably little or no difference in death (RR 0.96; 95% CI 0.69 to 1.36, moderate certainty). mTOR‐I substitution may make little or no difference to graft loss (RR 0.94, 95% CI 0.75 to 1.19; low certainty), probably makes little of no difference to hypertension (RR 0.86, 95% CI 0.64 to 1.15; moderate), and probably reduced the risk of cytomegalovirus (CMV) (RR 0.60, 95% CI 0.44 to 0.82; moderate certainty) and malignancy (RR 0.69, 95% CI 0.47 to 1.00; low certainty). Lymphoceles were increased with mTOR‐I substitution (RR 1.45, 95% CI 0.95 to 2.21; low certainty).
Low dose CNI combined with mTOR‐I probably increased glomerular filtration rate (GFR) (MD 6.24 mL/min, 95% CI 3.28 to 9.119; moderate certainty), reduced graft loss (RR 0.75, 95% CI 0.55 to 1.02; moderate certainty), and made little or no difference to acute rejection (RR 1.13 ; 95% CI 0.91 to 1.40; moderate certainty). Hypertension was decreased (RR 0.98, 95% CI 0.80 to 1.20; low certainty) as was CMV (RR 0.41, 95% CI 0.16 to 1.06; low certainty). Low dose CNI plus mTOR‐I makes probably makes little of no difference to malignancy (RR 1.22, 95% CI 0.42 to 3.53; low certainty) and may make little of no difference to death (RR 1.16, 95% CI 0.71 to 1.90; moderate certainty).
Authors' conclusions
CNI avoidance increased acute rejection and CNI withdrawal increases acute rejection but reduced graft loss at least over the short‐term. Low dose CNI with induction regimens reduced acute rejection and graft loss with no major adverse events, also in the short‐term. The use of mTOR‐I reduced CMV infections but increased the risk of acute rejection. These conclusions must be tempered by the lack of long‐term data in most of the studies, particularly with regards to chronic antibody‐mediated rejection, and the suboptimal methodological quality of the included studies.
Plain language summary
Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients
What is the issue?
Calcineurin inhibitors (CNI, cyclosporin and tacrolimus) are an important part of treatment to suppress the immune system to prevent rejection of transplanted kidneys. However, CNI can cause high blood pressure and kidney scarring which contribute to worsening of risk factors for heart attack, stroke, and loss of the transplanted organ over time.
There are conflicting data on the results of withdrawing these drugs from kidney transplant recipients; some studies suggest improved kidney function but others report a moderate risk of developing rejection. Because of this uncertainty, we assessed the benefits and harms of CNI withdrawal or tapering in kidney transplant recipients to identify which approach was more beneficial.
What did we do? We included 83 studies that involved more than 16,000 people in our review. Studies which compared standard dose CNI regimens with withdrawal, tapering or low dose CNI in the post‐transplant period were analysed.
What did we find? Although withdrawing CNI treatment resulted in more rejections in the short term, there was no clear change in transplanted organ failure, death, development of cancer, or infections. Replacing CNI with another group of drugs ‐ the mTOR inhibitors ‐ did not significantly change outcomes, except for fewer cytomegalovirus (CMV) infections. Lower CNI dose was associated with fewer episodes of kidney transplant rejection and loss, but only in the first year to up to five years after the transplant.
Conclusions We found that the long‐term outcomes for stopping or gradually reducing CNI therapy were not clear, and that mTOR inhibitors can reduce CMV infections with a higher risk of acute rejection. There were insufficient studies with long term follow‐up to clearly determine which treatment is better for people who receive kidney transplants.
Summary of findings
Summary of findings for the main comparison. Calcineurin inhibitor (CNI) withdrawal versus standard dose CNI for kidney transplant recipients.
| CNI withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: CNI withdrawal Comparison: standard dose CNI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with standard dose CNI | Risk with CNI withdrawal | ||||
| Death Follow‐up: range 9 months to 20 years | Study population | RR 1.09 (0.96 to 1.24) | 2010 (14 ) | ⊕⊕⊕⊝ MODERATE 1 2 3 4 | |
| 225 per 1,000 | 245 per 1,000 (216 to 279) | ||||
| Acute rejection Follow‐up: range 9 months to 15 years | Study population | RR 2.54 (1.56 to 4.12) | 1666 (15) | ⊕⊕⊕⊝ MODERATE 2 4 5 6 | |
| 137 per 1,000 | 348 per 1,000 (214 to 564) | ||||
| GFR Follow‐up: range 1 to 15 years | The mean GFR in the intervention group was 3.56 mL/min more (1.13 less to 8.25 more) than the control group |
‐ | 910 (8) | ⊕⊕⊝⊝ LOW 7 8 | |
| Graft loss Follow‐up: range 9 months to 20 years | Study population | RR 0.85 (0.74 to 0.98) | 2090 (16) | ⊕⊕⊝⊝ LOW 1 2 9 10 11 12 | |
| 236 per 1,000 | 201 per 1,000 (175 to 231) | ||||
| Adverse events: hypertension Follow‐up: range 1 to 15 years | Study population | RR 0.82 (0.71 to 0.95) | 950 (5 ) | ⊕⊕⊝⊝ LOW 2 10 | |
| 555 per 1,000 | 455 per 1,000 (394 to 527) | ||||
| Adverse events: CMV infection Follow‐up: range 9 months to 15 years | Study population | RR 0.87 (0.52 to 1.45) | 608 (7) | ⊕⊕⊝⊝ LOW 1 2 10 | |
| 98 per 1,000 | 86 per 1,000 (51 to 143) | ||||
| Adverse events: malignancy Follow‐up: range 1 to 15 years | Study population | RR 1.10 (0.93 to 1.30) | 1079 (6) | ⊕⊕⊝⊝ LOW 1 2 4 10 | |
| 257 per 1,000 | 282 per 1,000 (239 to 334) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 despite different follow up times, heterogeneity not noted on analysis
2 Most studies were ITT analysis, some small studies did not specify randomisation and allocation concealment
3 Larger studies closer to pooled estimate on funnel plot
4 Some studies were small with large confidence intervals, CI fails to exclude benefit or harm
5 Heterogeneity low when biopsy‐proven rejections were analysed in subgroup
6 Smaller studies not distributed around point estimate
7 Significant heterogeneity noted despite separating time periods of reporting GFR
8 Only few studies reported GFR with possible attrition bias
9 2 large studies had more than 2 comparison groups
10 Very few studies reported the outcome
11 Symmetric distribution studies around estimate of effect
12 2 studies with high event rates skew the effect
Summary of findings 2. Low dose calcineurin inhibitors (CNI) versus to standard dose CNI for kidney transplant recipients.
| Low dose CNI versus standard dose CNI for kidney transplant recipients | ||||||
| Patient or population: kidney transplant recipients Intervention: low dose CNI Comparison: standard dose CNI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard dose CNI | Risk with low dose CNI | |||||
| Death Follow‐up: range 6 months to 2 years | Study population | RR 0.79 (0.50 to 1.27) | 3462 (15) | ⊕⊕⊕⊝ MODERATE 1 2 3 | ||
| 23 per 1,000 | 19 per 1,000 (12 to 30) | |||||
| Acute rejection Follow‐up: range 6 months to 2 years | Study population | RR 0.87 (0.76 to 1.00) | 3757 (19) | ⊕⊕⊕⊝ MODERATE 1 2 4 | ||
| 183 per 1,000 | 159 per 1,000 (139 to 183) | |||||
| GFR Follow‐up: range 6 months to 2 years | The mean GFR in the intervention group was 4.1 mL/min more (2.07 more to 6.12 more) than the control group | ‐ | 2623 (13) | ⊕⊕⊕⊝ MODERATE 5 6 7 | ||
| Graft loss Follow‐up: range 6 months to 2 years | Study population | RR 0.75 (0.55 to 1.02) | 3286 (15) | ⊕⊕⊕⊝ MODERATE 1 2 3 6 | Sensitivity analysis after excluding 1 study which also involved steroid withdrawal; significant reduction in graft loss in the low dose regimen | |
| 58 per 1,000 | 44 per 1,000 (32 to 60) | |||||
| Adverse events: hypertension Follow‐up: range 6 months to 2 years | Study population | RR 0.84 (0.70 to 1.00) | 1877 (5) | ⊕⊕⊝⊝ LOW 2 7 8 9 | ||
| 218 per 1,000 | 184 per 1,000 (153 to 218) | |||||
| Adverse events: CMV infection Follow‐up: range 6 months to 2 years | Study population | RR 1.23 (0.94 to 1.62) | 1948 (6) | ⊕⊕⊕⊝ MODERATE 2 8 10 | ||
| 101 per 1,000 | 124 per 1,000 (95 to 163) | |||||
| Adverse events: malignancy Follow‐up: range 6 months to 2 years | Study population | RR 0.90 (0.41 to 1.97) | 1637 (5) | ⊕⊕⊝⊝ LOW 2 3 9 | ||
| 15 per 1,000 | 14 per 1,000 (6 to 30) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Most studies with ITT analysis, randomisation procedure and allocation concealment not clear from most publications
2 Minimal heterogeneity noted on analysis
3 Several small studies with wide confidence intervals
4 Despite studies with or without induction, sensitivity analysis made no difference to outcome
5 Heterogeneity noted only between subgroups
6 Only 2/15 studies had more than 2 comparison groups
7 Industry sponsored
8 1/6 studies did not report some outcomes due to high dropout
9 Only 5 studies reported the outcome and had wide CI
10 Few studies reported the outcome
Summary of findings 3. Calcineurin inhibitor (CNI) withdrawal + mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI for kidney transplant recipients.
| CNI withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: CNI withdrawal + mTORi Comparison: standard dose CNI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with standard dose CNI | Risk with CNI withdrawal + mTOR | ||||
| Death Follow‐up: range 6 months to 5 years | Study population | RR 0.99 (0.69 to 1.40) | 5427 (23) | ⊕⊕⊕⊝ MODERATE 1 2 3 4 | |
| 26 per 1,000 | 26 per 1,000 (18 to 36) | ||||
| Acute rejection Follow‐up: range 6 months to 5 years | Study population | RR 1.43 (1.15 to 1.78) | 5903 (30) | ⊕⊕⊕⊝ MODERATE 1 3 4 5 | |
| 134 per 1,000 | 191 per 1,000 (154 to 238) | ||||
| Graft loss Follow‐up: range 1 to 5 years | Study population | RR 0.94 (0.75 to 1.19) | 5446 (25) | ⊕⊕⊝⊝ LOW 2 4 6 | |
| 53 per 1,000 | 50 per 1,000 (40 to 64) | ||||
| Adverse events: hypertension Follow‐up: range 6 months to 5 years | Study population | RR 0.86 (0.64 to 1.15) | 2207 (7) | ⊕⊕⊝⊝ LOW 7 8 | |
| 218 per 1,000 | 187 per 1,000 (139 to 250) | ||||
| Adverse events: CMV Infection follow‐up: range 6 months to 5 years | Study population | RR 0.60 (0.44 to 0.82) | 2503 (13) | ⊕⊕⊕⊝ MODERATE 9 10 | |
| 150 per 1,000 | 90 per 1,000 (66 to 123) | ||||
| Adverse events: malignancy Follow‐up: range 6 months to 5 years | Study population | RR 0.69 (0.47 to 1.00) | 3699 (14) | ⊕⊕⊝⊝ LOW 2 4 10 | |
| 54 per 1,000 | 38 per 1,000 (26 to 54) | ||||
| Adverse events: lymphocele Follow‐up: range 6 months to 5 years | Study population | RR 1.45 (0.95 to 2.21) | 1926 (8) | ⊕⊕⊝⊝ LOW 6 8 11 | |
| 100 per 1,000 | 144 per 1,000 (95 to 220) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Randomisation method and allocation concealment performed in most studies
2 No significant heterogeneity noted in analysis
3 Only 2 studies had more than 2 comparison arms
4 Many studies with small events and wide CI
5 Significant heterogeneity in studies in biopsy‐proven acute rejection
6 Funnel plot skewed
7 Significant heterogeneity noted
8 Few studies reported this outcome
9 Moderate heterogeneity but follow‐up times are variable
10 Not all studies reported the outcome
11 Heterogeneity is not significant when 1 long‐term study was excluded
Summary of findings 4. Low dose CNI calcineurin inhibitor (CNI) + mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI for kidney transplant recipients.
| Low dose CNI + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: low dose CNI + mTORi Comparison: standard dose CNI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with standard dose CNI | Risk with low dose CNI + mTORi | ||||
| Death Follow‐up: range 6 months to 3 years | Study population | RR 1.16 (0.71 to 1.90) | 2750 (11) | ⊕⊕⊕⊝ MODERATE 1 2 3 4 | |
| 22 per 1,000 | 26 per 1,000 (16 to 42) | ||||
| Acute rejection Follow‐up: range 6 months to 3 years | Study population | RR 1.13 (0.91 to 1.40) | 3300 (16) | ⊕⊕⊕⊝ MODERATE 2 4 | |
| 132 per 1,000 | 149 per 1,000 (120 to 185) | ||||
| GFR Follow‐up: range 6 months to 2 years | The mean GFR in the intervention group was 6.24 mL/min more (3.28 more to 9.19 more) than the control group | ‐ | 1749 (11) | ⊕⊕⊕⊝ MODERATE 5 | |
| Graft loss Follow‐up: range 6 months to 3 years | Study population | RR 0.67 (0.45 to 1.01) | 3304 (16) | ⊕⊕⊕⊝ MODERATE 2 6 | |
| 38 per 1,000 | 25 per 1,000 (17 to 38) | ||||
| Adverse events: hypertension Follow‐up: range 6 months to 2 years | Study population | RR 0.98 (0.80 to 1.20) | 1421 (5) | ⊕⊕⊝⊝ LOW 7 8 | |
| 203 per 1,000 | 199 per 1,000 (162 to 243) | ||||
| Adverse events: CMV infection Follow‐up: range 1 to 3 years | Study population | RR 0.41 (0.16 to 1.06) | 1250 (5) | ⊕⊕⊝⊝ LOW 5 7 9 | |
| 105 per 1,000 | 43 per 1,000 (17 to 111) | ||||
| Adverse events: malignancy Follow‐up: range 1 to 3 years | Study population | RR 1.22 (0.42 to 3.52) | 1074 (5) | ⊕⊕⊝⊝ LOW 2 4 7 | |
| 11 per 1,000 | 14 per 1,000 (5 to 40) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Randomisation and allocation process not clear in some studies
2 No significant heterogeneity
3 Only 2 of the studies had more than 2 comparisons
4 Some small studies with wide CI
5 Substantial heterogeneity noted due to recording at different time periods
6 Small number of events and some small studies with wide CI
7 Only few studies reported this outcome
8 95% CI fails to exclude benefit or harm
9 Heterogeneity present but when abstract only studies are removed, heterogeneity is zero
Summary of findings 5. Calcineurin inhibitor (CNI) avoidance and late CNI withdrawal versus standard dose CNI.
| Subgroup analysis: CNI avoidance and late withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: CNI avoidance and late withdrawal Comparison: standard dose CNI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal | ||||
| Acute rejection: avoidance Follow‐up: range 1 to 12 years | Study population | RR 2.16 (0.85 to 5.49) | 238 (3) | ⊕⊕⊝⊝ LOW 1 2 | |
| 344 per 1,000 | 744 per 1,000 (293 to 1,000) | ||||
| Acute rejection: late withdrawal | Study population | RR 3.21 (1.59 to 6.48) | 1428 (12) | ⊕⊕⊕⊝ MODERATE 3 | |
| 102 per 1,000 | 328 per 1,000 (162 to 661) | ||||
| GFR: avoidance | The mean GFR for avoidance studies in the intervention group was 2.22 mL/min lower (14.84 less to 10.4 more) than the control group | ‐ | 242 (3) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |
| GFR: late withdrawal | The mean GFR for late withdrawal studies in the intervention group was 5.54 mL/min more (1.66 more to 9.43 more) than the control group | ‐ | 668 (5) | ⊕⊕⊝⊝ LOW 5 6 | |
| Graft loss: avoidance | Study population | RR 0.96 (0.79 to 1.16) | 566 (4) | ⊕⊕⊝⊝ LOW 7 8 | |
| 355 per 1,000 | 341 per 1,000 (281 to 412) | ||||
| Graft loss: late withdrawal | Study population | RR 0.84 (0.72 to 0.97) | 1831 (13) | ⊕⊕⊕⊝ MODERATE 3 9 10 | |
| 260 per 1,000 | 219 per 1,000 (187 to 252) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 3 small studies with one study including a non‐randomised arm
2 Significant heterogeneity
3 Several small studies with wide confidence intervals
4 Small numbers to make a judgement of difference
5 Skewed funnel plot
6 Substantial heterogeneity
7 2/4 are small studies with wide CI
8 4 studies included with one study with high event rate
9 No heterogeneity identified on analysis
10 Larger studies are not industry sponsored
Summary of findings 6. Calcineurin inhibitor (CNI) avoidance and late withdrawal with mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI.
| Subgroup analysis: CNI avoidance and late withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: CNI avoidance and late withdrawal + mTORi Comparison: standard dose CNI | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal + mTORi | ||||
| Acute rejection: avoidance Follow‐up: range 6 months to 3 years | Study population | RR 1.27 (0.98 to 1.65) | 1844 (11) | ⊕⊕⊕⊝ MODERATE 1 | |
| 234 per 1,000 | 297 per 1,000 (229 to 386) | ||||
| Acute rejection: late withdrawal Follow‐up: range 6 months to 5 years | Study population | RR 1.90 (1.44 to 2.51) | 3636 (17) | ⊕⊕⊕⊝ MODERATE 1 | |
| 65 per 1,000 | 124 per 1,000 (94 to 163) | ||||
| GFR: avoidance Follow‐up: range 6 months to 3 years | The mean GFR for avoidance studies in the intervention group was 6.45 mL/min higher (1.33 higher to 11.58 higher) than the control group | ‐ | 1748 (9) | ⊕⊕⊝⊝ LOW 1 2 | |
| GFR: late withdrawal Follow‐up: range 6 months to 5 years | The mean GFR for late withdrawal studies in the intervention group was MD 4.55 higher (0.26 higher to 8.85 higher) than for control group | ‐ | 2679 (14) | ⊕⊕⊝⊝ LOW 1 2 | |
| Graft loss: avoidance | Study population | RR 1.03 (0.72 to 1.48) | 1420 (8) | ⊕⊕⊕⊝ MODERATE 1 | |
| 74 per 1,000 | 76 per 1,000 (53 to 110) | ||||
| Graft loss: late withdrawal | Study population | RR 0.92 (0.65 to 1.30) | 4026 (17) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 46 per 1,000 | 42 per 1,000 (30 to 59) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Several smaller studies with wide CI
2 Significant heterogeneity
Background
Description of the condition
Standard immunosuppressive protocols to prevent acute graft rejection in kidney transplantation involve three major groups of drugs ‐ calcineurin inhibitor(s) (CNI), antimetabolites and steroids. CNI have been an important part of primary immunosuppression therapy together with adjunctive agents such as mycophenolate mofetil (MMF), azathioprine (AZA) and steroids in kidney transplant recipients (Hariharan 2000).
CNI inhibit the calcium‐dependent enzyme serine phosphatase calcineurin. This process prevents the dephosphorylation of nuclear factors of activated T lymphocytes (NFAT), which is essential for translocation into the nucleus leading to reduced activation of cytokine genes for interleukin‐2 (IL2) production. Cyclosporin (CsA) and tacrolimus (TAC) are CNI used for kidney transplant recipients (Melk 2003).
Description of the intervention
CNI have dramatically reduced the incidence of acute transplant rejection and decreased early graft loss (Ahsan 2001). However, CNI have been associated with significant adverse effects such as nephrotoxicity (Bennett 1996) causing decreased glomerular filtration rate (GFR), hypertension, hyperlipidaemia and a significant contribution to chronic allograft nephropathy. These effects could lead to subsequent graft loss and contribute directly or indirectly to patient morbidity and mortality by affecting the cardiovascular risk factors (Kasiske 1996). The immunological causes of graft loss have to be however considered. The potential risks of CNI use should be balanced against the risks of acute rejection and chronic antibody‐mediated rejection, especially in patients with a high immunological risk.
How the intervention might work
The significant toxicity profile of CNI have prompted many studies investigating CNI withdrawal and tapering strategies. However, some highlighted an increase in acute rejection following withdrawal (Abramowicz 2002) and others showed no effect on graft survival and a short term improvement in creatinine values (Gonwa 2002).
Why it is important to do this review
Despite the large number of studies conducted, uncertainty remains about tapering or withdrawing CNI. These strategies must be balanced with the significant benefits conferred by CNI in preventing early graft rejection. In the absence of a clear clinical consensus, this review aimed to assess the benefits and harms of CNI withdrawal or tapering for kidney transplant recipients.
Objectives
This review aimed to look at the benefits and harms of CNI tapering or withdrawal in terms of graft function and loss, incidence of acute rejection episodes, treatment‐related side effects (hypertension, hyperlipidaemia) and death.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) where standard dose CNI regimens were compared with CNI withdrawal or tapering for kidney transplant recipients were included. The first period of randomised cross‐over studies were also included.
Types of participants
Inclusion criteria
Patients with end‐stage kidney disease (ESKD), irrespective of age or gender, who received a first or subsequent cadaveric or living donor kidney transplant and received CNI (CsA or TAC) as the primary immunosuppression, were included.
Exclusion criteria
Recipients who received another solid organ in addition to a kidney transplant (e.g. pancreas) were excluded.
Types of interventions
Transplant recipients who received CNI (CsA or TAC) as the primary immunosuppression which was subsequently tapered or withdrawn completely were included.
All studies where tapering or withdrawal was compared with controls were included irrespective of the duration of treatment prior to the intervention. In cases of significant heterogeneity, subgroup analysis was performed.
All definitions of tapering mentioned in the studies were included irrespective of the duration of tapering; sensitivity analysis was used to differentiate between the tapering groups.
Studies that defined low dose either by exposure to CsA and TAC calculated using 12‐hour post‐dose nadir (trough; C0) blood levels, or studies which employed fixed doses (mg/kg) were included.
Specific comparisons were made between:
Standard dose CNI versus CNI withdrawal
Low dose CNI versus standard dose CNI
CNI withdrawal with conversion to mammalian target of rapamycin inhibitor (mTOR‐I) versus standard dose CNI
Low dose CNI with conversion to mTOR‐I versus normal dose CNI.
In case of significant heterogeneity among interventions, subgroup analysis was carried out in:
Duration of tapering or withdrawal
AZA and MMF groups.
Types of outcome measures
Graft loss (censored and not censored for death)
All‐cause mortality
Acute rejection episodes: both clinical and biopsy‐proven acute rejection (BPAR) were included
Graft kidney function at six months and at one, two and five years measured by serum creatinine (SCr), calculated GFR or creatinine clearance (CrCl)
Treatment‐related side effects (e.g. hyperlipidaemia, hypertension)
Rates of malignancy
Incidence of infections.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 11 October 2016 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that included relevant data or information on trials were retained initially. The same two authors independently assessed retrieved abstracts, and if necessary, the full text of studies which satisfied the inclusion criteria. Studies reported in non‐English language journals were translated before assessment. Discrepancies were resolved by discussion with a third author.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Where more than one publication of one study existed, reports were grouped together and the most recent or most complete data set were used. Any discrepancies between published versions were highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Results for dichotomous outcomes (e.g. incidence of acute rejections, graft loss, death) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. blood pressure, SCr, GFR), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Dealing with missing data
Further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using a Cochran Q test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). In case of significant heterogeneity, subgroup analysis was considered.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. interventions and study quality). Heterogeneity among participants could be related to age and renal pathology. Heterogeneity in treatments could be related to prior agent(s) used, the agent (CsA/TAC) and duration of therapy prior to withdrawal or tapering. Adverse effects are tabulated and assessed with descriptive techniques, as they are likely to be different for the various agents used.
Sensitivity analysis
Sensitivity analysis was used to differentiate between tapering groups.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Death
Graft loss
Acute rejection
GFR
Adverse events (e.g. hypertension, CMV infection, malignancy).
Results
Description of studies
Results of the search
Our search identified 2398 records. After title and abstract review we excluded 1605 records. The remaining 793 records were for 159 studies. We included only studies that compared standard dose CNI with tapering or withdrawal with or without mTOR‐I substitution which resulted in 83 studies (583 reports) being included in the analyses. We excluded 72 studies (202 records). Four studies (8 records) are ongoing (David‐Neto 2014; ERIC Study 2010; ISRCTN63298320; TRANSFORM Study 2013) and will be assessed in a future update of this review. See Figure 1.
1.
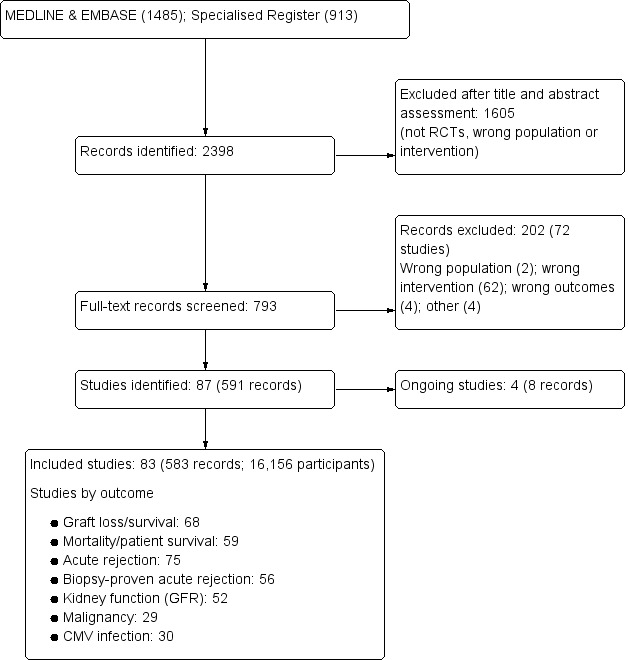
Flow chart showing number of studies identified
Included studies
See Characteristics of included studies.
The 83 studies included 16,156 randomised participants. Of these, 13 studies were available only in abstract form (2345 participants) (Alsina 1987; Bertoni 2007; Cockfield 2002; El‐Agroudy 2014; Heering 1993; HERAKLES Study 2012; Holm 2008; Kreis 2003; MODIFY Study 2012; Pacheco‐Silva 2013; Qazi 2014; Rossini 2007; Salvadori 2007).
CNI withdrawal or avoidance versus standard dose CNI regimens
We found 17 studies (81 reports, 1939 participants) that compared CNI withdrawal or avoidance with standard dose CNI regimens; four studies compared avoidance with standard dose CNI regimens (Asberg 2006; Garcia 2007; Grimbert 2002; Kosch 2003a), and one study with three arms and compared avoidance and withdrawal with standard dose CNI (Hall 1988). The remainder compared CNI withdrawal with standard dose CNI regimen.
Garcia 2007 and CTOT‐09 Study 2015 investigated TAC; two studies involved patients on either CsA or TAC (Pascual 2008; Suwelack 2002), and the remainder were CsA‐based studies (Abramowicz 2002; Asberg 2006; Dudley 2005; Grimbert 2002; Hall 1988; Hazzan 2005; Heering 1993; Hollander 1995; Isoniemi 1990; Kosch 2003a; MacPhee 1998; Pedersen 1991; Smak Gregoor 1999).
Standard versus low dose CNI
We included 18 studies (89 reports, 2904 participants) that compared standard dose CNI with low dose CNI. Of these, 15 were CsA‐based studies (Alsina 1987; Andres 2009; Baczkowska 2003; Budde 2007; Cai 2014; Chadban 2013; Cibrik 2007; de Sevaux 2001; DICAM Study 2010, Fangmann 2010; Ferguson 2006; Kreis 2003; Pascual 2003; REFERENCE Study 2006; Salvadori 2007); two investigated TAC (Chan 2012; MODIFY Study 2012); and OPTICEPT Study 2009 included either TAC or CsA. Of these, 12 studies involved introduction of low dose CNI regimen early in the post‐transplant period and six introduced low dose CNI later in the post‐transplant period (Cibrik 2007; DICAM Study 2010; Kreis 2003; MODIFY Study 2012; Pascual 2003; REFERENCE Study 2006).
Standard dose CNI versus CNI withdrawal or avoidance with mTOR‐I substitution
There were 29 studies (252 reports, 5012 participants) that compared standard dose CNI with CNI withdrawal or avoidance combined with mTOR‐I substitution (APOLLO Study 2015; Bansal 2013; Barsoum 2007; CALFREE Study 2010; CENTRAL Study 2012; CERTITEM Study 2015; Chhabra 2013; CONCEPT Study 2009; CONVERT Trial 2009; El‐Agroudy 2014; Flechner‐318 Study 2002; Grinyo 2004; Holm 2008; Martinez‐Mier 2006; Nafar 2012; ORION Study 2011; Pacheco‐Silva 2013; Pontrelli 2008; Rivelli 2015; RMR Study 2001; Rossini 2007; Schaefer 2006; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stallone 2004; Stegall 2003; Watson 2005; ZEUS Study 2011). Of these, nine compared CNI avoidance with mTOR‐I substitution versus conventional CNI regimen (CENTRAL Study 2012; Nafar 2012; Stegall 2003; Schaefer 2006; Barsoum 2007; CALFREE Study 2010; Flechner‐318 Study 2002; Martinez‐Mier 2006,SMART TX Study 2010). The rest looked at delayed CNI withdrawal with mTOR‐I substitution.
We included only five studies that investigated everolimus (APOLLO Study 2015; CENTRAL Study 2012; CERTITEM Study 2015; Pacheco‐Silva 2013; ZEUS Study 2011); the remainder investigated sirolimus. The CNI studied were:
TAC (eight studies: Chhabra 2013; El‐Agroudy 2014; Grinyo 2004; ORION Study 2011; Pacheco‐Silva 2013; Rivelli 2015; Schaefer 2006; Stegall 2003)
CsA (13 studies: Barsoum 2007; CALFREE Study 2010; CERTITEM Study 2015; CONCEPT Study 2009; Flechner‐318 Study 2002; Holm 2008; Martinez‐Mier 2006; CENTRAL Study 2012; Nafar 2012; RMR Study 2001; SMART TX Study 2010; Stallone 2003; ZEUS Study 2011)
TAC or CsA (seven studies: APOLLO Study 2015; Bansal 2013; CONVERT Trial 2009; Holm 2008; Spare‐the‐Nephron Study 2011; Rossini 2007; Stallone 2004; Watson 2005).
Standard dose CNI versus low dose CNI and mTOR‐I
We identified 14 studies (80 reports, 3110 participants) that compared standard dose CNI with combination of low dose CNI and mTOR‐I; (Bechstein‐193 2013; Bertoni 2007; Bertoni 2011; Chan 2008; Cockfield 2002; Muhlbacher 2014; Nashan 2004; Oh 2012; Paoletti 2012; Qazi 2014; Russ 2003; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001). Interventions were administered immediately post‐transplant in all studies.
There were nine studies that investigated everolimus as the mTOR‐I (Bertoni 2007; Bertoni 2011; Chan 2008; Nashan 2004; Oh 2012; Paoletti 2012; Qazi 2014; Takahashi 2013a; Tedesco‐Silva 2010); the remainder looked at sirolimus. TAC (CNI) was studied in five studies (Bechstein‐193 2013; Chan 2008; Cockfield 2002; Qazi 2014; Russ 2003) and the rest of the studies used CsA.
Low versus normal dose CNI with or without mTOR‐I (mixed studies)
Five studies (83 reports, 3191 participants) had more than two arms and compared low dose versus normal dose CNI with or without mTOR‐I (ASCERTAIN Study 2011; CAESAR Study 2007; HERAKLES Study 2012; MECANO Study 2009; SYMPHONY Study 2007). Each were split to form two studies comparing low dose or withdrawal with or without mTOR‐I.
Reporting of outcomes was variable, and definitions of outcomes were unclear in most studies. Acute rejection episodes were reported as biopsy proven (56 studies) or unspecified/mixed (19 studies). Most reported graft loss or failure (68 studies) and GFR (52 studies). Methods used to determine GFR varied: 15 studies applied the Nankivell formula; 17 used Cockcroft‐Gault; 12 used MDRD; six used nuclear GFR (iothalamate or Cr EDTA); and four did not state the method used. CMV infection rates were reported in 30 studies and malignancy rates were reported in 29 studies.
Excluded studies
We excluded 72 studies following full text assessment: two studies included populations that did not match our inclusion criteria; 62 investigated interventions that were not relevant to this review; four measured outcomes not relevant to this review; two were incomplete studies that stopped early; one was only published as an abstract 35 years ago; and one study converted patients from TAC to sirolimus, however 40% we converted back to TAC. See Characteristics of excluded studies.
This review excluded studies involving Belatacept as the intervention assessed efficacy of the new biologic agent rather than CNI withdrawal. The Belatacept studies has been analysed and published recently (Mason 2014).
Risk of bias in included studies
Study methodology reporting was incomplete in most studies. Randomisation methods and allocation concealment were clearly described in fewer than 50% of studies. Most were open‐label studies. Intention‐to‐treat (ITT) analysis was either not reported or did not contain adequate information in 20% of studies to assess reporting bias. Seven studies did not report all possible outcomes due to early termination. Details are summarised below and in Figure 2.
2.
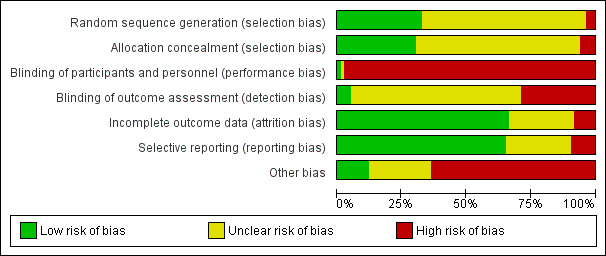
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
Randomisation methods were reported in detail in 27 studies (APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; CAESAR Study 2007; Cai 2014; CENTRAL Study 2012; Chan 2012; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; DICAM Study 2010; Dudley 2005; Fangmann 2010; Flechner‐318 Study 2002; Grinyo 2004; Hall 1988; MacPhee 1998; MECANO Study 2009; Paoletti 2012; REFERENCE Study 2006; Rivelli 2015; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; SYMPHONY Study 2007; Takahashi 2013a; Watson 2005; ZEUS Study 2011). Three studies were judged to be at high risk of bias; Pedersen 1991 randomised alternate participants to intervention and control groups, and Garcia 2007 and Schaefer 2006 included a third non‐randomised arm to the studies. The remaining 53 studies did not report randomisation methods.
Allocation concealment
Methods of allocation concealment were adequate in 25 studies (Abramowicz 2002; APOLLO Study 2015; Bansal 2013; CAESAR Study 2007; CENTRAL Study 2012; Chan 2008; Cibrik 2007; CONVERT Trial 2009; de Sevaux 2001; DICAM Study 2010; Dudley 2005; Fangmann 2010; Hall 1988; Isoniemi 1990; MacPhee 1998; MECANO Study 2009; Paoletti 2012; REFERENCE Study 2006; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; SYMPHONY Study 2007; Tedesco‐Silva 2010; Watson 2005; ZEUS Study 2011). Five studies were judged to be at high risk of bias (Barsoum 2007; Garcia 2007; Grinyo 2004; OPTICEPT Study 2009; Schaefer 2006) and the method of allocation concealment was not reported or unclear in 53 studies.
Blinding
Almost all studies were open‐label. Ferguson 2006 reported blinding of investigators and participants in one part of the study, and four studies (Cibrik 2007; DICAM Study 2010; Oh 2012; Rivelli 2015) reported blinding of outcome investigators.
Incomplete outcome data
Outcome data was reported or analysed as Intention‐ to‐treat in (ITT) in 55 studies (Abramowicz 2002; Andres 2009; APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; Barsoum 2007; Bertoni 2011; Budde 2007; CAESAR Study 2007; Cai 2014; CALFREE Study 2010; CENTRAL Study 2012; Chadban 2013; Chan 2008; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CTOT‐09 Study 2015; de Sevaux 2001; DICAM Study 2010; El‐Agroudy 2014; Fangmann 2010; Ferguson 2006; Flechner‐318 Study 2002; Garcia 2007; Grimbert 2002; Hall 1988; Hazzan 2005; HERAKLES Study 2012; Hollander 1995; Isoniemi 1990; Kosch 2003a; MacPhee 1998; Martinez‐Mier 2006; MODIFY Study 2012; Oh 2012; Paoletti 2012; Pascual 2003; Pontrelli 2008; Qazi 2014; REFERENCE Study 2006; Rivelli 2015; RMR Study 2001; Salvadori 2007; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stegall 2003; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001; Watson 2005; ZEUS Study 2011).
There was missing outcome data in seven studies (CENTRAL Study 2012; Cockfield 2002; Holm 2008; Heering 1993; Muhlbacher 2014; OPTICEPT Study 2009).
Attrition bias was judged to be unclear for the remaining 21 studies.
Selective reporting
There were 54 studies that reported prespecified outcomes (Abramowicz 2002; Andres 2009; APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; Barsoum 2007; Bertoni 2007; Bertoni 2011; Budde 2007; CAESAR Study 2007; Cai 2014; CENTRAL Study 2012; Chadban 2013; Chan 2008; Chan 2012; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; de Sevaux 2001; DICAM Study 2010; Dudley 2005; Fangmann 2010; Ferguson 2006; Flechner‐318 Study 2002; Garcia 2007; Grinyo 2004; Hall 1988; HERAKLES Study 2012; Isoniemi 1990; Kosch 2003a; MacPhee 1998; MODIFY Study 2012; Nashan 2004; Oh 2012; Pacheco‐Silva 2013; Pascual 2003; Pascual 2008; Qazi 2014; Pontrelli 2008; RMR Study 2001; Russ 2003; Salvadori 2007; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stallone 2004; Pontrelli 2008; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Watson 2005; ZEUS Study 2011).
Eight studies were judged to be at high risk of reporting bias. Three studies did not report all possible outcomes due to early termination (CTOT‐09 Study 2015; MECANO Study 2009; ORION Study 2011). Cockfield 2002 and CERTITEM Study 2015 did not report all prespecified outcomes. Full‐text publications had not been identified for three studies 10 years after the abstracts were first published (Holm 2008; Rossini 2007; Salvadori 2007).
Twenty four studies had insufficient information to ascertain reporting bias.
Other potential sources of bias
Of the 83 included studies, 49 received pharmaceutical industry funding, which is a potential source for bias (Abramowicz 2002; Andres 2009; APOLLO Study 2015; Asberg 2006; ASCERTAIN Study 2011; Bansal 2013; Bechstein‐193 2013; Budde 2007; CAESAR Study 2007; Cai 2014; CALFREE Study 2010; CENTRAL Study 2012; CERTITEM Study 2015; Chadban 2013; Chan 2008; Chan 2012; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; de Sevaux 2001; Dudley 2005; Ferguson 2006; Flechner‐318 Study 2002; Grinyo 2004; Hall 1988; MECANO Study 2009; Muhlbacher 2014; Nashan 2004; Oh 2012; OPTICEPT Study 2009; ORION Study 2011; Pascual 2003; Pascual 2008; Qazi 2014; REFERENCE Study 2006; RMR Study 2001; Russ 2003; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stegall 2003; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001; Watson 2005; ZEUS Study 2011).
In two studies, one study arm was terminated due to increased rates of acute rejection (MECANO Study 2009; ORION Study 2011) and in Heering 1993 and CTOT‐09 Study 2015 the studies were stopped due to increased acute rejections in the CNI withdrawal group.
Garcia 2007 included a third group of non‐randomised patients after the interim analysis of randomised patients.
Only preliminary data were reported in Cockfield 2002 and Muhlbacher 2014.
There was a high drop‐out rate in four studies (Grinyo 2004; OPTICEPT Study 2009, Stegall 2003, Tedesco‐Silva 2010) which resulted in protocol amendment in Grinyo 2004.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
CNI withdrawal (avoidance or late withdrawal) versus standard dose CNI
There was little or no difference in patient death between CNI withdrawal and standard dose CNI regimens (Analysis 1.1 (14 studies 2010 participants): RR 1.09, 95% CI 0.96 to 1.24; I2 = 0%; moderate certainty evidence).
1.1. Analysis.
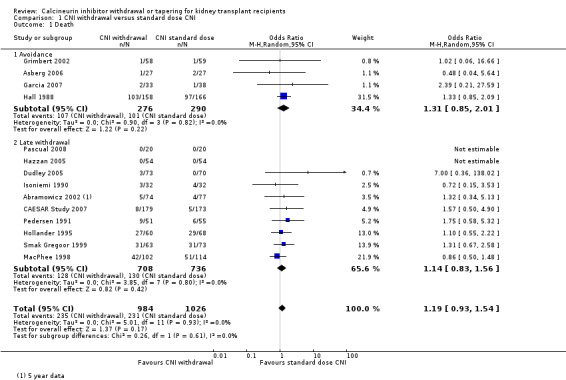
Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 1 Death.
Acute rejection episodes were higher with CNI withdrawal whether diagnosed by biopsy or clinically (Analysis 1.2 (15 studies, 1666 participants): RR 2.54, 95% CI 1.56 to 4.12; I2 = 70%; moderate certainty). However GFR increased (Analysis 1.3 (8 studies, 910 participants): MD 3.56 mL/min, 95% CI ‐1.25 to 8.25; I2 = 66%; low certainty) and graft loss decreased (Analysis 1.4 (16 studies, 2090 participants): RR 0.85, 95% CI 0.74 to 0.98; I2 = 0%; low certainty) with CNI withdrawal.
1.2. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 2 Acute rejection.
1.3. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 3 GFR.
1.4. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 4 Graft loss.
There was 18% reduction in hypertension noted with CNI withdrawal (Analysis 1.6.1 (5 studies, 950 participants): RR 0.82, 95% CI 0.71 to 0.95; I2 = 36%; low certainty). There was no differences in incidences of hyperlipidaemia (Analysis 1.6.2 (3 studies, 562 participants): RR 0.88, 95% CI 0.63 to 1.21; I2 = 2%), CMV infection (Analysis 1.6.3 (7 studies, 608 participants): RR 0.87, 95% CI 0.52 to 1.45; I2 = 0%; low certainty), diabetes mellitus (Analysis 1.6.4 (6 studies, 810 participants): RR 0.85, 95% CI 0.94 to 1.42; I2 = 0%), malignancy (Analysis 1.6.5 (6 studies, 1079 participants): RR 1.10, 95% CI 0.936 to 1.30; I2 = 0%; low certainty), or total infections (Analysis 1.6.6 (6 studies, 724 participants): RR 0.96, 95% CI 0.61 to 1.51; I2 = 46%) between the groups.
1.6. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 6 Adverse events.
Subgroup analyses
CNI avoidance versus standard dose CNI
There was more acute rejection episodes in CNI avoidance compared with standard dose CNI (Analysis 1.7.1 (3 studies, 238 participants): RR 2.16, 95% CI 0.85 to 5.49; I2 = 84%, low certainity). However, there was no difference in death (Analysis 1.1.1 (4 studies, 566 participants): RR 1.11, 95% CI 0.94 to 1.32; I2 = 0%), GFR (Analysis 1.8.1 (3 studies, 242 participant): MD ‐2.22 mL/min, 95% CI ‐14.84 to 10.40; I2 = 84%, very low certainity), and graft loss (Analysis 1.9.1 (4 studies, 566 participants): RR 0.96, 95% CI 0.79 to 1.16; I2 = 0%, low certainity).
1.7. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 7 Subgroup analysis: acute rejection.
1.8. Analysis.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 8 Subgroup analysis: GFR.
1.9. Analysis.
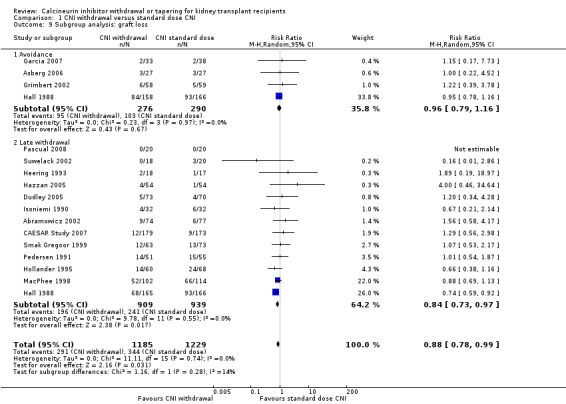
Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 9 Subgroup analysis: graft loss.
Late withdrawal versus standard dose CNI
Analysis of late withdrawal studies indicated that there was no difference in death (Analysis 1.1.2 (10 studies, 1444 participants): RR 1.06, 95% CI 0.88 to 1.29; I2 = 0%), however acute rejection episodes were higher in CNI withdrawal group (Analysis 1.7.2 (12 studies, 1428 participants): RR 3.21, 95% CI 1.59 to 6.48; I2 = 66%, moderate certainity). GFR was higher (Analysis 1.8.2 (5 studies, 668 participants): MD 5.54 mL/min, 95% CI 1.66 to 9.43; I2 = 29%, low certainity) and there was less graft loss (Analysis 1.9.2 (13 studies, 1848 participants): RR 0.84, 95% CI 0.72 to 0.97; I2 = 0%, low certainity) in the CNI withdrawal group.
Type of antimetabolite (MMF/MPA or AZA)
Subgroup analysis on antimetabolites found a higher acute rejection episodes associated with CNI withdrawal compared with standard dose CNI in the MMF/MPA studies (Analysis 2.1.1 (10 studies, 1110 participants): RR 3.51, 95% CI 1.79 to 6.88; I2 = 65%) but not in AZA studies (Analysis 2.1.2 (5 studies, 556 participants): RR 1.81, 95% CI 0.78 to 4.19; I2 = 72%).
2.1. Analysis.
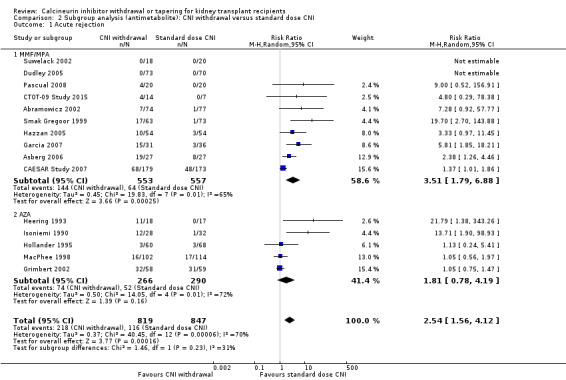
Comparison 2 Subgroup analysis (antimetabolite): CNI withdrawal versus standard dose CNI, Outcome 1 Acute rejection.
Type of CNI (CsA or TAC)
When classified by CNI type, acute rejection episodes increased in the withdrawal arm of CsA studies (Analysis 3.1.1 (11 studies, 1500 participants): RR 2.13, 95% CI 1.31 to 3.48; I2 = 71%), TAC (Analysis 3.1.2 (2 studies, 88 participants): RR 5.65, 95% CI 1.96 to 16.27; I2 = 0%), and in studies that investigated either CsA or TAC (Analysis 3.1.3 (2 studies, 78 participants): RR 9.00, 95% CI 0.52 to 156.9) compared with standard dose CNI.
3.1. Analysis.

Comparison 3 Subgroup analysis (CNI type): CNI withdrawal versus standard dose CNI, Outcome 1 Acute rejection.
Sensitivity analyses
On sensitivity analyses stratified for steroid‐free regimens the effects were not different from steroid regimens for death, acute rejection and GFR. When stratified for time of follow‐up, the reduction in graft loss observed in the CNI withdrawal group was not significant when the long‐term studies were excluded in the analysis (RR 1.07, 95% CI 0.72 to 1.57; forest plot not shown).
Low dose CNI versus standard dose CNI
There was little or no difference in patient death between low dose and standard dose CNI regimens (Analysis 4.1 (15 studies, 3462 participants): RR 0.79, 95% CI 0.50 to 1.27; I2 = 0%; moderate certainty).
4.1. Analysis.
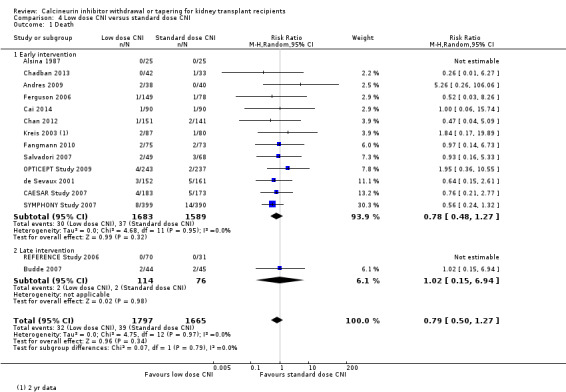
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 1 Death.
There was a lower incidence of acute rejection (Analysis 4.2 (19 studies, 3757 participants): RR 0.87, 95% CI 0.76 to 1.00; I2 = 0%; moderate certainty) and graft loss (Analysis 4.4 (15 studies, 3286 participants): RR 0.75, 95% CI 0.55 to 1.02; I2 = 0%; moderate certainty) in the low dose CNI group.
4.2. Analysis.
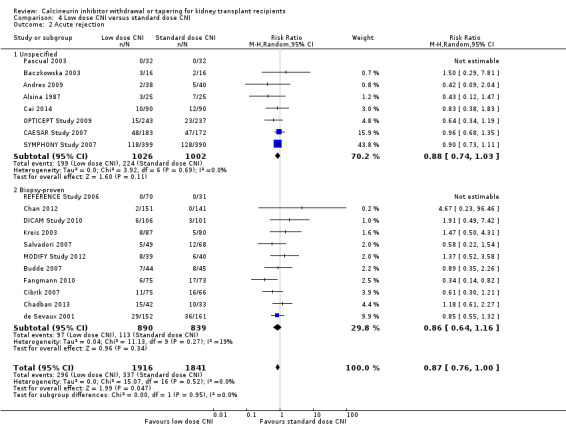
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 2 Acute rejection.
4.4. Analysis.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 4 Graft loss.
Patients treated with low dose CNI had higher GFR (Analysis 4.3 (13 studies, 2623 participants): MD 4.10, 95% CI 2.07 to 6.12; I2 = 16%; moderate certainty). Low dose CNI regimen probably slightly lowers SCr (Analysis 4.5 (6 studies, 742 participants): MD ‐4.28 µmol/L, 95% CI ‐14.65 to 6.10; I2 = 37%; low certainty).
4.3. Analysis.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 3 GFR.
4.5. Analysis.
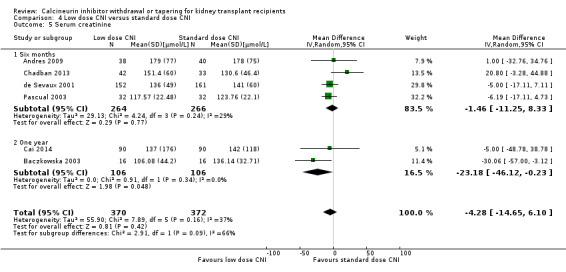
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 5 Serum creatinine.
Hypertension was probably reduced (Analysis 4.7.1 (5 studies, 1877 participants): RR 0.84, 95% CI 0.70 to 1.00; I2 = 0%; low certainty) in the low dose CNI group. There was no difference in hyperlipidaemia (Analysis 4.7.2 (3 studies, 1443 participants): RR 1.04, 95% CI 0.90 to 1.19; I2 = 12%), CMV infection (Analysis 4.7.3 (6 studies, 1948 participants): RR 1.23, 95% CI 0.94 to 1.62; I2 = 10%; moderate certainty), diabetes mellitus (Analysis 4.7.4 (5 studies, 1292 participants): RR 0.82, 95% CI 0.50 to 1.34; I2 = 53%), malignancy (Analysis 4.7.5 (5 studies, 1637 participants): RR 0.90, 95% CI 0.41 to 1.97; I2 = 0%; low certainty), and total infections (Analysis 4.7.6 (9 studies, 1437 participants): RR 0.95, 95% CI 0.84 to 1.07; I2 = 0%).
4.7. Analysis.
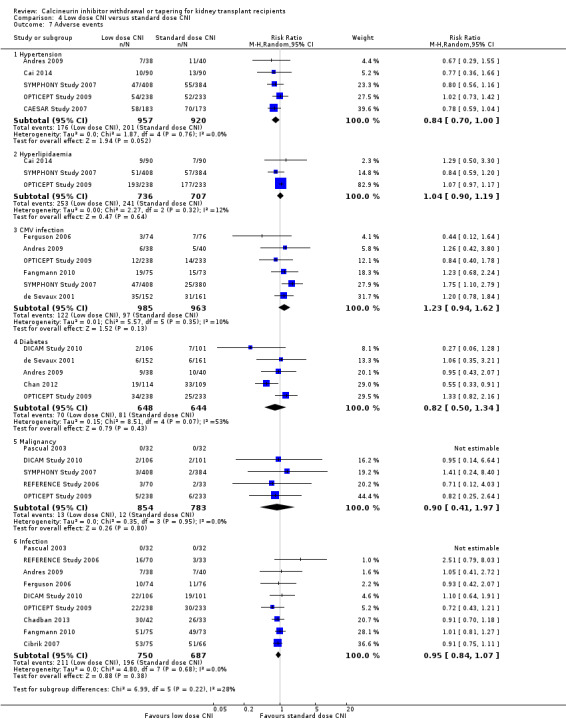
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 7 Adverse events.
Subgroup analyses
Low dose CNI immediately post‐transplant versus standard dose CNI
For studies which compared low dose CNI immediately post‐transplant with standard dose CNI regimens, there were less acute rejection episodes (Analysis 4.8.1 (12 studies, 2209 participants): RR 0.82, 95% CI 0.67 to 1.00; I2 = 0%) and graft loss (Analysis 4.10.1 (11 studies, 2800 participants): RR 0.75, 95% CI 0.55 to 1.03; I2 = 0%), and GFR improved (Analysis 4.9.1 (9 studies, 2200 participants): MD 3.09 mL/min, 95% CI 0.95 to 5.23; I2 = 4%) with the low dose regimen.
4.8. Analysis.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 8 Subgroup analysis: acute rejection.
4.10. Analysis.
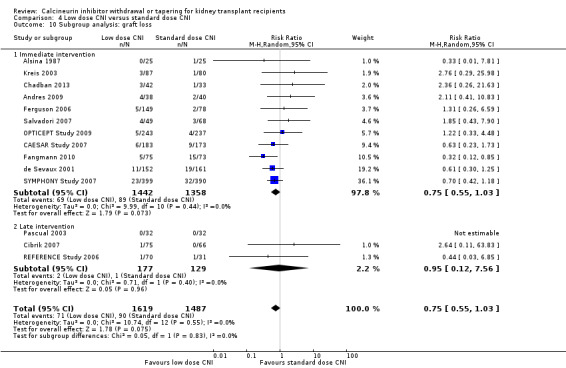
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 10 Subgroup analysis: graft loss.
4.9. Analysis.
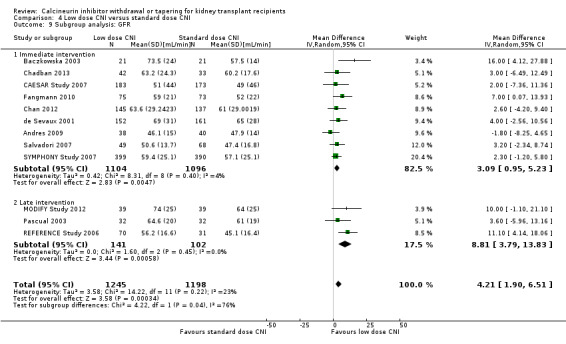
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 9 Subgroup analysis: GFR.
Late intervention with low dose CNI versus standard dose CNI
For studies which compared late intervention with low dose CNI, there was no difference acute rejection (Analysis 4.8.2 (6 studies, 759 participants): RR 1.05, 95% CI 0.61 to 1.81; I2 = 21%) or graft loss (Analysis 4.10.2 (3 studies, 306 participants): RR 0.95, 95% CI 0.12 to 7.56; I2 = 0%) however GFR was higher (Analysis 4.9.2 (3 studies, 243 participants): MD 8.81 mL/min, 95% CI 3.79 to 13.83; I2 = 0%).
Type of CNI (CsA or TAC)
When studies were classified on the type of CNI, there was less acute rejection in the low dose CsA (Analysis 5.1.1 (16 studies, 2906 participants): RR 0.87, 95% CI 0.76 to 1.01; I2 = 0%) compared to standard dose CsA but the results were not significant for low dose TAC (Analysis 5.1.2 (2 studies, 371 participants): RR 1.53, 95% CI 0.61 to 3.83; I2 = 0%) and for studies which used either CsA or TAC (Analysis 5.1.3 (1 study, 480 participants): RR 0.64, 95% CI 0.34 to 1.19).
5.1. Analysis.
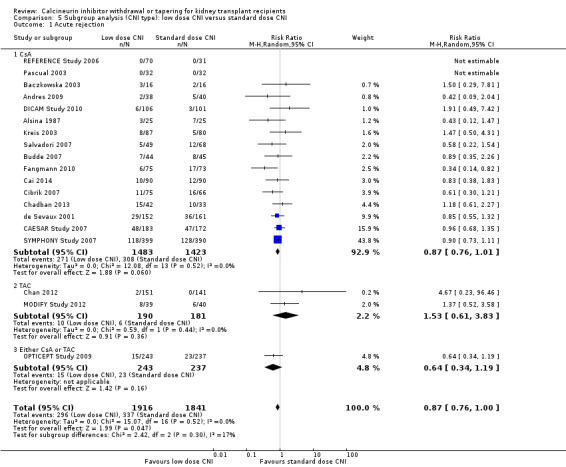
Comparison 5 Subgroup analysis (CNI type): low dose CNI versus standard dose CNI, Outcome 1 Acute rejection.
Sensitivity analysis
When stratified for steroid‐free regimens, the reduction in graft loss was significant when the study using a steroid‐free regimen was excluded from the analysis (RR 0.72, 95% CI 0.52 to 0.98; forest plot not shown).
When stratified for induction treatment with IL2RA or anti‐lymphocyte serum or globulin, the incidence of acute rejection was similar between the groups (12 studies: RR 0.84, 95% CI 0.66 to 1.07; forest plot not shown).
CNI withdrawal (avoidance or withdrawal) with mTOR‐I substitution versus standard dose CNI
There was little or no difference in death (Analysis 6.1 (23 studies, 5427 participants): RR 0.96, 95% CI 0.68 to 1.36; I2 = 0%; moderate certainty) and graft loss (Analysis 6.4 (25 studies, 5446 participants): RR 0.94, 95% CI 0.75 to 1.19; I2 = 0%; low certainty) between the CNI withdrawal with mTOR‐I and standard dose CNI regimens.
6.1. Analysis.
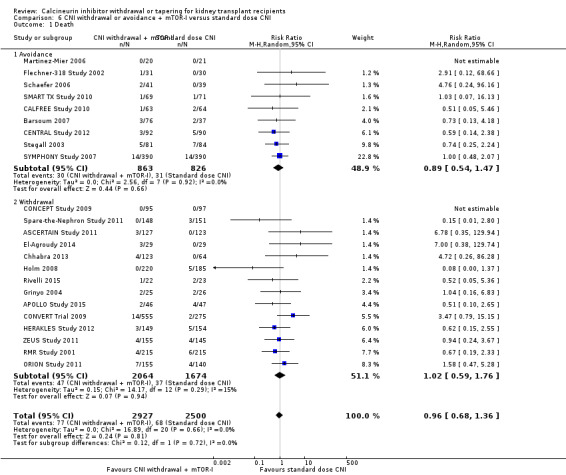
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 1 Death.
6.4. Analysis.
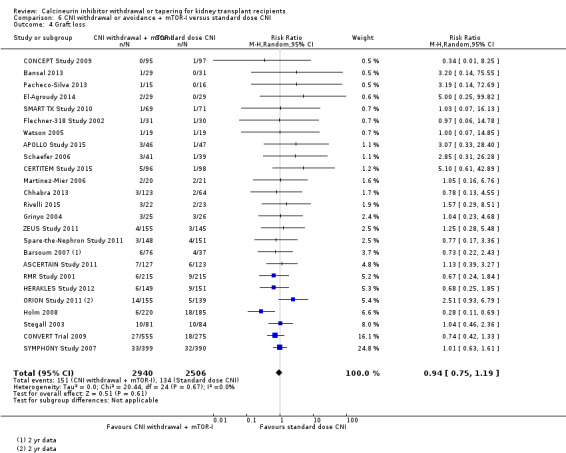
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 4 Graft loss.
There was an increase in acute rejection episodes (Analysis 6.2 (30 studies, 5903 participants): RR 1.43, 95% CI 1.15 to 1.78; I2 = 52%; moderate certainty) in the mTOR‐I group. Patients in the CNI withdrawal with mTOR‐I group had a higher GFR compared to standard dose CNI regimen (Analysis 6.3 (23 studies, 4427 participants): MD 5.29, 95% CI 2.08 to 8.51; I2 = 90%). SCr was lower at one year in the CNI withdrawal with mTOR‐I group (Analysis 6.5 (12 studies, 1702 participants): MD ‐17.10 µmol/L, 95% CI ‐26.95 to ‐7.25; I2 = 76%).
6.2. Analysis.
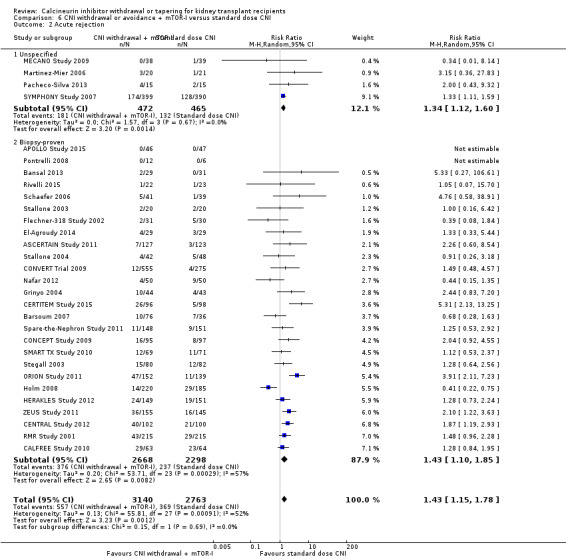
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 2 Acute rejection.
6.3. Analysis.
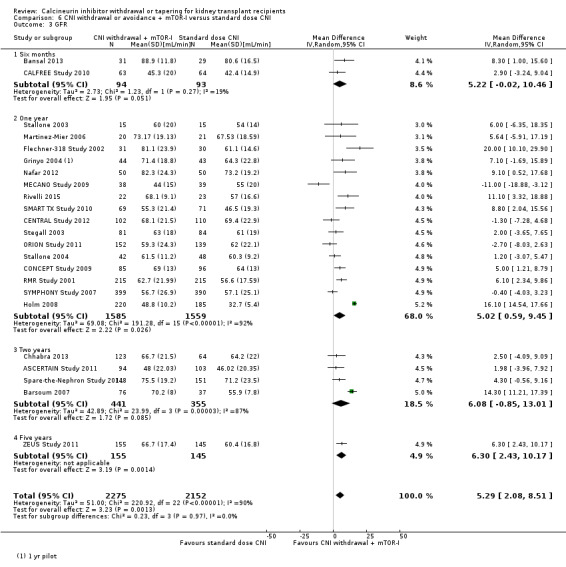
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 3 GFR.
6.5. Analysis.
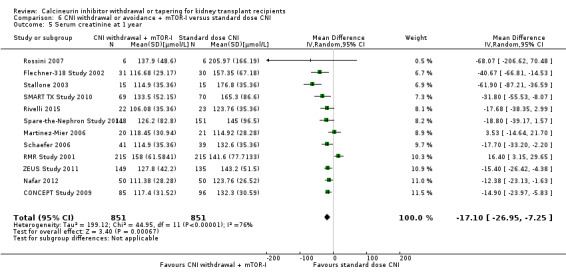
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 5 Serum creatinine at 1 year.
CNI withdrawal with mTOR‐I group had a higher incidence of hyperlipidaemia (Analysis 6.7.2 (13 studies 3494 participants): RR 1.76, 95% CI 1.40 to 2.20; I2 = 49%). There was little or no difference in hypertension Analysis 6.7.1 (7 studies, 2207 participants): RR 0.86, 95% CI 0.64 to 1.15; I2 = 79%), diabetes mellitus (Analysis 6.7.4 (11 studies, 2833 participants): RR 1.27, 95% CI 0.97 to 1.66; I2 = 0%), and infections (Analysis 6.7.6 (9 studies, 1624 participants): RR 0.99, 95% CI 0.92 to 1.07; I2 = 0%) between the two groups. There was a reduction in malignancy (Analysis 6.7.5 (14 studies, 3699 participants): RR 0.69, 95% CI 0.47 to 1.00; I2 = 19%; low certainty) and CMV infection (Analysis 6.7.3 (13 studies, 2503 participants): RR 0.60, 95% CI 0.44 to 0.82; I2 = 43%; moderate certainty) in the mTOR‐I group compared to those treated with standard dose CNI regimen. There was an increase in lymphoceles in the CNI withdrawal, mTOR‐I group (Analysis 6.7.7 (8 studies, 1926 participants): RR 1.45, 95% CI 0.95, 2.21; I2 = 56%; low certainty).
6.7. Analysis.
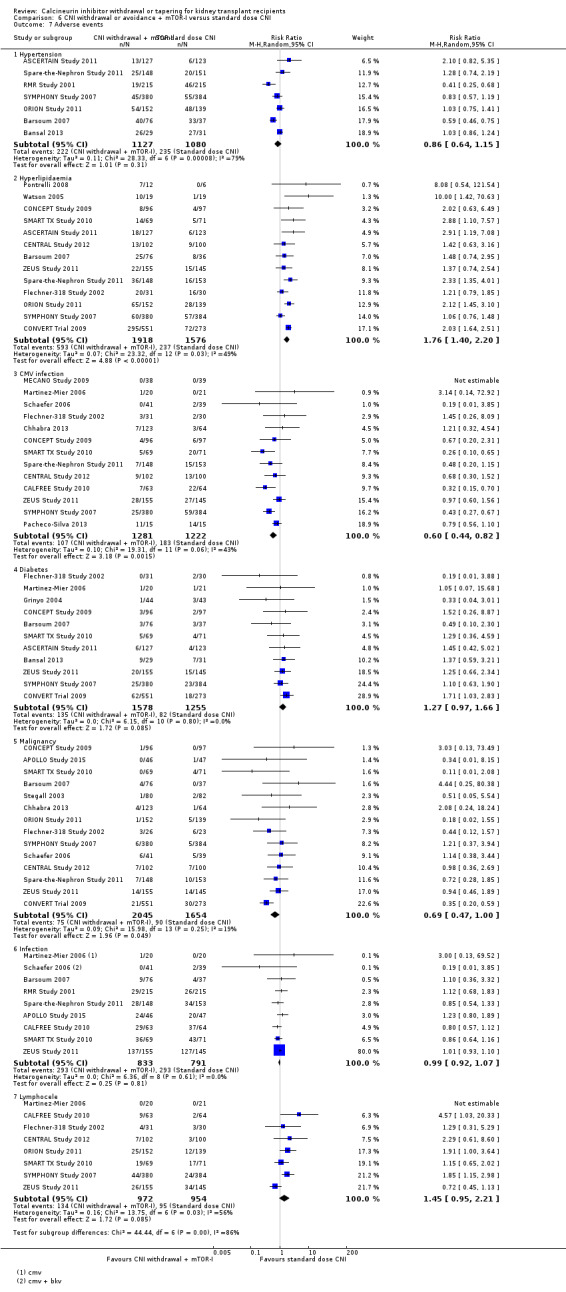
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 7 Adverse events.
Subgroup analysis
CNI avoidance with mTOR‐I substitution versus standard dose CNI
There was an increase acute rejection episodes (Analysis 6.8.1 (11 studies, 1844 participants): RR 1.27, 95% CI 0.98 to 1.65; I2 = 31%), while GFR was better (Analysis 6.9.1 (9 studies, 1748 participants): MD 6.45 mL/min, 95% CI 1.33 to 11.58; I2 = 86%) in the CNI avoidance with mTOR‐I regimen. Graft loss (Analysis 6.10.1 (8 studies, 1420 participants): RR 1.03, 95% CI 0.72 to 1.48; I2 = 0%) was similar in the two groups.
6.8. Analysis.
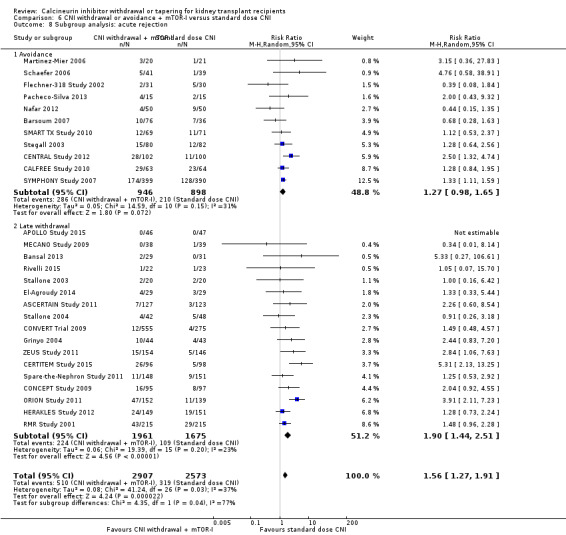
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 8 Subgroup analysis: acute rejection.
6.9. Analysis.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 9 Subgroup analysis: GFR.
6.10. Analysis.
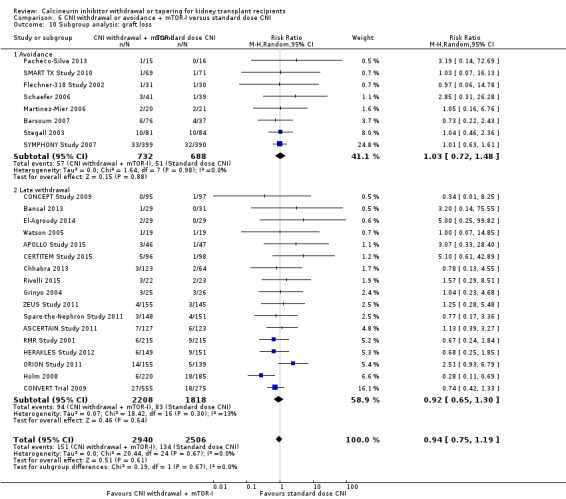
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 10 Subgroup analysis: graft loss.
Late CNI withdrawal with mTOR‐I substitution versus standard dose CNI
Acute rejection episodes were higher in the late CNI withdrawal with mTOR‐I substitution group (Analysis 6.8.2 (17 studies, 3636 participants): RR 1.90, 95% CI 1.44 to 2.51; I2 = 23%). GFR was not significantly higher (Analysis 6.9.2 (14 studies, 2679 participants): MD 4.55 mL/min, 95% CI 0.26 to 8.85; I2 = 92%) and there was no difference in graft loss (Analysis 6.10.2 (17 studies, 4026 participants): RR 0.92, 95% CI 0.65 to 1.30; I2 = 13%) in the late CNI withdrawal with mTOR‐I group.
Type of CNI (CsA or TAC)
There were more acute rejection episodes in the late CNI withdrawal with mTOR‐I group compared to standard dose CsA (Analysis 7.1.1 (18 studies, 5903 participants): RR 1.42, 95% CI 1.15 to 1.76; I2 = 37%) and standard dose TAC (Analysis 7.1.2 (7 studies, 753 participants): RR 2.23, 95% CI 1.43 to 3.49; I2 = 15%), however in studies which used either CsA or TAC (Analysis 7.1.3 (5 studies, 1687 participants): RR 0.97, 95% CI 0.40 to 2.33; I2 = 64%) there were no differences in acute rejection episodes.
7.1. Analysis.
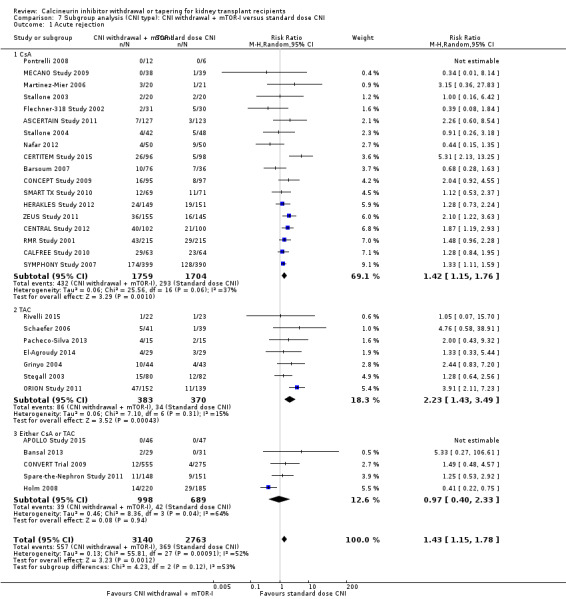
Comparison 7 Subgroup analysis (CNI type): CNI withdrawal + mTOR‐I versus standard dose CNI, Outcome 1 Acute rejection.
Sensitivity analyses
On sensitivity analyses stratified for steroid‐free regimens the effects were not different from steroid regimens for death, acute rejection, and GFR.
Low dose CNI with mTOR‐I versus standard dose CNI
There was little or no difference in patient deaths (Analysis 8.1 (11 studies, 2750 participants): RR 1.16, 95% CI 0.71 to 1.90; I2 = 0%; moderate certainty), acute rejection episodes (Analysis 8.2 (16 studies, 3300 participants): RR 1.13, 95% CI 0.91 to 1.40; I2 = 22%; moderate certainty), and graft loss (Analysis 8.4 (16 studies, 3304 participants): RR 0.67, 95% CI 0.45 to 1.01; I2 = 0%; moderate certainty) when low dose CNI with mTOR‐I was compared to standard dose CNI.
8.1. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 1 Death.
8.2. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 2 Acute rejection.
8.4. Analysis.
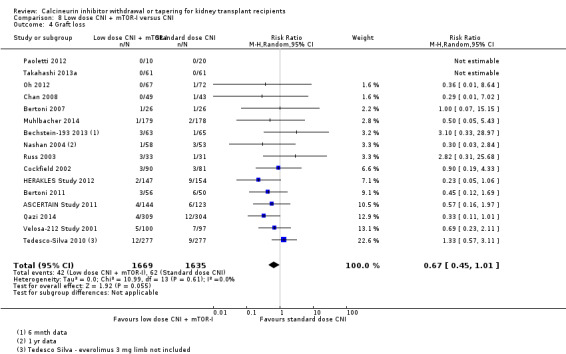
Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 4 Graft loss.
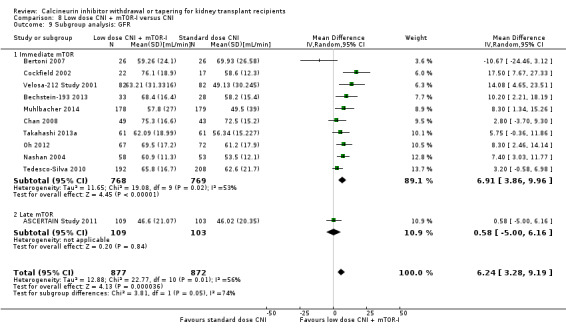
Patients treated with low dose CNI in combination with mTOR‐I had a higher GFR compared with standard dose CNI regimens (Analysis 8.3 (11 studies, 1749 participants): MD 6.24 mL/min, 95% CI 3.28 to 9.19; I2 = 56%; moderate certainty), and a lower SCr at one year (Analysis 8.5 (6 studies, 1320 participants): MD ‐14.14 µmol/L, 95% CI ‐22.55 to ‐5.72; I2 = 17%).
8.3. Analysis.
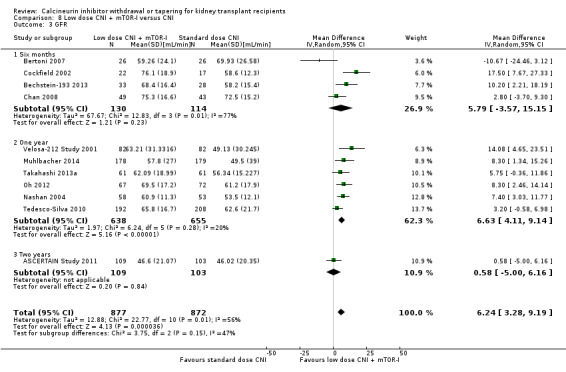
Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 3 GFR.
8.5. Analysis.
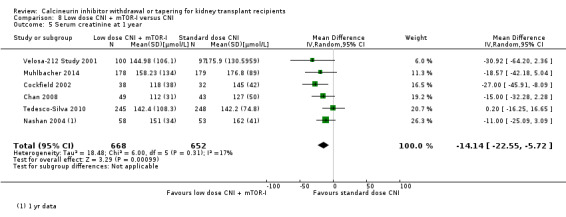
Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 5 Serum creatinine at 1 year.
Hypertension (Analysis 8.7.1 (5 studies, 1421 participants): RR 0.98, 95% CI 0.80 to 1.20; I2 = 0%, low certainity), hyperlipidaemia (Analysis 8.7.2 (8 studies, 1793 participants): RR 1.07, 95% CI 0.89 to 1.28; I2 = 30%), and diabetes mellitus (Analysis 8.7.4 (5 studies, 686 participants): RR 1.36, 95% CI 0.81 to 2.27; I2 = 0%) were noted to be similar in patients treated with either low dose CNI in combination with mTOR‐I or standard dose CNI regimens. There was no reduction in malignancy in the low CNI in combination with mTOR‐I group compared to those treated with standard dose CNI regimens (Analysis 8.7.5 (5 studies, 1074 participants): RR 1.22, 95% CI 0.42 to 3.52; I2 = 0%, low certainity). There was little or no difference in total Infections (Analysis 8.7.6 (5 studies, 1271 participants): RR 0.95, 95% CI 0.83 to 1.08; I2 = 28%) and CMV infection (Analysis 8.7.3 (5 studies, 1250 participants): RR 0.41, 95% CI 0.16 to 1.06; I2 = 74%; low certainty) between the two groups.
8.7. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 7 Adverse events.
Subgroup analysis
CNI and mTOR‐I combination with standard dose CNI regimen in the immediate post‐transplant period
GFR was higher in the low dose CNI with mTOR‐I group (Analysis 8.9.1 (10 studies, 1537 participants): MD 6.91 mL/min, 95% CI 3.86 to 9.96; I2 = 53%), however acute rejection (Analysis 8.10.1 (14 studies, 2736 participants): RR 1.09, 95% CI 0.86 to 1.39; I2 = 27%) and graft loss (Analysis 8.8.1 (14 studies, 2736 participants): RR 0.75, 95% CI 0.48 to 1.18; I2 = 0%) were similar in the two groups.
8.9. Analysis.
Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 9 Subgroup analysis: GFR.
8.10. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 10 Subgroup analysis: acute rejection.
8.8. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 8 Subgroup analysis: graft loss.
Late introduction of low dose CNI regimen with mTOR‐I substitution
Incidence of acute rejection was higher in the low dose CNI with mTOR‐I group (Analysis 8.10.2 (2 studies, 564 participants): RR 1.38, 95% CI 0.82 to 2.31; I2 = 0%), there was no difference in graft loss (Analysis 8.8.2 (2 studies, 568 participants): RR 0.40, 95% CI 0.15 to 1.04; I2 = 0%) and one study reported no difference in GFR in the late withdrawal group (Analysis 8.9.2 (1 study, 212 participants): MD 0.58 mL/min, 95% CI ‐5.00 to 6.16).
Type of CNI (CsA or TAC)
There was no difference in acute rejection in the low dose CsA with mTOR‐I compared to standard dose CsA (Analysis 9.1.1 (11 studies, 2232 participants): RR 0.97, 95% CI 0.78 to 1.22; I2 = 7%), however acute rejection was higher when low dose TAC with mTOR‐I was compared to standard dose TAC (Analysis 9.1.2 (5 studies, 1068 participants): RR 1.58, 95% CI 1.16 to 2.13; I2 = 0%).
9.1. Analysis.
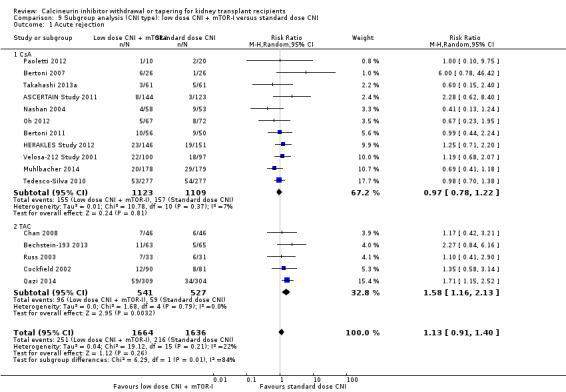
Comparison 9 Subgroup analysis (CNI type): low dose CNI + mTOR‐I versus standard dose CNI, Outcome 1 Acute rejection.
Sensitivity analysis
On sensitivity analyses stratified for steroid free regimens the effects were not different from steroid regimens for death, acute rejection, or GFR.
Discussion
Summary of main results
This review describes CNI withdrawal or tapering classified according to: CNI withdrawal, low dose CNI, CNI withdrawal with mTOR‐I substitution and low dose CNI with mTOR‐I compared to standard dose CNI regimens. The four groups were further stratified into CNI avoidance and withdrawal studies for major outcomes.
In the CNI withdrawal comparison with standard regimens, there was an increase in both clinical acute rejection and BPAR. GFR was higher in the withdrawal group especially over longer time periods. Death, diabetes mellitus, hyperlipidaemia, total and CMV infections were not significantly different between the groups. Standard dose CNI regimens were more likely to be associated with hypertension when compared to CNI withdrawal patients. Graft loss was lower in the CNI withdrawal group; however, when stratified for avoidance studies, there was no difference in graft loss between the groups. These protocols (late withdrawal or avoidance) resulted in an increase in acute rejection with no clear benefit in terms of reduced graft loss. There was also no difference in the type of CNI (TAC or CsA) used or steroid‐free regimens in causing acute rejection. The beneficial effects of CNI withdrawal in reducing graft loss were lost when studies with long‐term outcomes were excluded.
In the low dose CNI comparison with standard dose regimens, there was a reduction in acute rejection, however when studies which administered induction treatment (IL2RA or anti‐lymphocyte serum or globulin) were excluded from the analysis, acute rejection was similar in the low dose CNI and standard dose CNI regimens, both in the immediate and late introduction groups. GFR was higher in the low dose CNI group at both one and five years. There were no significant differences in death, diabetes mellitus, hyperlipidaemia, and CMV infection between the groups. Low dose CNI regimens had a marginal reduction in hypertension and total infections. Graft loss was reduced in the low dose CNI regimen, however when stratified for early and late intervention (taper), the effect was limited to the early intervention studies.
In the CNI avoidance or tapering with mTOR‐I substitution compared to standard dose CNI regimens, there was no difference in death between the two groups. The mTOR‐I substitution regimen however had more acute rejections (clinical and biopsy‐proven) and had more hyperlipidaemia. CMV infection and malignancy were significantly lower in the mTOR‐I substitution group. GFR was higher in the CNI avoidance with mTOR‐I subgroup but not in the late intervention subgroup. There was no difference in other outcomes when stratified for early or late intervention. Overall these protocols (avoidance or tapering) showed no major change compared to CNI alone except for the increase in acute rejection when compared with either CNI (CsA or TAC). The major benefit of mTOR‐I substitution is seen in the reduction in malignancies and CMV infections over time.
When low dose CNI was combined with mTOR‐I and compared to standard dose CNI regimens, there were no differences in death, graft loss or acute rejection. Adverse events including malignancy were not significantly different between the groups. GFR and SCr at one year favoured the low dose CNI with mTOR‐I regimen. However when stratified for early and late intervention there was increased acute rejection in the low dose CNI with mTOR‐I regimens.
This review investigated a large number of studies comparing different CNI regimens. Many studies and reports were published in multiple journals at various time points and were presented as abstracts at scientific meetings without acknowledging previous publications. The same studies were also published under different authors and this review combined these reports under a single study and reported outcomes systematically. The methodology was robust and the studies were also assessed for study quality and heterogeneity explored by subgroup and stratified analysis. The review classified interventions into four groups which reduced multiple comparisons due to several different regimens.
Overall completeness and applicability of evidence
Short time scales of most studies restrict the external validity of this review. Moving away from CNI may have multiple adverse long‐term effects that will not be measured by these studies. The studies also do not mention of antibody‐mediated rejection and pretransplant donor specific antibodies which could impact on short‐ and long‐term graft survival. Removal of CNI may remove one long‐term problem (CNI toxicity) but potentially cause worsening of other immunological issues which may in turn limit the duration of the graft. Low dose CNI seem the best option and mTOR‐I benefits appear to be limited to a reduction in the risks of malignancy and CMV infection, though these benefits are uncertain and are not the case when combined with CNI.
Quality of the evidence
The overall quality of the evidence was poor, with unclear risks of bias due to poor reporting (Figure 2); only 30% reported randomisation method and allocation concealment. Almost all studies were open‐label however for study outcomes such as death and graft loss they were not downgraded on GRADE assessment. Studies were analysed as intent to treat in 60% and all pre specified outcomes were reported in 54 studies. Almost half the studies received pharmaceutical funding which were classified as a high risk of bias.
The studies also used variable outcome measures and induction immunosuppression regimens. There is also variability in dosing, drug monitoring and time intervals of reporting outcomes. Most studies did not indicate baseline SCr or GFR to assess for changes due to the intervention. The follow‐up duration in majority of the included studies was between six months and three years which is a major limitation for concluding long‐term outcomes such as patient and graft survival.
Potential biases in the review process
There are multiple limitations of this review. The quality of data reporting was variable in terms of outcome and adverse effects. Most studies did not indicate the baseline creatinine or GFR to assess for changes due to the intervention. The standard deviation or confidence intervals were not noted when recording outcomes such as GFR and creatinine. Adverse effects were prevalent rather than incident cases which may affect outcomes such as diabetes mellitus, hyperlipidaemia and hypertension. The number of patients affected by individual outcomes were not indicated but mentioned as being significant with or without P values. Outcome reporting was not defined in cases of CMV, hypertension, hyperlipidaemia (total or low‐density lipoprotein) or diabetes mellitus. Different studies used different targets for CNI monitoring and also used either trough (C0) or two hour (C2) levels; some studies based the dose on mg/kg body weight and this review used the study author definitions to classify low dose and standard dose regimens. This may have some limitation in external validity of these recommendations. However we have tried to minimise this by subclassification into four groups and analyse them further into early and late interventions. Most studies were short‐term and did not capture long‐term hard outcomes such as graft survival, patient survival or adverse effects (such as cardiovascular outcomes) and malignancy. The duration of the majority of studies was between six months and three years with only three studies of up to five years duration. This raises the concern of how outcomes might be different after that time, particularly with regards to antibody‐mediated rejection which can be a complication of reduced immune suppression. The only studies that included more than 10 years of follow‐up tended to be much older studies, and compared immunosuppression such as azathioprine which is now largely obsolete or in very little use. The data from these studies is therefore limited by era effect. Studies with longer follow‐up are required to confirm the potential benefits of CNI reduction or risks of long‐term antibody‐mediated rejection, most studies also do not differentiate between patients with high versus low immunological risk.
Agreements and disagreements with other studies or reviews
This is the first review which sub classified studies into four different intervention groups and analysed them as low dose calcineurin inhibitor or CNI withdrawal with or without mTOR‐I substitution. The classification analysed the possible advantages noted in various studies with additional immunosuppressive agent such as mTOR‐I or continuation of CNI at a low dose.
Sharif 2011 (56 studies, 11,337 participants) showed a similar increase in acute rejection without affecting graft survival, infection, and patient survival, it also concluded an increase in graft failure when mTOR‐I was used. The review however did not classify studies into low dose or withdrawal as in our review but performed a pooled analysis which resulted in significant heterogeneity. In contrast to the conclusions of this review, Sharif 2011 reported lower NODAT in the CNI‐sparing group. Moore 2009 included only CNI‐sparing with MMF. The results were not stratified for mTOR‐I; however the studies were classified into those who had de novo CNI minimisation and elective minimisation or elimination of CNI. The results in the withdrawal group were similar to our review but the lower dose of CNI was not beneficial in reduction of acute rejection as we report. A systematic review by Lim 2014 (29 studies, 2350 participants) analysed conversion to an mTOR‐I based immunosuppression from CNI based therapy. They review reported short‐term improvements in GFR with mTOR‐I but increased acute rejections; there were no differences in graft loss or death. The conclusions of Lim 2014 are similar to our analysis of CNI withdrawal with mTOR‐I, however our review also analysed low dose CNI and mTOR‐I substitution.
Authors' conclusions
Implications for practice.
CNI avoidance increased acute rejection and CNI withdrawal increases acute rejection but reduced graft loss at least over the short‐term. Low dose CNI with induction regimens reduced acute rejection and graft loss with no major adverse events, also in the short‐term. The use of mTOR‐I reduced CMV infections but increased the risk of acute rejection. These conclusions must be tempered by the lack of long‐term data in most of the studies, particularly with regards to chronic antibody‐mediated rejection, and the suboptimal methodological quality of the included studies.
Implications for research.
Despite a large number of randomised multicentre studies, significant issues remain unanswered. Most study data highlighted short‐term outcomes due to the short follow‐up. Longer follow‐up will highlight hard end points such as cardiovascular outcomes, long‐term graft survival and effects on malignancy. Cost benefit analysis and quality of life surveys to assess the effect of lower immunosuppression may also be of significant benefit. Carefully structured longer term studies into immunosuppression of kidney transplant patients need to delineate patient death, malignancy risk in protocols with or without CNI, immunological risk will need to include acute rejection, donor‐specific antibodies and antibody‐mediated rejection.
History
Protocol first published: Issue 4, 2007 Review first published: Issue 7, 2017
| Date | Event | Description |
|---|---|---|
| 9 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Cochrane Kidney and Transplant for their advice and support in undertaking this review. We would also like to acknowledge the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| DATABASE | Search terms |
| CENTRAL | 1. Kidney Transplantation, MESH term 2. Tacrolimus, MESH 3. (tacrolimus):ti,ab,kw 4. "FK 506" or FK506:ti,ab,kw 5. Cyclosporine, MeSH term 6. (cyclosporin* or ciclosporin*):ti,ab,kw 7. (csa* or neoral* or cya* or restasis or sandimmun*):ti,ab,kw 8. (calcineurin inhibitor*):ti,ab,kw 9. (2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8) 10. (discontinu* or withdraw* or taper* or spar* or avoid* or minim* or remov* or stop* or reduction* or reduc* or free*):ti,ab,kw 11. (9 AND 10) 12. (1 AND 11) |
| MEDLINE | 1. Kidney Transplantation/ 2. Tacrolimus/ 3. tacrolimus.tw. 4. prograf$.tw. 5. ("FK 506" or FK506).tw. 6. fr‐900506.tw. 7. fujimycin.tw. 8. protopic.tw. 9. Cyclosporine/ 10. cyclosporin$.tw. 11. ciclosporin$.tw. 12. csa.tw. 13. neoral.tw. 14. cya$.tw. 15. sandimmun$.tw. 16. restasis.tw. 17. calcineurin inhibitor$.tw. 18. or/2‐17 19. (discontinu$ or withdraw$ or taper$ or spar$ or avoid$ or minim$ or remov$ or stop$ or reduction or reduc$ or free$).tw. 20. and/18‐19 21. and/1,20 |
| EMBASE | 1. Kidney Transplantation/ 2. Tsukubaenolide/ 3. tacrolimus.tw. 4. prograf$.tw. 5. ("FK 506" or FK506).tw. 6. fr‐900506.tw. 7. fujimycin.tw. 8. protopic.tw. 9. Cyclosporin/ 10. cyclosporin$.tw. 11. ciclosporin$.tw. 12. (csa or neoral or cya).tw. 13. (sandimmun$ or restaisi).tw. 14. Calcineurin Inhibitor/ 15. or/2‐14 16. (discontinu$ or withdraw$ or taper$ or spar$ or avoid$ or minim$ or remov$ or stop$ or reduction or reduc$ or free$).tw. 17. and/15‐16 18. and/1,17 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. CNI withdrawal versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 14 | 2010 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.93, 1.54] |
| 1.1 Avoidance | 4 | 566 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.85, 2.01] |
| 1.2 Late withdrawal | 10 | 1444 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.83, 1.56] |
| 2 Acute rejection | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 2.1 Unspecified | 7 | 1066 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.08, 2.75] |
| 2.2 Biopsy‐proven | 8 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 4.48 [2.10, 9.55] |
| 3 GFR | 8 | 910 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐1.13, 8.25] |
| 3.1 One year | 5 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐5.38, 4.94] |
| 3.2 Two years | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 7.90 [1.43, 14.37] |
| 3.3 Over 5 years | 2 | 149 | Mean Difference (IV, Random, 95% CI) | 11.09 [4.81, 17.37] |
| 4 Graft loss | 16 | 2090 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.98] |
| 5 Serum creatinine | 4 | 189 | Mean Difference (IV, Random, 95% CI) | 19.17 [5.89, 32.44] |
| 5.1 Six months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 31.78 [10.58, 52.98] |
| 5.2 One year | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 11.04 [‐5.99, 28.06] |
| 6 Adverse events | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hypertension | 5 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.95] |
| 6.2 Hyperlipidaemia | 3 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.21] |
| 6.3 CMV infection | 7 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.45] |
| 6.4 Diabetes | 6 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 6.5 Malignancy | 6 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.30] |
| 6.6 Infection | 6 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.51] |
| 7 Subgroup analysis: acute rejection | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 7.1 Avoidance | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [0.85, 5.49] |
| 7.2 Late withdrawal | 12 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [1.59, 6.48] |
| 8 Subgroup analysis: GFR | 8 | 910 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐1.13, 8.25] |
| 8.1 Avoidance | 3 | 242 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐14.84, 10.40] |
| 8.2 Late withdrawal | 5 | 668 | Mean Difference (IV, Random, 95% CI) | 5.54 [1.66, 9.43] |
| 9 Subgroup analysis: graft loss | 16 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 9.1 Avoidance | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.16] |
| 9.2 Late withdrawal | 13 | 1848 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.97] |
1.5. Analysis.
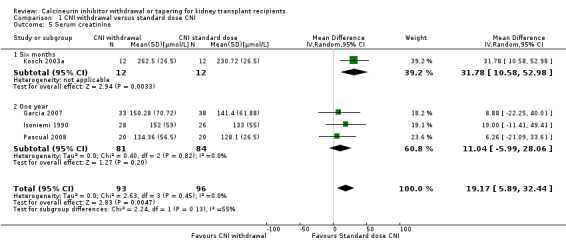
Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 5 Serum creatinine.
Comparison 2. Subgroup analysis (antimetabolite): CNI withdrawal versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 1.1 MMF/MPA | 10 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.79, 6.88] |
| 1.2 AZA | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.78, 4.19] |
Comparison 3. Subgroup analysis (CNI type): CNI withdrawal versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 1.1 CSA | 11 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.31, 3.48] |
| 1.2 TAC | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 5.65 [1.96, 16.27] |
| 1.3 Either CSA or TAC | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
Comparison 4. Low dose CNI versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 15 | 3462 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.27] |
| 1.1 Early intervention | 13 | 3272 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.27] |
| 1.2 Late intervention | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.15, 6.94] |
| 2 Acute rejection | 19 | 3757 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 2.1 Unspecified | 8 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.03] |
| 2.2 Biopsy‐proven | 11 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.16] |
| 3 GFR | 13 | 2623 | Mean Difference (IV, Random, 95% CI) | 4.10 [2.07, 6.12] |
| 3.1 Six months | 5 | 812 | Mean Difference (IV, Random, 95% CI) | 1.96 [‐1.35, 5.28] |
| 3.2 One year | 7 | 1710 | Mean Difference (IV, Random, 95% CI) | 4.30 [1.78, 6.82] |
| 3.3 Two years | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 11.10 [4.14, 18.06] |
| 4 Graft loss | 15 | 3286 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 5 Serum creatinine | 6 | 742 | Mean Difference (IV, Random, 95% CI) | ‐4.28 [‐14.65, 6.10] |
| 5.1 Six months | 4 | 530 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐11.25, 8.33] |
| 5.2 One year | 2 | 212 | Mean Difference (IV, Random, 95% CI) | ‐23.18 [‐46.12, ‐0.23] |
| 6 Change in GFR at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Adverse events | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 5 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 7.2 Hyperlipidaemia | 3 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.19] |
| 7.3 CMV infection | 6 | 1948 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.94, 1.62] |
| 7.4 Diabetes | 5 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.34] |
| 7.5 Malignancy | 5 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.97] |
| 7.6 Infection | 9 | 1437 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Subgroup analysis: acute rejection | 18 | 2968 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.70, 1.02] |
| 8.1 Immediate intervention | 12 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.00] |
| 8.2 Late intervention | 6 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.81] |
| 9 Subgroup analysis: GFR | 12 | 2443 | Mean Difference (IV, Random, 95% CI) | 4.21 [1.90, 6.51] |
| 9.1 Immediate intervention | 9 | 2200 | Mean Difference (IV, Random, 95% CI) | 3.09 [0.95, 5.23] |
| 9.2 Late intervention | 3 | 243 | Mean Difference (IV, Random, 95% CI) | 8.81 [3.79, 13.83] |
| 10 Subgroup analysis: graft loss | 14 | 3106 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10.1 Immediate intervention | 11 | 2800 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10.2 Late intervention | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.12, 7.56] |
4.6. Analysis.
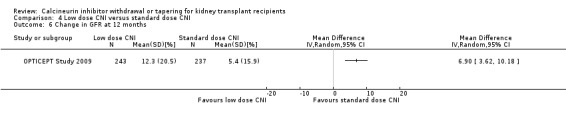
Comparison 4 Low dose CNI versus standard dose CNI, Outcome 6 Change in GFR at 12 months.
Comparison 5. Subgroup analysis (CNI type): low dose CNI versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection | 19 | 3757 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 1.1 CsA | 16 | 2906 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.01] |
| 1.2 TAC | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.61, 3.83] |
| 1.3 Either CsA or TAC | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.19] |
Comparison 6. CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 23 | 5427 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.36] |
| 1.1 Avoidance | 9 | 1689 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.47] |
| 1.2 Withdrawal | 14 | 3738 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.76] |
| 2 Acute rejection | 30 | 5903 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 2.1 Unspecified | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.12, 1.60] |
| 2.2 Biopsy‐proven | 26 | 4966 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.10, 1.85] |
| 3 GFR | 23 | 4427 | Mean Difference (IV, Random, 95% CI) | 5.29 [2.08, 8.51] |
| 3.1 Six months | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 5.22 [‐0.02, 10.46] |
| 3.2 One year | 16 | 3144 | Mean Difference (IV, Random, 95% CI) | 5.02 [0.59, 9.45] |
| 3.3 Two years | 4 | 796 | Mean Difference (IV, Random, 95% CI) | 6.08 [‐0.85, 13.01] |
| 3.4 Five years | 1 | 300 | Mean Difference (IV, Random, 95% CI) | 6.30 [2.43, 10.17] |
| 4 Graft loss | 25 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 5 Serum creatinine at 1 year | 12 | 1702 | Mean Difference (IV, Random, 95% CI) | ‐17.10 [‐26.95, ‐7.25] |
| 6 Change in GFR | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 One year | 1 | 246 | Mean Difference (IV, Random, 95% CI) | 6.10 [0.01, 12.19] |
| 6.2 Two years | 2 | 521 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐15.00, 15.56] |
| 7 Adverse events | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 7 | 2207 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.15] |
| 7.2 Hyperlipidaemia | 13 | 3494 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.40, 2.20] |
| 7.3 CMV infection | 13 | 2503 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.82] |
| 7.4 Diabetes | 11 | 2833 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 7.5 Malignancy | 14 | 3699 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.00] |
| 7.6 Infection | 9 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 7.7 Lymphocele | 8 | 1926 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.95, 2.21] |
| 8 Subgroup analysis: acute rejection | 28 | 5480 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.27, 1.91] |
| 8.1 Avoidance | 11 | 1844 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.98, 1.65] |
| 8.2 Late withdrawal | 17 | 3636 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.44, 2.51] |
| 9 Subgroup analysis: GFR | 23 | 4427 | Mean Difference (IV, Random, 95% CI) | 5.29 [2.08, 8.51] |
| 9.1 Avoidance | 9 | 1748 | Mean Difference (IV, Random, 95% CI) | 6.45 [1.33, 11.58] |
| 9.2 Late withdrawal | 14 | 2679 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.26, 8.85] |
| 10 Subgroup analysis: graft loss | 25 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 10.1 Avoidance | 8 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.72, 1.48] |
| 10.2 Late withdrawal | 17 | 4026 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
6.6. Analysis.
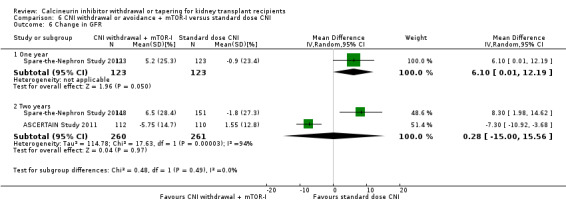
Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 6 Change in GFR.
Comparison 7. Subgroup analysis (CNI type): CNI withdrawal + mTOR‐I versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection | 30 | 5903 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 1.1 CsA | 18 | 3463 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.15, 1.76] |
| 1.2 TAC | 7 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.43, 3.49] |
| 1.3 Either CsA or TAC | 5 | 1687 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.40, 2.33] |
Comparison 8. Low dose CNI + mTOR‐I versus CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 11 | 2750 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.71, 1.90] |
| 1.1 Low dose CNI + immediate mTOR | 9 | 2182 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.62, 1.87] |
| 1.2 low dose CNI + late mTOR | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.52, 4.59] |
| 2 Acute rejection | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 2.1 Unspecified | 3 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.90, 2.09] |
| 2.2 Biopsy‐proven | 13 | 2804 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.83, 1.37] |
| 3 GFR | 11 | 1749 | Mean Difference (IV, Random, 95% CI) | 6.24 [3.28, 9.19] |
| 3.1 Six months | 4 | 244 | Mean Difference (IV, Random, 95% CI) | 5.79 [‐3.57, 15.15] |
| 3.2 One year | 6 | 1293 | Mean Difference (IV, Random, 95% CI) | 6.63 [4.11, 9.14] |
| 3.3 Two years | 1 | 212 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐3.00, 6.16] |
| 4 Graft loss | 16 | 3304 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| 5 Serum creatinine at 1 year | 6 | 1320 | Mean Difference (IV, Random, 95% CI) | ‐14.14 [‐22.55, ‐5.72] |
| 6 Change in GFR at 2 years | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Adverse events | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 5 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.20] |
| 7.2 Hyperlipidaemia | 8 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.28] |
| 7.3 CMV infection | 5 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.06] |
| 7.4 Diabetes | 5 | 686 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.81, 2.27] |
| 7.5 Malignancy | 5 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.42, 3.52] |
| 7.6 Infection | 5 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.08] |
| 8 Subgroup analysis: graft loss | 16 | 3304 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| 8.1 Immediate mTOR | 14 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.18] |
| 8.2 Late mTOR | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.15, 1.04] |
| 9 Subgroup analysis: GFR | 11 | 1749 | Mean Difference (IV, Random, 95% CI) | 6.24 [3.28, 9.19] |
| 9.1 Immediate mTOR | 10 | 1537 | Mean Difference (IV, Random, 95% CI) | 6.91 [3.86, 9.96] |
| 9.2 Late mTOR | 1 | 212 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐3.00, 6.16] |
| 10 Subgroup analysis: acute rejection | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 10.1 Immediate mTOR | 14 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.39] |
| 10.2 Late mTOR | 2 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.82, 2.31] |
8.6. Analysis.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 6 Change in GFR at 2 years.
Comparison 9. Subgroup analysis (CNI type): low dose CNI + mTOR‐I versus standard dose CNI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 1.1 CsA | 11 | 2232 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.78, 1.22] |
| 1.2 TAC | 5 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.16, 2.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abramowicz 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed centrally using the minimisation method, with frequency matching for variables |
| Allocation concealment (selection bias) | Low risk | Central randomisation performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up completed and reported as per ITT |
| Selective reporting (reporting bias) | Low risk | All relevant outcome data reported |
| Other bias | High risk | Pharmaceutical industry funded: Hoffman La‐Roche |
Alsina 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome reporting complete; 3/6 of our outcomes of interest reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Abstract‐only publications |
Andres 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
All groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported; AR was clinical and BPAR |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Modified ITT analysis |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | First author employee of Novartis; funding source not clarified |
APOLLO Study 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a validated, automated, central system |
| Allocation concealment (selection bias) | Low risk | Investigators notified of the treatment group by fax from central system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | study not feasible to blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Pharma funded (Funding source: Novartis), study terminated early, 5 year outcome awaited |
Asberg 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Expected outcomes reported, ITT analysis |
| Other bias | High risk | Funded by a grant from Roche |
ASCERTAIN Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation, stratified by centre, was performed using a validated, automated system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data at 24 weeks was reported as ITT |
| Selective reporting (reporting bias) | Low risk | Data at 24 weeks was reported as ITT |
| Other bias | High risk | Funded by Novartis Pharma AG |
Baczkowska 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bansal 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with the help of a computer generated Bernoulli random number table |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by opaque sequentially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement, ITT not specified |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Biocon Nephrology, India |
Barsoum 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process of generating random numbers not clear |
| Allocation concealment (selection bias) | High risk | Sequentially randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT performed and outcome reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported completely |
| Other bias | Low risk | Appears free of other biases |
Bechstein‐193 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funded by Wyeth |
Bertoni 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bertoni 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Protocol not specified but study reports all possible outcomes |
| Other bias | Low risk | No other apparent biases |
Budde 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | All patient outcome data reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study design may not allow for blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | prespecified outcomes reported |
| Other bias | High risk | Funded by Novartis |
CAESAR Study 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code for the CAESAR study (M67005) was generated in the Oracle Clinical randomisation module, Each site was supplied with a list of unique patient numbers |
| Allocation concealment (selection bias) | Low risk | Treatment assignment, corresponding to patient number, was provided on a sheet sealed inside a randomisation envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis and had minimal missing data |
| Selective reporting (reporting bias) | Low risk | The report include all possible outcomes |
| Other bias | High risk | Funded by Roche, Switzerland |
Cai 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Novartis |
CALFREE Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, minimal lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by grants from Wyeth Pharma |
CENTRAL Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally using a validated automated system |
| Allocation concealment (selection bias) | Low risk | Investigators notified of the randomisation group via the electronic case record form system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals high in the EVL group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported in 3 year follow‐up |
| Other bias | High risk | Funded by Novartis Scandinavia |
CERTITEM Study 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcome comparison of pathology pre and post randomisation not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal lost to follow‐up at 2 year period |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes not reported |
| Other bias | High risk | Funded by Novartis Pharma |
Chadban 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes |
| Other bias | High risk | Funded by Novartis Australia. Australian sub protocol part of a global trial |
Chan 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Centrally generated sequential sealed treatment allocation cards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Data were collected by investigators via a validated electronic system and transferred to an electronic database for analysis |
| Other bias | High risk | Funded by Novartis, USA |
Chan 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by using a validated automated system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results stated to be ITT but are different from the randomised number |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Novartis Pharma AG, Basel, Switzerland |
Chhabra 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis reporting complete with minimal loss of follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified in methods |
| Other bias | High risk | Funded by Pfizer Pharmaceuticals |
Cibrik 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated randomisation |
| Allocation concealment (selection bias) | Low risk | Numbers on the outside with concealed information about maintenance group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Investigators remained blinded but not clear if patients were blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators remained blinded until the end of the 2nd month post‐transplant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes noted |
| Selective reporting (reporting bias) | Low risk | Pre specified outcomes reported |
| Other bias | High risk | Funded by Novartis Pharma |
Cockfield 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Preliminary data only |
| Selective reporting (reporting bias) | High risk | Outcomes not complete and reported as preliminary data |
| Other bias | High risk | Preliminary data only; no full text publication 15 years after abstracts published |
CONCEPT Study 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation at week 12 was centralized and balanced (1:1). Data collections were ensured by an electronic case report form and the centralized randomisation was ensured via Internet |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, minimal withdrawal |
| Selective reporting (reporting bias) | Low risk | The report included all expected outcomes |
| Other bias | High risk | Funded by Roche |
CONVERT Trial 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomisation/enrolment system used |
| Allocation concealment (selection bias) | Low risk | Automatic transtelephonic randomisation was used to assign study treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High dropout, however ITT |
| Selective reporting (reporting bias) | Low risk | Report included all expected outcomes |
| Other bias | High risk | Funded by Wyeth Research |
CTOT‐09 Study 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Initial treatment for 6 months (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nature of the study does not let for physician blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Prespecified outcomes reported in randomised patients |
| Selective reporting (reporting bias) | High risk | Study was prematurely stopped and TAC was introduced in more than half of the patients |
| Other bias | High risk | Only 21 of the planned 210 patients were randomised |
de Sevaux 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group Conventional CsA trough levels: 300 ng/mL (1st 3 months), 150 ng/mL (3 to 6 months) Both groups
|
|
| Outcomes |
All end points also were assessed at 6 months after transplant |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out by opening a sealed envelope with the lowest available study number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data noted |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Roche Pharmaceuticals, The Netherlands |
DICAM Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was generated and maintained by the Biostatistics Department at the University of Rouen |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed independently at each centre using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pathologists were blinded for biopsy interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most patients completed the trial |
| Selective reporting (reporting bias) | Low risk | All expected outcome data reported |
| Other bias | Unclear risk | MPA concentration measurements were funded by Roche |
Dudley 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomisation |
| Allocation concealment (selection bias) | Low risk | Computerized touch‐tone system stratified for centre, was used for treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data, analysed ITT |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded and authored (2) by Hoffmann‐La Roche |
El‐Agroudy 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fangmann 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation lists |
| Allocation concealment (selection bias) | Low risk | After verification through the central office, centres were notified by fax |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis and all outcomes reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | None identified |
Ferguson 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
C2 levels difference 50 to 70% between reduced and full dose group. |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Partial blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Novartis Pharma AG |
Flechner‐318 Study 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via computer‐generated cards |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No identifiable missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by Wyeth‐Ayerst Pharma |
Garcia 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Enrolment was interrupted in 2002 and a 3rd group of patients were enrolled in a non‐randomised fashion Group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study was described as randomised, method of randomisation was not reported; a 3rd group of non‐randomised patients included after interim analysis |
| Allocation concealment (selection bias) | High risk | Process not clarified, also a 3rd group of non‐randomised patients included after interim analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | A 3rd group of non‐randomised patients included after the interim analysis |
Grimbert 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (immediately post‐transplant)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, no missing outcomes despite long duration |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Grinyo 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | High risk | Envelopes for randomisation prepared by Wyeth |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate resulting in protocol change |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Wyeth; high drop‐out resulting in protocol amendment mid trial |
Hall 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was centrally generated by computer and stratified by centre |
| Allocation concealment (selection bias) | Low risk | Patient assignment was delivered to each of the centres opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, ITT analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Funded by Sandoz to 10 years |
Hazzan 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (1st 3 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data noted |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Heering 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
Both groups (to 9 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study was stopped early due to increased rejection |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Study stopped early due to significant increase in acute rejection in group 3 |
HERAKLES Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
Both groups (to 3 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hollander 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (to 3 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Long term follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Holm 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Antibody induction
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up data not published as planned |
| Selective reporting (reporting bias) | High risk | No full‐text publication 10 years after abstract publication |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Isoniemi 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
Group 4
All groups (1st 10 weeks)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious missing data |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Kosch 2003a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (to 6 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Kreis 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
MacPhee 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "allocation was concealed in opaque numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Martinez‐Mier 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
MECANO Study 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups (1st 6 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated with “Random Allocation Software” Version 1.0 2004 tripod.com |
| Allocation concealment (selection bias) | Low risk | A sealed opaque envelope was used, containing a sheet with the number of the treatment arm. All patients received an envelope after recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study prematurely terminated after increased rejection in one arm |
| Selective reporting (reporting bias) | High risk | Study prematurely terminated after increased rejection in one arm |
| Other bias | High risk | Funded by Novartis Pharma. Early termination of the study |
MODIFY Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | ITT analysis, all outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Muhlbacher 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis only reported |
| Selective reporting (reporting bias) | Unclear risk | Interim analysis, outcomes reported as prespecified |
| Other bias | High risk | Funded by Wyeth; interim analysis report |
Nafar 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Adverse effects mentioned only for the initial one year |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned for 1st year but only efficacy subsequently |
| Other bias | Unclear risk | Safety data limited to the 1st year |
Nashan 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis for efficacy but not safety data |
| Selective reporting (reporting bias) | Low risk | Published data included all expected outcomes |
| Other bias | High risk | Funded by Novartis. High drop‐out rates, safety data was not ITT |
Oh 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were recorded and entered onto an electronic database and re‐evaluated by external monitors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data was handled by ITT analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in final analysis |
| Other bias | High risk | Funded by Novartis |
OPTICEPT Study 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocated sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Roche |
ORION Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data accounted by modified ITT analysis |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes reported however group 2 of the study limbs reported high BPAR and was terminated |
| Other bias | High risk | Funded by Wyeth; one of the study groups was terminated |
Pacheco‐Silva 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Included prespecified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Paoletti 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation was implemented using sequentially numbered, opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | 3 year data yet to be reported |
| Other bias | Unclear risk | Study not powered, initially planned for 36 patients, ITT was for 30 |
Pascual 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data noted |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Funded by Roche Laboratories |
Pascual 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 year data not available |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by ILEX Inc, San Antonio (makers of Alemtuzumab). |
Pedersen 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (on entry)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised consecutively |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Not all adverse events recorded in outcomes |
| Other bias | Low risk | Study appears free of biases |
Pontrelli 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Qazi 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Novartis Pharmaceuticals were sponsors, study directors, and authors |
REFERENCE Study 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was centralised and stratified |
| Allocation concealment (selection bias) | Low risk | "centralized randomization was ensured via Internet" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data accounted for outcomes and analysed as ITT |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Roche |
Rivelli 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned to groups by random numbers generated by computer immediately before surgery |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pathologist analysing the biopsies was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing patient data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | This study was supported by: The Brazilian Council for Scientific and Technological Development |
RMR Study 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data accounted for outcome reporting |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Wyeth‐Ayerst Research |
Rossini 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Biopsy documented at 2 years for all 12 patients |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes noted; no full text publication by 2017 |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Russ 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No further data reported after 6 months |
| Selective reporting (reporting bias) | Low risk | 6 month data was reported as specified in methods |
| Other bias | High risk | Funded by Wyeth |
Salvadori 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes reported; no full text publication by 2017 |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Schaefer 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Smak Gregoor 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
All groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes with random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data noted |
| Selective reporting (reporting bias) | Low risk | Prespecified expected outcomes reported |
| Other bias | High risk | Funded by Roche Pharmaceuticals |
SMART TX Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permuted block randomisation scheme was used to assign trial participants to one of the treatment groups |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was secured by a centralized distribution of sequentially numbered, opaque, sealed envelopes, and a confirmatory randomisation fax to the clinical research organization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All possible outcomes reported as ITT |
| Selective reporting (reporting bias) | Low risk | Pre specified outcomes reported |
| Other bias | High risk | Funded by Wyeth and Fresenius |
Spare‐the‐Nephron Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks, numbers generated by study sponsor |
| Allocation concealment (selection bias) | Low risk | Accessed through interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, ITT analysis |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funding by Roche |
Stallone 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups (to 3 months)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Insufficient information to permit judgement |
Stallone 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Stegall 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, reported ITT |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Wyeth, Genzyme, and Roche; high drop‐out rate |
Suwelack 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data noted |
| Selective reporting (reporting bias) | Low risk | Pre specified outcomes reported |
| Other bias | High risk | Funded by Hoffman‐La Roche |
SYMPHONY Study 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
Group 4
All groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was described as randomised and stratified, "A minimization algorithm was used to optimize the balance of characteristics of patients in study groups, overall and across the strata." |
| Allocation concealment (selection bias) | Low risk | Central randomisation, voice interactive allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Outcomes that are of interest reported |
| Other bias | High risk | Funded by Hoffmann‐La Roche |
Takahashi 2013a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent validated system that automated the random assignment of treatment arms |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Most outcomes were objective, however there was no blinding of assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis all patients analysed |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | High risk | Funded by Novartis who also helped in manuscript development |
Tedesco‐Silva 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
Group 3
All groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned a randomisation number but procedure not clarified which was linked to one of the three treatment groups, using an interactive voice‐response system. The randomisation scheme was reviewed and approved by the Biostatistics Quality Assurance Group. |
| Allocation concealment (selection bias) | Low risk | Patient allocation was based on an interactive voice‐response system centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data outcome despite high drop‐out rates due to ITT analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Novartis; high drop‐out rates |
Velosa‐212 Study 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | One of the randomised groups did not receive the drug and were in a 3rd group |
| Other bias | High risk | Funded by Wyeth. Patients with ATN‐DGF that resolved later than post‐transplantation day 7 were not randomised but were assigned to a 3rd group (non‐randomised) |
Watson 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Determined by random numbers generated by a Microsoft Excel Software program |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes but concealed from the members who were involved in the enrolment of the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis complete reporting |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Funded by Wyeth |
ZEUS Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 1:1 ratio by use of a central, validated system that automated the random assignment of treatment groups to randomisation numbers (stratified according to living‐donor or deceased donor status) |
| Allocation concealment (selection bias) | Low risk | Central automated random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported and missing data accounted |
| Selective reporting (reporting bias) | Low risk | prespecified variables reported |
| Other bias | High risk | Funded by Novartis |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ACR ‐ albumin:creatinine ratio; ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; ATN ‐ acute tubular necrosis; AR ‐ acute rejection; ARB ‐ angiotensin II receptor blocker; AUC ‐ area under the curve; AZA ‐ azathioprine; BMI ‐ body mass index; BP blood pressure; BPAR ‐ biopsy‐proven acute rejection; BPM ‐ beats per minute; C2 ‐ drug concentration 2 hours post ingestion; CAN ‐ chronic allograft nephropathy; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CrCl ‐ creatinine clearance; CsA ‐ cyclosporin A; CPA ‐ cyclophosphamide; DGF ‐ delayed graft function; ECG ‐ electrocardiogram; EC‐MPS ‐ encapsulated mycophenolate sodium; ESKD ‐ end‐stage kidney disease; EVL ‐ everolimus; FSGS ‐ focal segmental glomerulosclerosis; (e or m)GFR ‐ (estimated or measured) glomerular filtration rate; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; IL2RA ‐ interleukin 2 receptor antagonist; ITT ‐ intention‐to‐treat; M/F ‐ male/female; MMF ‐ mycophenolate mofetil; MPS ‐ mycophenolate sodium; MMF ‐ mycophenolate mofetil; MPA ‐ mycophenolic acid; mTOR‐I ‐ mammalian target of rapamycin inhibitors; NODAT ‐ new‐onset diabetes after transplantation; PRA ‐ panel reactive antibodies; PRED ‐ prednisone/prednisolone; PTLD ‐ post‐transplant lymphoproliferative disease; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SRL ‐ sirolimus; TAC ‐ tacrolimus; Treg ‐ regulatory T cells; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abouna 1991 | Wrong intervention: abrupt CNI withdrawal compared to slow withdrawal, no standard dose CNI comparison group |
| Alexander 2006 | Wrong intervention: determined if steroids can be eliminated with early discontinuation of CsA and later discontinuation of MMF |
| Alpay 2013 | Wrong outcomes: study outcomes were effects of switch from CNI to EVL on serum/urinary markers of fibrosis (TGF‐beta), inflammation, glomerular and tubular injury. the follow‐up data was at 3 months of conversion |
| Artz 2002 | Wrong intervention: conversion from CsA to TAC |
| Asberg 2013 | Wrong intervention: CNI withdrawal versus mycophenolate withdrawal |
| Baboolal 2003 | Wrong intervention: CsA elimination versus withdrawal, no standard dose |
| Baboolal 2004 | Wrong intervention: CsA elimination versus withdrawal, no standard dose |
| Baxter 1982 | Other: abstract more than 30 years old; no full text publication |
| Brady 1990 | Wrong intervention: low dose CNI pre‐operatively |
| Burkhalter 2012 | Wrong intervention: low dose CNI versus CNI withdrawal, no standard dose comparison |
| CAMPASIA Study 2005 | Wrong intervention: Intervention also included steroid withdrawal |
| Cattaneo 2005 | Wrong intervention: low dose SRL versus low dose CsA; part of a study to evaluate campath and MMF |
| Chapman 1985 | Wrong intervention: CsA withdrawal compared to avoidance, no continuation arm |
| CIS Trial 2014 | Wrong intervention: randomised to either trough CsA monitoring or by residual NFAT‐regulated gene expression |
| CONCERTO Study 2005 | Wrong intervention: evaluated C2 monitoring in the context of a quadruple immunosuppressive regimen and planned to assess the efficacy and safety of two C2 targets for patients 3 to 6 months post‐transplantation. However, because differences between the groups post‐month 2 were not discernible; the secondary endpoints were highlighted as combined outcome |
| David‐Neto 2001 | Wrong intervention: compared low versus high dose CsA in presence and absence of antibody induction |
| de Sandes Freitas 2011 | Wrong intervention: steroid withdrawal versus CNI withdrawal |
| de Sevaux 1998 | Wrong intervention: CNI + AZA converted to CNI + PRED |
| EVEREST Study 2009 | Wrong intervention: both arms included low dose CsA, no standard dose arm |
| Flechner 2004 | Wrong intervention: compared 2 doses of MMF |
| Fleming 2016 | Wrong intervention: compared mTOR‐I based CNI withdrawal with CNI minimisation, no standard dose CNI for comparison |
| Forwell 1986 | Wrong intervention: compared normal CsA dose with historical controls on azathioprine |
| Fries 1988 | Wrong intervention: CsA with AZA regimen was compared with CsA, antilymphocyte antibody and steroids |
| Fries 1988a | Wrong intervention: CsA with AZA regimen was compared with CsA, antilymphocyte antibody and steroids |
| Fruchaud 1996 | Wrong intervention: CNI based immunosuppression with and without antilymphocyte antibody compared |
| Gaber 2003 | Wrong intervention: compared CNI sparing with withdrawal |
| Gelens 2006 | Wrong intervention: TAC and SRL versus TAC and SRL versus SRL and MMF intervention |
| Ghafari 2007 | Wrong intervention: compared standard versus high dose which was tapered to standard dose at 3 months, not relevant to this review |
| Gotti 2003 | Wrong population: CNI versus steroid tapering based on biopsy |
| Griffin 1993 | Wrong intervention: Timing of CNI |
| Grino 1991 | Wrong intervention: compared 2 induction regimens (OKT3 and ALG) |
| Hamdy 2005 | Wrong intervention: did not compare low dose/withdrawal to standard dose regimen |
| Hariran 2015 | Other: conversion of TAC based regimen to SRL in DGF; 6/15 randomised patients shifted back to TAC |
| Henny 1986 | Wrong intervention: Study compared very high dose of CsA in the arm with high dose had CsA withdrawal |
| Hernandez 2007 | Wrong intervention: low dose CNI with MMF and normal dose with AZA |
| Hiesse 1991 | Wrong Intervention: multivariate analysis of various doses |
| Hilbrands 1993 | Wrong intervention: CNI versus steroid withdrawal |
| Hourmant 1987 | Wrong intervention: delayed introduction of CNI with monoclonal antibodies |
| Hricik 1990 | Wrong intervention: low dose CNI versus withdrawal, no standard dose comparison |
| Infante 2008 | Wrong intervention: compared withdrawal with low dose CNI, no standard comparison group |
| Jain 2001 | Wrong intervention: no standard CsA group comparison, both arms were low dose |
| Jindal 2002 | Wrong intervention: compared CNI elimination and withdrawal, no standard dose comparison |
| John 1999 | Wrong outcome: compared high and low dose CsA with single outcome (lipid profile) |
| Kamar 2012 | Wrong intervention: compared different doses of MMF |
| Kandaswamy 2005 | Wrong intervention: multiple comparisons not relevant to this review; compared CsA + MMF with high and low dose TAC with variable SRL |
| Keitel 1999 | Wrong intervention: compared early versus late CNI withdrawal, no standard dose comparison |
| Kovarik 2001 | Wrong intervention: early versus delayed CsA |
| Kovarik 2003 | Wrong intervention: compared early versus delayed introduction of CNI |
| Kovarik‐2306 2004 | Wrong intervention: did not include standard dose |
| Liu 2002a | Wrong intervention: compared CNI reduction versus withdrawal with mTOR‐I, no standard dose comparison |
| Liu 2007b | Wrong intervention: compared CNI reduction versus withdrawal with mTOR‐I, no standard dose comparison |
| Maiorano 2006 | Wrong intervention: compared CsA reduction with CsA withdrawal and SRL, no standard dose comparison |
| McGrath 2001 | Wrong intervention: CsA withdrawal and substitution with another CNI (TAC) versus mycophenolate |
| McMaster 1983 | Wrong intervention: CsA use alone in one arm |
| Meier 2006 | Wrong intervention: compared two different CNIs (TAC and CsA) |
| Messa 2009 | Wrong outcome: evaluated Treg changes between the SRL and TAC group, not relevant outcomes noted for this review |
| Metcalfe 2002 | Wrong intervention: compared MMF and AZA |
| Miserlis 2008 | Wrong intervention: variable co‐intervention (MMF and EVL) |
| Mourad 2004a | Wrong intervention: Simulect versus ATG |
| Mourad 2005 | Wrong intervention: early versus late introduction of CNI |
| Mourer 2012 | Wrong intervention: mycophenolate withdrawal versus CNI withdrawal |
| Noris 2007 | Wrong intervention: compared low dose SRL with low dose CNI does not satisfy inclusion criteria of this review |
| Novoa 2011 | Wrong intervention: standard dose CNI not part of comparison |
| OPTIMA‐TX Study 2008 | Wrong outcomes: reported CsA versus TAC, not relevant to this review |
| Pankewycz 2011 | Wrong intervention: primary intervention to study low dose ATG irrespective of maintenance immunosuppression and included low dose SRL and low dose TAC, no standard dose TAC for comparison |
| Ponticelli 1988 | Wrong intervention: double therapy compared to 3 drug CNI regimen |
| Rahamimov 2008 | Other: incomplete study, stopped prematurely |
| Ritz 1998 | Wrong intervention: CsA was withdrawn with ATG support and reintroduced within a week immediate transplant for ATN |
| Saunders 2003 | Wrong intervention: CNI dose reduction in both arms |
| SOCRATES Study 2014 | Wrong intervention: CNI withdrawal versus steroid withdrawal |
| Westhoff 1995 | Other: study discontinued before conclusion |
| Wu 2007d | Wrong population: randomisation only if GFR < 40 mL/min |
ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; ATN ‐ acute tubular necrosis; AZA ‐ azathioprine; CNI ‐ calcineurin inhibitor; CsA ‐ cyclosporin A; C2 ‐ drug dose levels 2 hours after ingestion; EVL ‐ everolimus; GFR ‐ glomerular filtration rate: MMF ‐ mycophenolate mofetil; mTOR ‐ mammalian target of rapamycin; NFAT ‐ nuclear factor of activated T‐cells; SRL ‐ sirolimus; TAC ‐ tacrolimus; TGF ‐ transforming growth factor; Treg ‐ regulatory T cells
Characteristics of ongoing studies [ordered by study ID]
David‐Neto 2014.
| Trial name or title | A randomized, prospective study comparing everolimus/low tacrolimus with regular tacrolimus/MPS for the elderly renal transplant recipients |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Both groups
|
| Outcomes |
|
| Starting date | 36 patients have been evaluated of the total 90 planned |
| Contact information | David‐Neto, E |
| Notes |
ERIC Study 2010.
| Trial name or title | An appraisal on the convenience of early everolimus introduction and calcineurin inhibitor withdrawal in Kidney recipients: THE ERIC STUDY |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | July 2010 |
| Contact information | JC Ruiz |
| Notes |
ISRCTN63298320.
| Trial name or title | A prospective randomised trial of the use of cellcept to allow early tacrolimus withdrawal in live donor kidney transplantation |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 01/01/2002 |
| Contact information | M Nicholson, University Hospitals of Leicester c/o Research and Development Office Leicester General Hospital NHS Trust LE1 4PW, Leicester, UK |
| Notes | Recruitment dates 1/1/2002 to 1/6/2003 ‐ not study results published by February 2017 |
TRANSFORM Study 2013.
| Trial name or title | Advancing renal TRANSplant eFficacy and safety Outcomes with an eveRolimus‐based regiMen (TRANSFORM) |
| Methods | Multicentre, open‐label RCT |
| Participants | Recipient of a primary (or secondary, if 1st graft is not lost due to immunological reasons) kidney transplant from a deceased heart beating, living‐unrelated, living‐related non‐HLA identical or an expanded criteria donor. Randomised within 24 h of completion of transplant surgery |
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | December 2013 |
| Contact information | Novartis Pharmaceuticals |
| Notes |
AR ‐ acute rejection; ATG ‐ antithymocyte globulin; BPAR ‐ biopsy‐proven acute rejection; CKD ‐ chronic kidney disease; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CsA ‐ cyclosporin A; DM ‐ diabetes mellitus; EVL ‐ everolimus; (e)GFR ‐ (estimated) glomerular filtration rate; MMF ‐ mycophenolate mofetil; MPS ‐ mycophenolate sodium; NODAT ‐ new onset diabetes after transplantation; PRED ‐ prednisone/prednisolone; RCT ‐ randomised controlled trial; TAC ‐ tacrolimus
Differences between protocol and review
Cochrane's risk of bias assessment tool has replaced the quality assessment checklist.
Contributions of authors
Writing of protocol and review: KK, GW, GT Screening of titles and abstracts: KK, GW Assessment for inclusion: KK, GW Quality assessment: KK, GW Data extraction: KK, GW Data entry into RevMan: KK, GW Data analysis: KK, GW Disagreement resolution: GT
Declarations of interest
Krishna M Karpe: none known
Girish S Talaulikar: none known
Giles Walters: none known.
New
References
References to studies included in this review
Abramowicz 2002 {published data only}
- Abramowicz D, Carmen Rial M, Vitko S, Castillo D, Manas D, Lao M, et al. Cyclosporine withdrawal from a mycophenolate mofetil‐containing immunosuppressive regimen: results of a five‐year, prospective, randomized study. Journal of the American Society of Nephrology 2005;16(7):2234‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abramowicz D, Manas D, Lao M, Vanrenterghem Y, Castillo D, Wijngaard P, et al. Cyclosporine withdrawal from a mycophenolate mofetil‐containing immunosuppressive regimen in stable kidney transplant recipients: a randomized, controlled study. Transplantation 2002;74(12):1725‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Castillo D, Abramowicz D, Manas D, Lao M, Vanrenterghem Y, Barker D, et al. Benefits of cyclosporine (CsA) withdrawal in stable renal transplant recipients, receiving mycophenolate mofetil (MMF) and steroids (S): a multicenter, randomised, controlled study [abstract no: A3674]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):726A. [CENTRAL: CN‐00550730] [Google Scholar]
Alsina 1987 {published data only}
- Alsina J, Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, et al. Low dose cyclosporine (CsA), ALG and steroids in first cadaveric renal transplants [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:606. [CENTRAL: CN‐00583779]
- Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, Andres E, et al. Low dose cyclosporine (CyA), ALG and steroids in first cadaveric renal transplants [abstract]. Kidney International 1988;34(2):301. [CENTRAL: CN‐00644138] [Google Scholar]
- Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, Andres E, et al. Low‐dose cyclosporin (CsA), ALG and steroids in first cadaveric renal transplants [abstract]. Nephrology Dialysis Transplantation 1988;3(1):99‐100. [Google Scholar]
Andres 2009 {published data only}
- Andres A, Marcen R, Sanchez J, Valdes F, Errasti P, Sola R, et al. Comparative study of three different patterns of immunosuppression based on induction with basiliximab and low initial doses or delayed initiation of neoral in kidney transplant recipients at high risk of delayed graft function [abstract no: 1629]. American Journal of Transplantation 2005;5(Suppl 11):570. [CENTRAL: CN‐00644164] [Google Scholar]
- Andres A, Marcen R, Valdes F, Plumed JS, Sola R, Errasti P, et al. A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function. Clinical Transplantation 2009;23(1):23‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marcen R, Andres A, Sanchez‐Plumed J, Valdes F, Errasti P, Sola R, et al. A randomized study to compare immunosuppression based on basiliximab induction and low initial doses or delayed neoral in kidney transplant recipients at high risk for delayed graft function [abstract no: PO‐266]. Transplant International 2005;18(Suppl 1):114. [Google Scholar]
APOLLO Study 2015 {published data only}
- Arns W, Rath T, Sommerer C, Reinke P, Haller H, Suwelack B, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 5 year data of the APOLLO trial [abstract]. Transplantation 2014;98(Suppl 1):145. [EMBASE: 71543988] [Google Scholar]
- Budde K, Rath T, Sommerer C, Haller H, Reinke P, Witzke O, et al. Renal, efficacy and safety outcomes following late conversion of kidney transplant patients from calcineurin inhibitor therapy to everolimus: the randomized APOLLO study. Clinical Nephrology 2015;83(1):11‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Sommerer C, Haller H, Suwelack B, May C, Paulus E, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 3 year data of the APOLLO trial [abstract no: 928]. American Journal of Transplantation 2012;12(Suppl S3):298. [EMBASE: 70746881] [Google Scholar]
- Budde K, Sommerer C, Rath T, Reinke P, Haller H, Witzke O, et al. Renal function to 5 years after late conversion of kidney transplant patients to everolimus: a randomized trial. Journal of Nephrology 2015;28(1):115‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Sommerer C, Reinke P, Haller H, Arns W, Witzke O, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 4 year data of the APOLLO Trial [abstract no: B396]. American Journal of Transplantation 2013;13(Suppl S5):311‐2. [EMBASE: 71057512] [Google Scholar]
- Budde K, Zeier M, Haller H, Arns W, Kramer S, Vogel EM, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients [abstract no: 1639]. American Journal of Transplantation 2010;10(Suppl 4):504. [EMBASE: 70465014] [Google Scholar]
- Haller H, Rath T, Zeier M, Arns W, Kramer S, Vogel E, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients [abstract no: Sa645]. NDT Plus 2010;3(Suppl 3):iii259. [EMBASE: 70484111] [Google Scholar]
- Rath T, Budde K, Sommerer C, Haller H, Suwelack B, May C, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 3 year data of the APOLLO trial [abstract no: 1684]. Transplantation 2012;94:994. [EMBASE: 71251767] [Google Scholar]
- Rath T, Sommerer C, Suwelack B, Arns W, Haller H, Reinke P, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 5 year data of the APOLLO trial [abstract]. Transplantation International 2014;98:50. [EMBASE: 71664130] [Google Scholar]
- Reinke P, Haller H, Rath T, Arns W, Paulus E, Scheidf S, et al. Two year data of the APOLLO trial: Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients [abstract no: P141]. Transplant International 2011;24:50. [EMBASE: 70537441] [Google Scholar]
- Rhat T, Sommerer C, Haller H, Reinke P, Witzke O, Suwelack B, et al. Outcome on renal function of everolimus conversion in maintenance KTX patients: 4 years APOLLO trial [abstract no: P271]. Transplant International 2013;26(Suppl 2):240. [EMBASE: 71359891] [Google Scholar]
- Sommerer C, Rath T, Budde K, Haller H, Arns W, Scheidl S, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 2 year follow‐up data of the APOLLO trial [abstract no: RO‐185]. Transplant International 2011;24(Suppl 2):180. [EMBASE: 70527714] [Google Scholar]
- Sommerer C, Rath T, Haller H, Arns W, Suwelack B, Reinke P, et al. 4 year data of the APOLLO trial: outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients [abstract no: V79]. Transplant International 2013;26(Suppl 1):21. [EMBASE: 71356263] [Google Scholar]
Asberg 2006 {published data only}
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR‐matched de novo kidney transplant recipients. Transplantation 2006;82(1):62‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor‐avoidance with daclizumab, mycophenolate mofetil and prednisolone in DR matched de novo kidney transplant recipients [abstract no: SP737]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv264. [DOI] [PubMed] [Google Scholar]
- Asberg A, Midtvedt K, Voytovich MH, Line PD, Narverud J, Reisaeter AV, et al. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation. Clinical Transplantation 2009;23(4):511‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asberg A, Midtvedt K, Voytovich MH, Line PD, Narverud J, Reisaeter AV, et al. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation [abstract no: 529]. American Journal of Transplantation 2008;8(Suppl 2):319. [DOI] [PubMed] [Google Scholar]
ASCERTAIN Study 2011 {published data only}
- Holdaas H, Midtvedt K, Seron D, O'Connell P, Thiagarajan CM, Fassett R, et al. Everolimus in maintenance renal transplant recipients: the ASCERTAIN Study [abstract no: P‐309]. Transplant International 2009;22(Suppl 2):172. [Google Scholar]
- Holdaas H, Midtvedt K, Seron D, O'Connell P, Thiagrajan C, Fassett R, et al. Assessment of everolimus in maintenance renal transplant recipients: the Ascertain Study [abstract no: 1646]. Transplantation 2008;86(Suppl 2):546. [CENTRAL: CN‐00740482] [Google Scholar]
- Holdaas H, Rostaing L, Midtvedt K, Seron D, Cole E, Chapman J, Fellstrom B, et al. Conversion of long‐term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24‐month study.[Erratum appears in Transplantation. 2011 Nov 27;92(10):e61 Note: multiple investigator names added.]. Transplantation 2011;92(4):410‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Long‐term post transplantation switch to an everolimus‐based therapy with CNI elimination/minimization does not overall impact graft function: the ASCERTAIN study [abstract no: 8]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:42.
- O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Post‐hoc analysis of the ASCERTAIN trial: everolimus based therapy with CNI elimination improves renal function in select populations [abstract no: 7]. Transplantation Society of Australia & New Zealand (TSANZ).29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:41.
Baczkowska 2003 {published data only}
- Baczkowska T, Durlik M, Perkowska A, Sadowska A, Cieciura T, Nowacka‐Cieciura E, et al. Cytokines and growth factors serum level and renal allograft function (preliminary report) [Cytokiny i czynniki wzrostu w surowicy u biorcow nerki allogenicznej a losy przeszczepu (doniesienie wstepne)]. Polski Merkuriusz Lekarski 2003;15(88):356‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska A, Cieciura T, Wierzbicki P, Klosowka D, Matlosz B, et al. Daclizumab allows for a protocol with low‐dose cyclosporine in low rejection‐risk kidney recipients ‐ preliminary data [abstract no: T435]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):309. [CENTRAL: CN‐00400168] [Google Scholar]
- Baczkowska T, Perkowska‐Francka A, Durlik M, Cieciura T, Nowacka‐Cieciura E, Pazik J, et al. The role of the protocol biopsies in renal allograft recipients. Transplantation Proceedings 2003;35(6):2179‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Cieciura T, Pazik J, Nowacka‐Cieciura E, Lewandowski Z, et al. Untreated subclinical, borderline rejection in 12 and 36‐month protocol biopsies is not associated with progressive loss of GFR at 5‐year's follow‐up [abstract no: 1755]. Transplantation 2008;86(2 Suppl):581. [CENTRAL: CN‐00671803] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, Pazik J, et al. Serum TGF‐b1 correlates with chronic histopathological lesions in protocol biopsies in kidney allograft recipients [abstract no: P354]. Transplantation 2004;78(2 Suppl):319. [CENTRAL: CN‐00583398] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Lewandowski Z, Nowacka‐Cieciura E, Pazik J, Cieciura T, et al. Increased renal TFG‐b protein expression in human allograft specimens correlates with renal allograft function and chronic histopathological changes [abstract no: SaP437]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi382. [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Pazik J, Cieciura T, Nowacka‐Cieciura E, Lewandowski Z, et al. The role of the protocol biopsies in renal allograft recipients: five‐years' follow‐up [abstract no: P093]. Transplant International 2007;20(Suppl 2):118. [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Pazik J, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, et al. The role of the protocol biopsies in renal allograft recipients: three‐years' follow‐up [abstract no: SP736]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00602089] [Google Scholar]
- Baczkowska T, Perkowska‐Ptasinska A, Sadowska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, et al. Serum TGF‐beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplantation Proceedings 2005;37(2):773‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Sadowska A, Perkowska‐Ptasinska A, Lewandowski Z, Cieciura T, Pazik J, et al. Optimal mycophenolic acid and mycophenolic acid glucuronide levels at the early period after kidney transplantation are the key contributors to improving long‐term outcomes. Transplantation Proceedings 2009;41(8):3019‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baczkowska T, Wierzbicki P, Perkowska‐Ptasinska A, Klosowska D, Nowaczyk M, Kowalska GK, et al. Pharmacodynamic monitoring in kidney allograft recipients with and without calcineurin inhibitors [abstract no: P‐593]. Transplant International 2009;22(Suppl 2):242. [Google Scholar]
Bansal 2013 {published data only}
- Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V. Deferred pre‐emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of t‐regulatory cell population: a randomized, controlled trial. PLoS ONE [Electronic Resource] 2013;8(10):e75591. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barsoum 2007 {published data only}
- Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo Kidney Center protocol for rapamycin‐based sequential immunosuppression in kidney transplant recipients: 2‐year outcomes. Experimental & Clinical Transplantation 2007;5(2):649‐57. [MEDLINE: ] [PubMed] [Google Scholar]
Bechstein‐193 2013 {published data only}
- Bechstein W, Paczek L. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415252] [Google Scholar]
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus + steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00444369] [Google Scholar]
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, Zygmunt AJ, European Rapamune Tacrolimus Study Group. A comparative, randomized trial of concentration‐controlled sirolimus combined with reduced‐dose tacrolimus or standard‐dose tacrolimus in renal allograft recipients. Transplantation Proceedings 2013;45(6):2133‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paczek L, Bechstein W, Wramner L, Squifflet JP. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002.
- Paczek L, Bechstein WO, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus +steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):464. [Google Scholar]
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351] [Google Scholar]
Bertoni 2007 {published data only}
- Bertoni E, Becherelli P, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolate acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. A monocentric experience [abstract no: F‐PO614]. Journal of the American Society of Nephrology 2007;18(Abstracts):234A. [CENTRAL: CN‐00716078] [Google Scholar]
- Rosati A, Bertoni E, Rosso G, Larti A, Mehmetaj A, Salvadori M. Triple therapy with Neoral, steroids and enteric coated mycophenolic acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. A monocentric experience [abstract no: SaP477]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi396. [CENTRAL: CN‐00725011] [Google Scholar]
Bertoni 2011 {published data only}
- Bertoni E, Larti A, Farsetti S, Rosso G, Maria L, Zanazzi M. Cyclosporine (CyA) very low dose with everolimus (E) high dose is associated with better outcomes in renal transplant patients with respect to standard treatment with EC‐MPS (M) [abstract]. Transplant International 2009;22(Suppl 2):91. [Google Scholar]
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. Journal of Nephrology 2011;24(5):613‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Budde 2007 {published data only}
- Budde K, Bosmans J, Zeier M, Sennesael J, Hopt U, Fischer WH, et al. Safety and efficacy of reduced or full dose of cyclosporine (neoral®) in combination with mycophenolate sodium (Myfortic®), basiliximab (Simulect®), and steroids in de novo kidney transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):83. [CENTRAL: CN‐00527096] [Google Scholar]
- Budde K, Bosmans JL, Sennesael J, Zeier M, Hopt U, Fischer W, et al. Reduced cyclosporine exposure is safe and efficacious in combination with basiliximab, enteric‐coated mycophenolate‐sodium, and steroids [abstract no: 1195]. American Journal of Transplantation 2005;5(Suppl 11):461. [CENTRAL: CN‐00644165] [Google Scholar]
- Budde K, Bosmans JL, Sennesael J, Zeier M, Pisarski P, Schutz M, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab. Clinical Nephrology 2007;67(3):164‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Zeier M, Bosmans JL, Sennesael J, Glander P, Fischer W, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab [abstract no: F‐PO1088]. Journal of the American Society of Nephrology 2006;17(Abstracts):565A. [CENTRAL: CN‐00644166] [DOI] [PubMed] [Google Scholar]
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms Study Group. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464. [CENTRAL: CN‐00602063] [Google Scholar]
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre CH, Rostaing L, Kirchherr B, MyProms Study Group. Tolerability of enteric coated mycophenolate sodium (EC‐MPS) in combination with neoral and steroids in de novo kidney transplant recipients: a 12 months prospective trial [abstract no: 1209]. American Journal of Transplantation 2005;5(Suppl 11):464‐5. [CENTRAL: CN‐00602066] [Google Scholar]
- Pietruck F, Budde K, Salvadori M, Sollinger H, Bourbigot B, Gentil MA, et al. Efficacy and safety of enteric‐coated mycophenolate sodium in renal transplant patients with diabetes mellitus: post hoc analyses from three clinical trials. Clinical Transplantation 2007;21(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Legendre CH, Cohen D, Budde K, Zeier M, Kirchherr B, et al. Safety and tolerability of enteric coated mycophenolate sodium in combination with neoral and steroids in de novo kidney transplant recipients: 12 months analysis of a prospective trial [abstract no: T‐PO50030]. Nephrology 2005;10(Suppl 1):A215. [CENTRAL: CN‐00644301] [Google Scholar]
CAESAR Study 2007 {published data only}
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, CAESAR Study Group. Low‐dose cyclosporine in conjunction with daclizumab, mycophenolate mofetil and corticosteroids is safe and effective in contrast to early cyclosporine withdrawal [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550672]
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Calleja E, et al. The use of daclizumab and mycophenolate mofetil in combination with corticosteroids and cyclosporine (low dose versus low dose followed by withdrawal) to optimize renal function in recipients of renal allografts [abstract]. Transplantation 2004;78(2 Suppl):458. [CENTRAL: CN‐00509171] [Google Scholar]
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. American Journal of Transplantation 2007;7(3):560‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Spleiss O, et al. Association of three polymorphisms with acute rejection after kidney transplantation: an exploratory pharmacogenetic analysis of a randomized multicenter clinical trial (the CAESAR study) [abstract no: 1020]. American Journal of Transplantation 2006;6(Suppl 2):410. [CENTRAL: CN‐00678972] [Google Scholar]
- Kuypers DR, Ekberg H, Grinyo J, Nashan B, Vincenti F, Snell P, et al. Mycophenolic acid exposure after administration of mycophenolate mofetil in the presence and absence of cyclosporin in renal transplant recipients. Clinical Pharmacokinetics 2009;48(5):329‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Vanrenterghem Y, Nashan B, Grinyo J, Ekberg H, Nasmyth‐Miller C, et al. The use of mycophenolate mofetil, daclizumab and corticosteroids with cyclosporine (low dose, low dose/withdrawal and standard dose) to optimize renal function in renal allograft recipients ‐ 18 month results [abstract no: 1507]. American Journal of Transplantation 2005;5(Suppl 11):539. [CENTRAL: CN‐00644286] [Google Scholar]
Cai 2014 {published data only}
- Cai L, Zeng F, Liu B, Wei L, Chen Z, Jiang J. A single‐centre, open‐label, prospective study of an initially short‐term intensified dosing regimen of enteric‐coated mycophenolate sodium with reduced cyclosporine A exposure in Chinese live‐donor kidney transplant recipients. International Journal of Clinical Practice. Supplement 2014;68(181):23‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CALFREE Study 2010 {published data only}
- Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus‐ and cyclosporine‐based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. American Journal of Kidney Diseases 2010;55(2):335‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Giannini O, Dickenmann M, Kim MJ, Franz S, Mayr M, Mihatsch MJ, et al. The CALFREE Study ‐ an open, prospective, randomized single center study to investigate calcineurin free immunosuppression in 100 de novo, normal risk renal transplant recipients: preliminary results [abstract no: P04.02]. Kidney & Blood Pressure Research 2004;27(5‐6):329. [CENTRAL: CN‐00615861] [Google Scholar]
CENTRAL Study 2012 {published data only}
- Mjornstedt L, Schwartz Sorensen S, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transplant International 2015;28(1):42‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mjornstedt L, Sorensen SS, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. American Journal of Transplantation 2012;12(10):2744‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murbraech K, Holdaas H, Massey R, Undset LH, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients: An echocardiographic substudy of the randomized controlled CENTRAL trial. Transplantation 2014;97(2):184‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murbraech K, Massey R, Undset LH, Midtvedt K, Holdaas H, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients ‐ a three‐yr serial echocardiographic substudy of the randomized controlled CENTRAL trial. Clinical Transplantation 2015;29(8):678‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CERTITEM Study 2015 {published data only}
- Hertig A, Kamar N, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract]. Transplantation 2014;98:81. [EMBASE: 71543793] [Google Scholar]
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: O5]. Transplant International 2013;26(Suppl 3):2. [EMBASE: 71356080] [Google Scholar]
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Ligny BH, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: P081]. Transplant International 2013;26(Suppl 2):201. [EMBASE: 71359712] [Google Scholar]
- Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Fibrosis progression according to epithelial‐mesenchymal transition profile: a randomized trial of everolimus versus CsA. American Journal of Transplantation 2015;15(5):1303‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chadban 2013 {published data only}
- Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one‐year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric‐coated mycophenolate sodium (EC‐MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA‐ME), basiliximab and steroids in de novo kidney transplantation [abstract no: 32]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand (TSANZ); 2006 Mar 29‐31; Canberra, Australia. 2006:51. [CENTRAL: CN‐00583470]
- Chadban S, Eris J, Russ G, Campbell S, Chapman J, Pussell B, et al. Enteric‐coated mycophenolate sodium in combination with full dose or reduced dose cyclosporine, basiliximab and corticosteroids in Australian de novo kidney transplant patients. Nephrology 2013;18(1):63‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2008 {published data only}
- Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85(6):821‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Everolimus (RAD001) concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients [abstract no: SA‐PO2529]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):678A. [CENTRAL: CN‐00716072] [Google Scholar]
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Optimal everolimus concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients. Transplantation 2010;90(1):31‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Asderakis A, Chan L. Renal function with enteric‐coated mycophenolate sodium in combination with reduced and standard tacrolimus levels: results of a 6‐month study in de novo renal transplant recipients [abstract no: O111]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
- Chan L, Andres A, Bunnapradist S, Gugliuzza K, Parasuraman R, Peddi VR, et al. Renal function and NODM in de novo renal transplant recipients treated with standard and reduced levels of tacrolimus in combination with EC‐MPS. Journal of Transplantation 2012;2012:941640. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L, Hart M, Andres A, Gugliuzza K, Bunnapradist S, Parasumaran R. Renal function and incidence of NODM with enteric‐coated mycophenolate sodium in combination with reduced and standard tacrolimus levels: results of a 6‐month comparative study in de novo renal transplant recipients [abstract no: 718]. Transplantation 2008;86(2S):251. [Google Scholar]
- Nicholson ML, Chan L. Incidence of new onset diabetes mellitus in de novo renal transplant recipients treated with enteric‐coated mycophenolate sodium in combination with reduced or standard tacrolimus target levels: results of a 6‐month randomized study [abstract no: P266]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
Chhabra 2013 {published data only}
- Alvarado A, Chhabra D, Wang E, Najafian N, Friedewald J, Ho B, et al. Prospective randomized study to evaluate the feasability of CNI elimination with conversion to sirolimus in prednisone‐free immunosuppressive regimen [abstract no: 53]. American Journal of Transplantation 2012;12(Suppl S3):42. [EMBASE: 70746000] [Google Scholar]
- Alvarado A, Shetty A, Traitanon O, Leventhal J, Mas V, Chhabra D, et al. Calcineurin‐inhibitor conversion to mTOR inhibitor in renal transplant recipients leads to worse long term clinical outcomes [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953109] [Google Scholar]
- Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento‐Reodica N, et al. Impact of calcineurin‐inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone‐free regimen. American Journal of Transplantation 2013;13(11):2902‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallon L, Traitanon O, Sustento‐Reodica N, Leventhal J, Ansari MJ, Gehrau RC, et al. Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus. Kidney International 2014;87(4):828‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah G, Xu L, Dalal P, Chhabran D, Friedewald J, Ho B, et al. Conversion from CNI to SRL in a pred‐free immunosuppressive regimen: interim report of a prospective randomized study [abstract no: 1641]. American Journal of Transplantation 2010;10(Suppl 4):504. [Google Scholar]
Cibrik 2007 {published data only}
- Bresnahan B, Cibrik D, Jensik S, Whelchel J, Klintmalm G, Cohen D, et al. Treatment of high‐risk renal transplant recipients with EC‐MPS (Myfortic®) is safe and efficacious [abstract no: PUB216]. Journal of the American Society of Nephrology 2005;16:829A. [CENTRAL: CN‐00644277] [Google Scholar]
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms Study Group. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464. [CENTRAL: CN‐00602063] [Google Scholar]
- Cibrik D, Jensik S, Bresnahan B, Whelchel J, Klintmalm G, ERL2405‐US01 Study Group. Safety and efficacy of EC‐MPS in combination with simulect and neoral in de novo renal transplant high‐risk recipients [abstract no: 135]. American Journal of Transplantation 2005;5(Suppl 11):190. [CENTRAL: CN‐00644170] [Google Scholar]
- Cibrik D, Jensik S, Meier‐Kriesche H, Bresnahan B, Lieberman B, Myfortic US01 Renal Transplant Group. Enteric‐coated mycophenolate sodium in combination with optimized neoral dosing, basiliximab, and steroids results in good efficacy and renal function in renal transplant recipients in the first six months [abstract no: 220]. American Journal of Transplantation 2004;4(Suppl 8):218. [CENTRAL: CN‐00644278] [Google Scholar]
- Cibrik D, Meier‐Kriesche HU, Bresnahan B, Wu YM, Klintmalm G, Kew CE, et al. Renal function with cyclosporine C2 monitoring, enteric‐coated mycophenolate sodium and basiliximab: a 12‐month randomized trial in renal transplant recipients. Clinical Transplantation 2007;21(2):192‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre CH, Rostaing L, Kirchherr B, MyProms Study Group. Tolerability of enteric coated mycophenolate sodium (EC‐MPS) in combination with neoral and steroids in de novo kidney transplant recipients: a 12 months prospective trial [abstract no: 1209]. American Journal of Transplantation 2005;5(Suppl 11):464‐5. [CENTRAL: CN‐00602066] [Google Scholar]
- Meier‐Kriesche H, Cibrik D, Bresnahan B, Cohen D, Lieberman B. Optimized Neoral C2 monitoring in combination with enteric‐coated mycophenolic acid, basiliximab and steroids is effective, safe and tolerable: 12‐month results of a multicenter, randomized, prospective trial [abstract no: F‐PO1068]. Journal of the American Society of Nephrology 2004;15(Oct):299A. [CENTRAL: CN‐00583408] [Google Scholar]
- Pietruck F, Budde K, Salvadori M, Sollinger H, Bourbigot B, Gentil MA, et al. Efficacy and safety of enteric‐coated mycophenolate sodium in renal transplant patients with diabetes mellitus: post hoc analyses from three clinical trials. Clinical Transplantation 2007;21(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Legendre CH, Cohen D, Budde K, Zeier M, Kirchherr B, et al. Safety and tolerability of enteric coated mycophenolate sodium in combination with neoral and steroids in de novo kidney transplant recipients: 12 months analysis of a prospective trial [abstract no: T‐PO50030]. Nephrology 2005;10(Suppl 1):A215. [CENTRAL: CN‐00644301] [Google Scholar]
Cockfield 2002 {published data only}
- Cockfield S, Whelchel J, MacDonald A. An open‐label, concentration‐controlled, randomized, 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 1021]. American Journal of Transplantation 2002;2(Suppl 3):395. [MEDLINE: ] [Google Scholar]
- Daloze P, Whelchel J, Cockfield S, MacDonald A. A 6‐month multicenter randomized concentration‐controlled study of reduced‐dose tacrolimus (TAC)+sirolimus(SRL)+corticosteroids(CS) compared to standard‐dose TAC+SRL+CS in clinical kidney transplantation [abstract no: 0555]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415490]
CONCEPT Study 2009 {published data only}
- Chun DX, Alexandre H, Sandrine GS, Olivier T, Isabelle E, Christophe L, et al. The phenotype of tubular epithelial cells does not recover after a conversion from cyclosporine A to siroliumus [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii517. [EMBASE: 70766851] [Google Scholar]
- Joannides R, Monteil C, Ligny BH, Westeel PF, Iacob M, Thervet E, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. American Journal of Transplantation 2011;11(11):2414‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Etienne I, Touchard G, Thervet E, Westell P, Toupance O, et al. Comparison of efficacy and safety of cyclosporine (CsA) discontinuation with introduction of sirolimus (SRL) at week 12 to standard strategy in renal transplant recipients receiving mycophenolate mofetil (MMF) [abstract no: 53]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00724873] [Google Scholar]
- Lebranchu Y, Thierry A, Thervet E, Buchler M, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF‐four‐year results of the Postconcept study. American Journal of Transplantation 2011;11(8):1665‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: Concept study. American Journal of Transplantation 2009;9(5):1115‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Mazouz H, et al. Impact on renal function of early conversion at 3 months from cyclosporine (CSA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) in kidney transplantation: 30 months follow up of a multicenter randomized controlled trial: The CONCEPT Study [abstract no: Sa706]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00765353]
- Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Mazouz H, et al. Impact on renal function of early conversion at 3 months from cyclosporine (CsA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) in kidney transplantation: 30‐months follow up of a multicenter randomized controlled trial: the Concept Study [abstract no: 241]. American Journal of Transplantation 2009;9(Suppl 2):260. [EMBASE: 70010114] [Google Scholar]
- Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Westeel PF, et al. Impact of early conversion at 3 months from cyclosporine (CSA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) on renal function ‐ "results at 48 months of follow up of a multicenter randomized controlled trial: The Concept Study" [abstract no: 376]. American Journal of Transplantation 2010;10(Suppl 4):151. [EMBASE: 70463737] [Google Scholar]
- Servais A, Meas‐Yedid V, Lebranchu Y, Etienne I, Touchard G, Legendre C, et al. Comparison at one year of interstitial fibrosis (IF) by automatic quantification in renal transplant recipients with cyclosporine (CsA) discontinuation and sirolimus (SRL) introduction [abstract no: 527]. American Journal of Transplantation 2008;8(Suppl 2):319. [CENTRAL: CN‐00716068] [Google Scholar]
- Servais A, Meas‐Yedid V, Lebranchu Y, Etienne I, Touchard G, Westeel PF, et al. Comparison of interstitial fibrosis (IF) by automatic quantification at one year in renal transplant recipients with cyclosporine (CsA) discontinuation and sirolimus (SRL) introduction [abstract no: TH‐FC040]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):10A. [CENTRAL: CN‐00716074] [Google Scholar]
- Servais A, Meas‐Yedid V, Toupance O, Lebranchu Y, Thierry A, Moulin B, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. American Journal of Transplantation 2009;9(11):2552‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Servais A, Meas‐Yedid V, Toupance O, Lebranchu Y, Touchard G, Westeel PF, et al. Impact of interstitial fibrosis (IF) by automatic quantification at one year on the evolution of renal function in transplant recipients with cyclosporine discontinuation and sirolimus introduction [abstract no: 1103]. American Journal of Transplantation 2009;9(Suppl 2):502. [CENTRAL: CN‐00775138] [Google Scholar]
- Thervet E, Servais A, Meas‐Yedid V, Lebranchu Y, Etienne I, Touchard G, et al. Comparison at one year of interstitial fibrosis (IF) by automatic quantification in renal transplant recipients (RTR) with cyclosporine (CSA) discontinuation and sirolimus (SRL) introduction [abstract no: 450]. Transplantation 2008;86(Suppl 2):159. [CENTRAL: CN‐00740483] [Google Scholar]
- Thierry A, Lebranchu Y, Toupance O, Westeel PF, Etienne E, Thervet E, et al. Impact of inflammatory subclinical lesions (ISCL) at M12 on renal function at M48: results from Post‐Concept Study [abstract no: 1648]. American Journal of Transplantation 2010;10(Suppl 4):506. [Google Scholar]
- Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long‐term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. American Journal of Transplantation 2011;11(10):2153‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vuiblet V, Birembaut P, Francois A, Cordonnier C, Noel LH, Goujon JM, et al. Sirolimus‐based regimen is associated with decreased expression of glomerular vascular endothelial growth factor. Nephrology Dialysis Transplantation 2012;27(1):411‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vuiblet V, Birembaut P, Francois A, Jaureguy M, Thierry A, Thervet E, et al. Sirolimus‐based regimen is associated with decreased expression of vascular endothelial growth factor in kidneys: ancillary study of the CONCEPT Study [abstract no: 1652]. American Journal of Transplantation 2010;10(Suppl 4):507. [Google Scholar]
- Xu‐Dubois YC, Hertig A, Lebranchu Y, Hurault de Ligny B, Thervet E, Jaureguy M, et al. Progression of pulse pressure in kidney recipients durably exposed to CsA is a risk factor for epithelial phenotypic changes: an ancillary study of the CONCEPT trial. Transplant International 2014;27(4):344‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Xu‐Dubois YC, Lebranchu Y, Hurault de Ligny B, Thervet E, Mazouz H, Lepogamp P, et al. Conversion from cyclosporine to sirolimus at M3 after renal transplantation does not reduce the score of epithelial to mesenchymal transition at M12: ancillary study of the CONCEPT Study [abstract no: 1664]. American Journal of Transplantation 2010;10(Suppl 4):510. [Google Scholar]
CONVERT Trial 2009 {published data only}
- Alberu J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus‐based, calcineurin inhibitor‐free immunotherapy: 24‐month results from the CONVERT trial. Transplantation 2011;92(3):303‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Alberu J, Schena FP, Wali R, Pascoe M, Carmen Rial M, The Sirolimus CONVERT Trial Study Group. Lower malignancy rates in renal allograft recipients converted to sirolimus (SRL)‐based, calcineurin inhibitor‐free immunotherapy: 24 month results from the CONVERT trial [abstract no: TH‐FC082]. Journal of the American Society of Nephrology 2006;17(Abstracts):18A. [CENTRAL: CN‐00601954] [Google Scholar]
- Brennan D, Schena FP, Wali R, The Sirolimus CONVERT Trial Study Group. Factors contributing to the development of proteinuria after conversion from calcineurin inhibitors to sirolimus: results from the multicenter CONVERT trial [abstract no: TH‐FC083]. Journal of the American Society of Nephrology 2006;17(Abstracts):18A. [CENTRAL: CN‐00601955] [Google Scholar]
- Chapman J, Campistol JM, Grinyo JM, Morales JM, Mota A, Schena FP. Results from a long‐term extension study in renal allograft recipients show that sirolimus‐based therapy without cyclosporine is a safe alternative to sirolimus plus cyclosporine therapy. [abstract no: F‐PO1050]. Journal of the American Society of Nephrology 2004;15(Oct):295A. [CENTRAL: CN‐00583164] [Google Scholar]
- Oberbauer R, Schena FP, Wali R, Pascoe M, Alberu J, Carmen Rial M, et al. Conversion from calcineurin inhibitors to sirolimus compared with continued use of calcineurin inhibitors in renal allograft recipients: 24‐month safety and efficacy results from the CONVERT trial [abstract no: F‐FC154]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601952] [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Brown F, et al. A randomised, open‐label, comparative evaluation of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients [abstract no: PS71]. Nephrology 2005;10(Suppl 3):A397. [CENTRAL: CN‐00644366] [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Conversion from calcineurin inhibitors to sirolimus compared with continued use of calcineurin inhibitors in renal allograft recipients: 24‐month safety and efficacy results from the CONVERT trial study group [abstract]. Immunology & Cell Biology 2007;85(4):A27‐8. [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: 18‐month efficacy and safety results from a large, randomized, open‐label, comparative trial [abstract no: 106]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:91. [CENTRAL: CN‐00644368]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Efficacy and safety of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: results of the CONVERT trial [abstract no: 2006]. Nephrology 2006;11(Suppl 2):A35. [CENTRAL: CN‐00583835] [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Factors contributing to the development of urinary protein excretion after conversion from calcineurin inhibitors to sirolimus: results from the multicenter CONVERT trial [abstract]. Immunology & Cell Biology 2007;85(4):A21. [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Reduced incidence of malignancy in renal transplant recipients converted from calcineurin inhibitors (CNIS) to sirolimus (SRL) compared with those who continued CNI therapy [abstract no: 1582]. Nephrology 2006;11(Suppl 2):A24. [CENTRAL: CN‐00583837] [Google Scholar]
- Pussell B, Russ G, Walker R, Campbell S, O'Connell P, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus‐based, calcineurin inhibitor‐free immunotherapy: 24 month results from the CONVERT Trial [abstract]. Immunology & Cell Biology 2007;85(4):A20. [Google Scholar]
- Pussell B, Russ G, Walker R, O'Connell P, Kanellis J. Determinants of proteinuria after conversion from a calcineurin inhibitor to sirolimus‐based immunosuppressive regimen: results from the CONVERT Trial [abstract no: 1572]. Nephrology 2006;11(Suppl 2):A21. [CENTRAL: CN‐00583834] [Google Scholar]
- Russ G, Pussell B, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Analysis of the incidence of anaemia and its treatment after sirolimus conversion versus calcineurin inhibitor continuation in renal allograft recipients: 2‐year results from the ongoing CONVERT trial [abstract]. Immunology & Cell Biology 2007;85(4):A20. [Google Scholar]
- Russ G, Schena FP, Oberbauer R, The Sirolimus CONVERT Trial Study Group. Incidence and treatment of anemia after conversion to sirolimus vs. continuation of calcineurin inhibitors in renal allograft recipients: 2‐year results from the CONVERT Trial [abstract no: TH‐FC081]. Journal of the American Society of Nephrology 2006;17(Abstracts):18A. [CENTRAL: CN‐00601953] [Google Scholar]
- Schena FP, Pascoe MD, Alberu J, Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24‐month efficacy and safety results from the CONVERT trial. Transplantation 2009;87(2):233‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schena FP, Wali RK, Pascoe MD, Alberu J, Carmen Rial M. A randomized, open‐label, comparative evaluation of the safety and efficacy of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients [abstract no: PO‐456]. Transplant International 2005;18(Suppl 1). [Google Scholar]
- Schena FP, Wali RK, Pascoe MD, Alberu J, Carmen Rial M, The Sirolimus CONVERT Trial Study Group. Efficacy and safety of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: 12‐month results from a large, randomized, open‐label, comparative trial [abstract no: TH‐FC155]. Journal of the American Society of Nephrology 2005;16:33A. [CENTRAL: CN‐00601956] [Google Scholar]
- Schena FP, Wali RK, Pascoe MD, Alberu J, Carmen Rial M, The Sirolimus Renal Conversion Trial Study Group. A randomized, open‐label, comparative evaluation of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients [abstract no: 1008]. American Journal of Transplantation 2005;5(Suppl 11):413. [CENTRAL: CN‐00689212] [Google Scholar]
- Schena FP, Wali RK, Pascoe MD, Alberu J, Carmen Rial M, The Sirolimus Renal Conversion Trial Study Group. Conversion to sirolimus in kidney transplant patients receiving maintenance calcineurin inhibitor immunosuppression: a randomized, open‐label, comparative clinical trial [abstract no: SP440]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v165. [CENTRAL: CN‐00689740] [Google Scholar]
- Steinberg S, Schena FP, Wali R, The Sirolimus CONVERT Trial Study Group. Multivariate and univariate analyses of factors influencing renal allograft function after conversion to sirolimus (SRL)‐based immunotherapy from calcineurin inhibitors (CI) [abstract no: TH‐PO582]. Journal of the American Society of Nephrology 2006;17(Abstracts):231A. [CENTRAL: CN‐00601958] [Google Scholar]
- Vathsala A, Schena F, Wali RK, Pascoe MD, Alberu J, Carmen Rial M, et al. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: a randomized, open‐label, comparative trial [abstract no: T‐PO50036]. Nephrology 2005;10(Suppl 1):A217. [CENTRAL: CN‐00601967] [Google Scholar]
CTOT‐09 Study 2015 {published data only}
- Formica R, Nickerson P, Poggio E, Tinckam K, Rush D, Gibson I, et al. Immune monitoring and tacrolimus (TAC) withdrawal in low risk recipients of kidney transplants‐results of CTOT09 [abstract]. Transplantation 2014;98(Suppl 1):225. [EMBASE: 71544207] [Google Scholar]
- Hricik DE, Formica RN, Nickerson P, Rush D, Fairchild RL, Poggio ED, et al. Adverse outcomes of tacrolimus withdrawal in immune‐quiescent kidney transplant recipients. Journal of the American Society of Nephrology 2015;26(12):3114‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P, Wiebe C, Formica R, Tinckam K, Poggio E, Bunnapradist S, et al. Epitope mismatch predicts de novo DSA after CNI withdrawal in low‐risk kidney transplant recipients [abstract]. Transplantation 2014;98(Suppl 1):137. [EMBASE: 71543963] [Google Scholar]
de Sevaux 2001 {published data only}
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hilbrands LB, Gelder T, Hoitsma AJ, et al. The cyclosporine sparing effect of mycophenolate mofetil, a randomized study [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):699A. [CENTRAL: CN‐00447776] [Google Scholar]
- Sevaux RG, Gregoor PJ, Hene RJ, Hoitsma AJ, Vos P, Weimar W, et al. A controlled trial comparing two doses of cyclosporine in conjunction with mycophenolate mofetil and corticosteroids. Journal of the American Society of Nephrology 2001;12(8):1750‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sevaux RG, Smak Gregoor PJ, Hene RJ, Hoitsma AJ, Vos P, et al. Addition of mycophenolate mofetil to cyclosporine and prednisone allows for a lower dose of cyclosporine after renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00583239]
- Sévaux RG, Smak Gregoor PJ, Hené RJ, Weimar W, Hoitsma AJ, Gelder T, et al. A randomised study of conventional vs low dose cyclosporine in renal transplant recipients treated with cyclosporine, mycophenolate mofetil and prednisone [abstract no: 418]. Transplantation 1998;65(12):S107. [CENTRAL: CN‐00583548] [Google Scholar]
DICAM Study 2010 {published data only}
- Etienne I, Toupance O, Benichou J, Thierry A, Al Najjar A, Hurault dL, et al. A 50% reduction in cyclosporine exposure in stable renal transplant recipients: renal function benefits. Nephrology Dialysis Transplantation 2010;25(9):3096‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dudley 2005 {published data only}
- Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Marshall S, et al. MMF substitution for CSA in chronic allograft dysfunction: 2 year follow‐up of a multi‐center randomized controlled study [abstract no: 1513]. American Journal of Transplantation 2005;5(Suppl 11):541. [Google Scholar]
- Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, et al. Mycophenolate mofetil substitution for cyclosporine a in renal transplant recipients with chronic progressive allograft dysfunction: the "creeping creatinine" study. Transplantation 2005;79(4):466‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dudley CR, MMF 'Creeping Creatinine' Study Group. MMF substitution for CSA is an effective and safe treatment of chronic allograft dysfunction; results of a multi‐center randomized controlled study [abstract no: 41]. American Journal of Transplantation 2002;2(Suppl 3):148. [Google Scholar]
- Tedesco de Silva H. MMF replacing CSA reverses the "creeping creatinine" of renal transplant recipients of a multi‐center randomized controlled study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416751]
El‐Agroudy 2014 {published data only}
- El‐Agroudy A, Alarrayed S, Ghareeb S, Farid E, Alhellow H, Abdulla S. Long‐term outcome of a prospective randomized trial of conversion from tacrolimus to sirolimus treatment after renal transplantation [abstract no: B964]. Transplantation 2014;98(Suppl 1):539. [EMBASE: 71545346] [Google Scholar]
Fangmann 2010 {published data only}
- Fangmann J, Arns W, Marti H, Budde K, Beckurts T, Hauss J. Low dose cyclosporine regimen with daclizumab induction and mycophenolate mofetil after kidney transplantation ‐ impact on renal function and rejection episodes [abstract no: 113]. American Journal of Transplantation 2005;5(Suppl 11):185. [CENTRAL: CN‐00644197] [Google Scholar]
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract no: 715]. American Journal of Transplantation 2004;4(Suppl 8):353. [CENTRAL: CN‐00509182] [Google Scholar]
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract no: P249]. Transplantation 2004;78(2 Suppl):280. [Google Scholar]
- Fangmann J, Arns W, Marti HP, Hauss J, Ketteler M, Beckurts T, et al. Impact of daclizumab, low‐dose cyclosporine, mycophenolate mofetil and steroids on renal function after kidney transplantation. Nephrology Dialysis Transplantation 2010;25(1):283‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2006 {published data only}
- Ferguson R, Mulgaonkar S, Tedesco Silva H, Oppenheimer F, Walker R, et al. FTY720 with reduced‐exposure (R‐E) cyclosporine [neoral] (CsA): acute rejection prophylaxis in de novo renal transplant (Tx) recipients [abstract]. Transplantation Society of Australia and New Zealand. 21st Annual Meeting; 2003 Apr 9‐11; Canberra (ACT). 2003:63. [CENTRAL: CN‐00445317]
- Ferguson RM, Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Kunzendor U, et al. FTY720 with reduced‐exposure neoral provides adequate rejection prophylaxis in de novo renal transplant recipients. Interim results [abstract no: 0049]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415638]
- Ferguson RM, Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Russ G, et al. High efficacy of FTY720 with reduced cyclosporine dose in preventing rejection in renal transplantation: 12‐month preliminary results [abstract no: 624]. American Journal of Transplantation 2003;3(Suppl 5):311. [CENTRAL: CN‐00445316] [Google Scholar]
- Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Kunzendorf U, Russ G, et al. FTY720/cyclosporine regimens in de novo renal transplantation: a 1‐year dose‐finding study.[Erratum appears in Am J Transplant. 2006 Dec;6(12):3044]. American Journal of Transplantation 2006;6(8):1848‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Russ G, Ferguson RM, Mulgaonkar S, Tedesco‐Silva H, Oppenheimer F, Walker R, et al. FTY720 with reduced dose Neoral (RDN) may offer a safety/tolerability profile advantage over conventional immunosuppressive therapies [abstract no: 1000]. American Journal of Transplantation 2004;4(Suppl 8):432. [CENTRAL: CN‐00509451] [Google Scholar]
Flechner‐318 Study 2002 {published data only}
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351] [Google Scholar]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract no: 1317]. American Journal of Transplantation 2002;2(Suppl 3):470. [Google Scholar]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415657]
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil‐based immunosuppression: 5‐year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83(7):883‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Kurian S, Solez K, Cook DJ, Burke JT, Rollin H, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function at two years compared to calcineurin inhibitor drugs [abstract no: O361]. Transplantation 2004;78(2 Suppl):141. [CENTRAL: CN‐00509193] [Google Scholar]
- Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. American Journal of Transplantation 2004;4(11):1776‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Solez K, Cook DJ, Burke JT, Rollin H, Mastoianni B, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function compared to calcineurin inhibitor (CNI) drugs [abstract]. American Journal of Transplantation 2004;4(Suppl 8):296. [CENTRAL: CN‐00509194] [Google Scholar]
Garcia 2007 {published data only}
- Garcia R, Hanzawa NM, Machado PG, Moreira SR, Prismich G, Felipe CR, et al. A calcineurin inhibitor‐free regimen for low risk kidney transplant recipients [abstract no: 2379]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00520337]
- Garcia R, Machado PG, Felipe CR, Park SI, Spinelli GA, Franco MF, et al. Exploratory calcineurin inhibitor‐free regimens in living‐related kidney transplant recipients. Brazilian Journal of Medical & Biological Research 2007;40(4):457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grimbert 2002 {published data only}
- Grimbert P, Baron C, Fruchaud G, Hemery F, Desvaux D, Buisson C, et al. Long‐term results of a prospective randomized study comparing two immunosuppressive regimens, one with and one without CsA, in low‐risk renal transplant recipients. Transplant International 2002;15(11):550‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grinyo 2004 {published data only}
- Campistol JM, Grinyo JM, Paul J, Garcia J, Arias M, Morales JM, et al. Sirolimus, TAC and corticosteroids in the postoperative period. Preliminary results [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002.
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract no: SA‐PO0491]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):363A. [CENTRAL: CN‐00445561] [Google Scholar]
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (TAC) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, tac and corticosteroids in the postoperative period. Preliminary results [abstract]. American Journal of Transplantation 2002;2(Suppl 3):394. [Google Scholar]
- Grinyo JM, Campistol JM, Paul J, Garcia‐Martinez J, Morales JM, Prats D, et al. Pilot randomized study of early tacrolimus withdrawal from a regimen with sirolimus plus tacrolimus in kidney transplantation. American Journal of Transplantation 2004;4(8):1308‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Grinyo JM, Campistol JM, Garcia J, Arias M, Paul J, et al. FK‐506 withdrawal from a regimen of sirolimus plus FK‐506 results at 2 years in an improvement in renal function, blood pressure and proteinuria in ongoing kidney recipients [abstract no: 1192]. American Journal of Transplantation 2005;5(Suppl 11):460. [CENTRAL: CN‐00724880] [Google Scholar]
- Morales JM, Grinyo JM, Campistol JM, Garcia‐Martinez J, Arias M, Paul J, et al. Improved renal function, with similar proteinuria, after two years of early tacrolimus withdrawal from a regimen of sirolimus plus tacrolimus. Transplantation 2008;86(4):620‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paul J, Grinyo JM, Campistol JM, Garcia J, Morales JM, Arias M, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (TAC) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, TAC and corticosteroids in the postoperative period: preliminary results [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):18‐9. [CENTRAL: CN‐00550490] [Google Scholar]
Hall 1988 {published data only}
- Gallagher M, Jardine M, Cass A, Perkovic V, Petrie J, McDonald S, et al. 20 year outcomes of the Australian multicentre randomised trial of cyclosporine withdrawal in renal transplantation [abstract no: 1075]. Nephrology 2007;12(Suppl 2):A19. [CENTRAL: CN‐00653709] [Google Scholar]
- Gallagher M, Jardine M, Cass A, Perkovic V, Petrie J, McDonald S, et al. 20 year outcomes of the Australian multicentre randomised trial of cyclosporine withdrawal in renal transplantation [abstract]. Immunology & Cell Biology 2007;85(4):A19. [Google Scholar]
- Gallagher M, Jardine M, Perkovic V, Cass A, McDonald S, Petrie J, et al. Cyclosporine withdrawal improves long‐term graft survival in renal transplantation. Transplantation 2009;87(12):1877‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallagher M, Webster A, Jardine M, Perkovic V, Cass A, Eris J. Twenty year cancer outcomes of a randomized trial of immunosuppression in kidney transplant recipients: results of the Australian multicentre trial of cyclosporine withdrawal [abstract no: 078]. Nephrology 2008;13(Suppl 3):A119. [CENTRAL: CN‐00758532] [Google Scholar]
- Gallagher MP, Eris JM, Tiller DJ, Hall BM. Long term outcome of Australian multicentre trial of cyclosporin withdrawal in cadaveric renal transplantation [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415694]
- Gallagher MP, Hall B, Craig J, Berry G, Tiller DJ, Eris J, et al. A randomized controlled trial of cyclosporine withdrawal in renal‐transplant recipients: 15‐year results. Transplantation 2004;78(11):1653‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallagher MP, Kelly PJ, Jardine M, Perkovic V, Cass A, Craig JC, et al. Long‐term cancer risk of immunosuppressive regimens after kidney transplantation. Journal of the American Society of Nephrology 2010;21(5):852‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MP, Webster AC, Jardine MJ, Perkovic V, Cass A, Eris JM. Twenty year cancer outcomes of a randomized trial of immunosuppression in kidney transplant recipients: results of the Australian multicentre trial of cyclosporine withdrawal [abstract no: 288]. Transplantation 2008;86(2 Suppl):102. [Google Scholar]
- Hall BM, Tiller DJ, Hardie I, Mahony J, Mathew T, Thatcher G, et al. Comparison of three immunosuppressive regimens in cadaver renal transplantation: long‐term cyclosporine, short‐term cyclosporine followed by azathioprine and prednisolone, and azathioprine and prednisolone without cyclosporine. New England Journal of Medicine 1988;318(23):1499‐507. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hazzan 2005 {published data only}
- Hazzan M, Buob D, Labalette M, Provot F, Glowacki F, Hoffmann M, et al. Assessment of the risk of chronic allograft dysfunction after renal transplantation in a randomized cyclosporine withdrawal trial. Transplantation 2006;82(5):657‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hazzan M, Copin MC, Labalette M, Glowacki F, Provot F, Roumilhac D, et al. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients receiving mycophenolate mofetil: results from a prospective, randomized trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):197. [CENTRAL: CN‐00445673] [DOI] [PubMed] [Google Scholar]
- Hazzan M, Hertig A, Buob D, Noel C, Copin M, Rondeau E, et al. Cyclosporin induces epithelial to mesenchymal transition in renal grafts [abstract no: 131]. American Journal of Transplantation 2008;8(Suppl 2):214. [Google Scholar]
- Hazzan M, Labalette M, Copin MC, Glowacki F, Provot F, Pruv FR, et al. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients who receive mycophenolate mofetil: results from a prospective, randomized trial. Journal of the American Society of Nephrology 2005;16(8):2509‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heering 1993 {published data only}
- Heering P, Westhoff A, Ivens K K, Grabensee B. Comparison between the effects of CSA and azathioprine on functional renal parameters in randomized study after renal transplantation [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):940. [CENTRAL: CN‐00484304] [Google Scholar]
- Heering P, Westhoff A, Ivens K, Helmchen U, Grabensee B. Increased risk in conversion of stable renal allografts from cyclosporin to azathioprine: a randomized controlled study [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:194.
- Heering P, Westhoff K, Ivens B, Kutkuhn B, Grabensee H. Increased risk in conversion of stable renal allografts from cyclosporin to azathioprine [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1041. [CENTRAL: CN‐00260889] [Google Scholar]
- Ivens K, Heering P, Withold W, Koch M, Reinauer H, Grabensee B. Withdrawal of cyclosporine improves the cardio‐vascular risk profile in renal graft recipients [abstract]. Journal of the American Society of Nephrology 1994;5(3):1013. [Google Scholar]
HERAKLES Study 2012 {published data only}
- Arns W, Budde K, Sommerer C, Witzke O, Guba M, Jacobi J, et al. Month 48 follow‐up results of HERAKLES trial on three different treatment regimen and switching off behaviour in de novo renal transplant patients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953873] [Google Scholar]
- Arns W, Neumayer H, Lehner F, Witzke O, Sommerer C, Kliem V, et al. HERAKLES at month 24: follow‐up results on efficacy and safety of three different treatment regimens in de novo renal transplant patients demonstrate options for individualized immunosuppression [abstract no: BO148]. Transplant International 2013;26(Suppl 2):21. [EMBASE: 71356261] [Google Scholar]
- Arns W, Sommerer C, Witzke O, Lehner F, Zeier M, Neumayer HH, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: results of the HERAKLES trial [abstract no: 1722]. Transplantation 2012;94(10):995. [EMBASE: 71251769] [Google Scholar]
- Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/ mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 36 [abstract no: 716]. Transplantation 2014;98(Suppl 1):81. [EMBASE: 71543792] [Google Scholar]
- Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 24 [abstract no: B932]. American Journal of Transplantation 2013;13(Suppl 5):310‐1. [EMBASE: 71057508] [Google Scholar]
- Budde K, Sommerer C, Weithofer P, Witzke O, Guba M, Jacobi J, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 48 [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71954274] [Google Scholar]
- Budde K, Witzke O, Lehner F, Zeier M, Neumayer H, Stangl M, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: the HERAKLES Study [abstract no: 50]. American Journal of Transplantation 2012;12(Suppl S3):41. [EMBASE: 70745997] [Google Scholar]
- Guba M, Budde K, Sommerer C, Neumayer HH, Lehner F, Reinke P, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 36 [abstract no: V62]. Transplant International 2014;27(Suppl 3):22. [EMBASE: 71664029] [Google Scholar]
- Guba M, Witzke O, Lehner F, Arns W, Sommerer C, Neumayer H, et al. The HERAKLES study at 24 month: Superior renal function in an everolimus‐based CNI free regimen [abstract no: O219]. Transplant International 2013;26(Suppl 2):110. [EMBASE: 71359365] [Google Scholar]
- Hauser IA, Witzke O, Arns W, Reinke P, Sommerer C, Neumayer HH, et al. Efficacy and safety of three different treatment regimens in de novorenal transplant patients: month 36 follow‐up results of HERAKLES trial [abstract no: P132]. Transplant International 2014;27(Suppl 3):51. [EMBASE: 71664135] [Google Scholar]
- Hoerning A, Kohler S, Jun C, Lu J, Fu J, Tebbe B, et al. Cyclosporin but not everolimus inhibits chemokine receptor expression on CD4+ T cell subsets circulating in the peripheral blood of renal transplant recipients. Clinical & Experimental Immunology 2012;168(2):251‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner F, Guba M, Arns W, Sommerer C, Neumayer HH, Jacobi J, et al. Follow‐up data from HERAKLES study at month 24: maintained superior renal function in patients on an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus [abstract no: P04]. Transplant International 2013;26(Suppl 1):28. [EMBASE: 71356287] [Google Scholar]
- Lehner F, Sommerer C, Witzke O, Arns W, Kliem V, Neumayer HH, et al. HERAKLES at month 24: efficacy and safety of 3 different regimens in de novo renal transplant patients [abstract no: BO148]. Transplant International 2013;26(Suppl 2):82. [EMBASE: 71359263] [Google Scholar]
- Sommerer C, Budde K, Kliem V, Witzke O, Guba M, Jacobi J, et al. Efficacy and safety of three different treatment regimen in de novo renal transplant patients: month 48 follow‐up results of the HERAKLES trial [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953341] [Google Scholar]
- Sommerer C, Lehner F, Arns W, Reinke P, Eisenberger U, Heller K, et al. Post HOC analysis of ZEUS and HERAKLES, two prospective, open‐label, multicenter, randomized trials: onset and progression of diabetes in kidney transplant recipients on cyclosporine standard or converted to CNI‐free or CNI‐low everolimus regimen [abstract no: 2921]. Transplantation 2014;98(Suppl 1):145‐6. [EMBASE: 71543989] [Google Scholar]
- Sommerer C, Lehner F, Budde K, Arns W, Hauser IA, Suwelack B, et al. Post hoc analysis of ZEUS and HERAKLES, two prospective, open‐label, multicenter, randomized trials: onset and progression of diabetes in kidney transplant recipients on cyclosporine standard or converted to CNI‐free or CNI‐low everolimus regimen [abstract no: P127]. Transplant International 2014;27(Suppl 3):50. [EMBASE: 71664131] [Google Scholar]
- Sommerer C, Witzke O, Lehner F, Zeier M, Neumayer H, Stangl M, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: results of the HERAKLES Trial [abstract no: 51]. American Journal of Transplantation 2012;12(Suppl S3):41. [EMBASE: 70745998] [Google Scholar]
- Witzke O, Budde K, Lehner F, Zeier M, Neumayer HH, Stangl M, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: the HERAKLES study [abstract no: 1675]. Transplantation 2012;94(Suppl):993. [EMBASE: 71251766] [Google Scholar]
- Zeier M, Budde K, Arns W, Guba M, Sommerer C, Neumayer H, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: follow‐up results of the HERAKLES trial at month 24 [abstract no: 495]. American Journal of Transplantation 2013;13(Suppl S5):183. [Google Scholar]
- Zeier M, Budde K, Arns W, Guba M, Sommerer C, Neumayer H, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: month 36 follow‐up results of HERAKLES trial [abstract no: 718]. Transplantation 2014;98(Suppl 1):81‐2. [EMBASE: 71543794] [Google Scholar]
Hollander 1995 {published data only}
- Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, Fijter JW. Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney International 2003;64(3):1027‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hollander AA, Saase JL, Kootte AM, Dorp WT, Bockel HJ, Es LA, et al. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. Lancet 1995;345(8950):610‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holm 2008 {published data only}
- Holm A, Hernandez M, Camarena A, Perez L, Santos M, Porras MA. Efficacy, tolerability and safety of mycophenolate mofetil (Cellcept) + sirolimus (Rapamune) as maintenance therapy after calcineurin inhibitor withdrawal in LRD and CAD, adults and pediatric renal transplant recipients (experience with 405 patients) [abstract no: 627]. Transplantation 2008;86(Suppl 2):220. [CENTRAL: CN‐00740576] [Google Scholar]
Isoniemi 1990 {published data only}
- Isoniemi H. Renal allograft immunosuppression V: glucose intolerance occurring in different immunosuppressive treatments. Clinical Transplantation 1991;5(3):268‐72. [EMBASE: 21188067] [Google Scholar]
- Isoniemi H. Renal allograft immunosuppression. III. Triple therapy versus three different combinations of double drug treatment: two year results in kidney transplant patients. Transplant International 1991;4(1):31‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi H, Ahonen J, Eklund B, Hockerstedt K, Salmela K, Willebrand E, et al. Renal allograft immunosuppression. II. A randomized trial of withdrawal of one drug in triple drug immunosuppression. Transplant International 1990;3(3):121‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Ahonen J, Krogerus L, Eklund B, Hockerstedt K, Salmela K, et al. Chronic rejection of renal allografts with four immunosuppressive regimens. Transplantation Proceedings 1992;24(6):2716‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Eklund B, Hockerstedt K, Korsback C, Salmela K, Willebrand E, et al. Discontinuation of one drug in triple drug treatment of renal allograft patients: 1‐year results. Transplantation Proceedings 1990;22(4):1365‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Krogerus L, Willebrand E, Taskinen E, Gronhagen‐Riska C, Ahonen J, et al. Renal allograft immunosuppression. VI. Triple drug therapy versus immunosuppressive double drug combinations: histopathological findings in renal allografts. Transplant International 1991;4(3):151‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Tikkanen M, Hayry P, Eklund B, Hockerstedt K, Salmela K, et al. Lipid profiles with triple drug immunosuppressive therapy and with double drug combinations after renal transplantation and stable graft function. Transplantation Proceedings 1991;23(1 (Pt 2)):1029‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Tikkanen MJ, Ahonen J, Hayry P. Renal allograft immunosuppression. IV. Comparison of lipid and lipoprotein profiles in blood using double and triple immunosuppressive drug combinations. Transplant International 1991;4(3):130‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Willebrand E, Ahonen J, Eklund B, Hockerstedt K, Krogerus L, et al. Late histopathological findings in renal allografts with four immunosuppressive regimens. Transplant International 1992;5 Suppl 1:S6‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi HM, Ahonen J, Tikkanen MJ, Willebrand EO, Krogerus L, Eklund BH, et al. Long‐term consequences of different immunosuppressive regimens for renal allografts.[Erratum appears in Transplantation 1998 Sep 15;66(5):678]. Transplantation 1993;55(3):494‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi HM, Krogerus L, Willebrand E, Taskinen E, Ahonen J, Hayry P. Histopathological findings in well‐functioning, long‐term renal allografts. Kidney International 1992;41(1):155‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kosch 2003a {published data only}
- Hausberg M, Lang D, Levers A, Suwelack B, Kisters K, Tokmak F, et al. Sympathetic nerve activity in renal transplant patients before and after withdrawal of cyclosporine. Journal of Hypertension 2006;24(5):957‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hausberg M, Suwelack B, Kosch M, Barenbrock M, Gerhardt U, Hohage H, et al. Effects of calcineurin‐inhibitor withdrawal on sympathetic nerve activity and blood pressure in renal transplant recipients [abstract]. Deutsche Medizinische Wochenschrift 2001;126:S193. [CENTRAL: CN‐00383083] [Google Scholar]
- Kosch M, Hausberg M, Suwelack B. Studies on effects of calcineurin inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients. Transplantation 2003;76(10):1516‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kosch M, Suwelack B, Barenbrock M, Kobelt V, Hohage H, Rahn KH, et al. Studies on effects of calcineurin‐inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients. Deutsche Medizinische Wochenschrift 2001;126:S185. [CENTRAL: CN‐00383367] [DOI] [PubMed] [Google Scholar]
Kreis 2003 {published data only}
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Daclizumab and mycophenolate mofetil in renal transplant recipients: 2‐year outcome after early reduction of cyclosporine [abstract no: 1266]. American Journal of Transplantation 2003;3(Suppl 5):476. [CENTRAL: CN‐00446199] [Google Scholar]
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Lowering cyclosporine dose is not associated with an increased risk of gastro‐intestinal adverse events nor the need for dosage decrease of mycophenolate mofetil [abstract no: P743]. Transplantation 2004;78(2 Suppl):462. [CENTRAL: CN‐00509292] [Google Scholar]
MacPhee 1998 {published data only}
- Joss N. Randomised study comparing cyclosporin with azathioprine one year after renal transplantation ‐ 15 year outcome [abstract]. Scottish Medical Journal 2005;50(2):82. [CENTRAL: CN‐00653805] [Google Scholar]
- Joss N, Glasgow Transplant Group. Randomised study comparing cyclosporin with azathioprine one year after renal transplantation ‐ 15 year outcome [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:197. [CENTRAL: CN‐00636135]
- Joss N, Rodger RS, McMillan MA, Junor BJ. Randomized study comparing cyclosporine with azathioprine one year after renal transplantation‐15‐year outcome data. Transplantation 2007;83(5):582‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MacPhee IA, Bradley JA, Briggs JD, Junor BJ, Macpherson SG, McMillan MA, et al. Long‐term outcome of a prospective randomized trial of conversion from cyclosporine to azathioprine treatment one year after renal transplantation. Transplantation 1998;66(9):1186‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MacPhee IA, Briggs JD, Junor BJ, McMillan MA, Rodger RS, Watson MA. A prospective randomised controlled trial of long‐term outcome for renal transplant recipients converted from cyclosporin to azathioprine treatment one year after transplantation [abstract]. Journal of the American Society of Nephrology 1996;7(9):1915. [CENTRAL: CN‐00602085] [Google Scholar]
Martinez‐Mier 2006 {published data only}
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: Initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MECANO Study 2009 {published data only}
- Baas MC, Gerdes VE, Berge IJ, Heutinck KM, Florquin S, Meijers JC, et al. Treatment with everolimus is associated with a procoagulant state. Thrombosis Research 2013;132(2):307‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baas MC, Kers J, Florquin S, Fijter JW, Heide JJ, Bergh Weerman MA, et al. Cyclosporine versus everolimus: effects on the glomerulus. Clinical Transplantation 2013;27(4):535‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baas MC, Struijk GH, Moes DJ, Berk IA, Jonkers RE, Fijter JW, et al. Interstitial pneumonitis caused by everolimus: a case‐cohort study in renal transplant recipients. Transplant International 2014;27(5):428‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bemelman FJ, Maar EF, Press RR, Kan HJ, Berge IJ, Homan van der Heide JJ, et al. Minimization of maintenance immunosuppression early after renal transplantation: an interim analysis. Transplantation 2009;88(3):421‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Havenith SH, Yong SL, Donselaar‐van der Pant KA, Lier RA, Berge IJ, Bemelman FJ. Everolimus‐treated renal transplant recipients have a more robust CMV‐specific CD8+ T‐cell response compared with cyclosporine‐ or mycophenolate‐treated patients. Transplantation 2013;95(1):184‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Struijk GH, Minnee RC, Koch SD, Zwinderman AH, Donselaar‐van der Pant KA, Idu MM, et al. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney International 2010;78(9):934‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dijk M, Roon A, Sanders JS. Intima media thickness (IMT) and major adverse cardiac events (MACE) in patients after kidney transplantation [abstract no: P226]. Transplant International 2015;28(Suppl 4):431. [EMBASE: 72112220] [Google Scholar]
- Berge IJ, Maar E, Press R, Heide JH, Fijter H, Bemelman FJ. Minimization of immunosuppressive drug therapy at 6 months after renal transplantation: everolimus or cyclosporin superior to mycophenolate sodium [abstract no: 532]. Transplantation 2008;86(Suppl 2):186. [CENTRAL: CN‐00740475] [Google Scholar]
- Heide JJ, Fijter JW, Maar EF, Berge I, Bemelman FJ. Low acute rejection rate and superior renal function 2 years after early CsA withdrawal and overnight switch to everolimus [abstract no: 1654]. American Journal of Transplantation 2010;10(Suppl 4):508. [Google Scholar]
MODIFY Study 2012 {published data only}
- David‐Neto E, Lemos F, Antonopoulos I, Souza P, Piovesan A, Ventura C, Kanashiro H, et al. Five‐year follow‐up of the MoDIFY study (modification of doses to improve function through the years) shows benefit of low tacrolimus and regular MMF doses [abstract]. Journal of Urology 2011;185(4 Suppl 1):e828. [EMBASE: 70378695] [Google Scholar]
- David‐Neto E, Lemos FC, Souza PS, Ventura CG, Castro MCR\, Agena F, et al. Five‐year follow‐up of the MoDIFY Study (Modification of Doses to Improve Function through the Years) shows benefit of low tacrolimus and regular MMF doses [abstract no: 1655]. American Journal of Transplantation 2010;10(Suppl 4):508. [EMBASE: 70465030] [Google Scholar]
- David‐Neto E, Pereira LM, Castro MC, Mattos RM, Sumita NM, Mendes E, et al. Interim analysis of the MODIFY study in renal transplantation [abstract]. Transplantation 2004;78(2 Suppl):278‐9. [CENTRAL: CN‐00509408] [Google Scholar]
- David‐Neto E, Pereira LM, Castro MC, Mattos RM, Sumita NM, Mendes E, et al. Therapeutic levels of mycophenolic acid under fixed doses of MMF on the first two months after kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):278. [Google Scholar]
- David‐Neto E, Pereira LM, Castro CR, Mattos RM, Sumita NM, Mendes ME, et al. Interim analysis of the MODIFY study in renal transplantation (Modification of Doses to Improve Function through the Years) [abstract]. American Journal of Transplantation 2004;4(Suppl 8):234‐5. [CENTRAL: CN‐00509150] [Google Scholar]
- David‐Neto E, Pereira LM, Castro MC, Mattos RM, Ventura CG, Sumita NM, et al. Therapeutic levels of mycophenolic acid under fixed doses of MMF on the first two months after kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):255. [CENTRAL: CN‐00509409] [Google Scholar]
- David‐Neto E, Souza PS, Panajotopoulos N, Rodrigues H, Ventura CG, David DS, et al. The impact of pretransplant donor‐specific antibodies on graft outcome in renal transplantation: a six‐year follow‐up study. Clinics 2012;67(4):355‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LM, Castro MC, Ventura CG, Reis FS, Sumita NM, Saito MI, et al. The MODIFY Study in renal transplantation (Modification of Doses to Improve Function Through the Years) [abstract]. American Journal of Transplantation 2005;5(Suppl 11):466. [Google Scholar]
- Pereira LM, Castro MC, Ventura CG, Soares PS, Ferreira GF, Saldanha LB, et al. A prospective, randomized and controlled trial of tacrolimus minimization in renal transplantation: 1‐year result of the MODIFY study [abstract no: 53]. American Journal of Transplantation 2006;6(Suppl 2):84. [CENTRAL: CN‐00671794] [Google Scholar]
Muhlbacher 2014 {published data only}
- Muehlbacher F, Neumayer HH, Castillo D, Stefoni S, European Sirolimus CsA Minimisation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: 1224]. American Journal of Transplantation 2003;3(Suppl 5):465. [CENTRAL: CN‐00446852] [Google Scholar]
- Muhlbacher F, Neumayer HH, Castillo D, Stefoni S, Zygmunt AJ, Budde K, et al. The efficacy and safety of cyclosporine reduction in de novo renal allograft patients receiving sirolimus and corticosteroids: results from an open‐label comparative study. Transplant International 2014;27(2):176‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muhlbacher F, Paczek L. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: 399]. American Journal of Transplantation 2002;2(Suppl 3):238. [Google Scholar]
- Neumayer H, Muhlbacher F, Stefoni S, Castillo D, Myfortic Maintenance Renal Transplantation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416320]
- Neumayer HH, Muehlbacher F, Castillo D, Stefoni S, European Sirolimus CsA Minimisation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: W738]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00446926] [Google Scholar]
Nafar 2012 {published data only}
- Nafar M, Alipour B, Ahmadpoor P, Pour‐Reza‐Gholi F, Samadian F, Samavat S, et al. Sirolimus versus calcineurin inhibitor‐based immunosuppressive therapy in kidney transplantation: a 4‐year follow‐up. Iranian Journal of Kidney Diseases 2012;6(4):300‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Nashan 2004 {published data only}
- Curtis J, Nashan B, Poniicelli C, Mourad G, Boger R, RAD 156 Study Group. One year result of a multicenter, open‐label trial on safety and efficacy of certican (RAD) used in combination with simulect, corticosteroids, and full or reduced dose neoral in renal transplant [abstract no: 1335]. American Journal of Transplantation 2001;1(Suppl 1):474. [CENTRAL: CN‐00444958] [Google Scholar]
- Curtis J, Nashan B, Ponticelli C, Mourad G, Boger R, RAD 156 Study Group. Everolimus (RAD) in combination with reduced dose with reduced dose neoral® maintains effective immunosuppression with improved tolerability compared to full dose neoral [abstract no: A4625]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):884A. [CENTRAL: CN‐00550720] [Google Scholar]
- Mourad G, Boger R, Curtis J, Nashan B, Ponticelli C, RAD B 156 Study Group. CERTICAN (TM) (RAD; EVEROLIMUS), the proliferation signal inhibitor, is complementary with a reduced dose NEORAL® based quadruple immunosuppressive regimen [abstract no: 1622]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487738]
- Nashan B, Curtis J, Ponticelli C, Mourad G, Boger R, RAD B156 Study Group. Certican (RAD; everolimus), the proliferation signal inhibitor, is complementary with a reduced dose neoral based quadruple immunosuppressive regimen [abstract no: 398]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487741]
- Nashan B, Mourad G, Boger R, Curtis J, Ponticelli C, Haas T. Everolimus and reduced‐exposure cyclosporine in de novo renal‐transplant recipients: a three‐year phase II, randomized, multicenter, open‐label study. Transplantation 2004;78(9):1332‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Curtis J, Kovarik JM, Nashan B, Mourad G, Figueiredo J, et al. Everolimus pharmacokinetics are unaltered with full‐dose versus reduced‐dose cyclosporine [abstract no: 318]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487748]
- RADB156 Study Group, Boger R, Frei U, Glotz D, Kahan B, Ponticelli C, et al. CERTICAN(TM) with reduced dose NEORAL® results in a significantly higher GFR in de novo renal transplant recipients over the first 12 Months compared to CERTICAN(TM)‐full dose NEORAL® as Assessed by Iohexol Clearance [abstract no:1623]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487751]
Oh 2012 {published data only}
- Oh CK, Ha JW, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus (Certican) with low dose of cyclosporine in de novo kidney recipients after 1 month of transplantation (Preliminary results). Journal of the Korean Society for Transplantation 2012;26(2):83‐91. [DOI: 10.4285/jkstn.2012.26.2.83] [DOI] [Google Scholar]
- Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced‐exposure cyclosporine a in de novo kidney recipients. Transplantation 2015;99(1):180‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPTICEPT Study 2009 {published data only}
- Bloom R, Kaplan B, Meier‐Kriesche H, Shaw L, Shah T, Patel D, et al. Opticept Trial: Efficacy and safety of monitored MMF in combination with CNI in renal transplantation at 1 and 2 years [abstract no: 862]. Transplantation 2008;86(2 Suppl):301. [CENTRAL: CN‐00740509] [Google Scholar]
- Gaston R, Bloom R, Shah T, Cibrik D, Angelis M, Mulgaonkar S, et al. 6‐month outcomes of monitored mycophenolate mofetil (MMF) in combination with calcineurin inhibitors (CNI) in renal allograft recipients: an interim analysis of the OPTICEPT trial [abstract no: TH‐PO565]. Journal of the American Society of Nephrology 2006;17(Abstracts):228A. [CENTRAL: CN‐00602020] [Google Scholar]
- Gaston R, Kaplan B, Meier‐Kriesche H, Shaw L, Shah T, Patel D, et al. Opticept Trial: efficacy and safety of monitored MMF in combination with CNI in renal transplantation at 12 months [abstract no: 526]. American Journal of Transplantation 2008;8(Suppl 2):319. [CENTRAL: CN‐00653708] [Google Scholar]
- Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed‐ or controlled‐dose mycophenolate mofetil with standard‐ or reduced‐dose calcineurin inhibitors: the OPTICEPT trial. American Journal of Transplantation 2009;9(7):1607‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kaplan B, Gaston R, Meier‐Kriesche H, Bloom R, Patel D, Shaw L. Mycophenolate mofetil may require dose modification based on weight: an analysis of MPA exposure in the OptiCept Trial [abstract no: 787]. American Journal of Transplantation 2009;9(Suppl 2):419. [CENTRAL: CN‐00794654] [Google Scholar]
- Kaplan B, Gaston RS, Meier‐Kriesche HU, Bloom RD, Shaw LM. Mycophenolic acid exposure in high‐ and low‐weight renal transplant patients after dosing with mycophenolate mofetil in the Opticept trial. Therapeutic Drug Monitoring 2010;32(2):224‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shaw L, Kaplan B, Angelis M, Bloom R, Gaston R, Meier‐Kriesche H, et al. Correlation between mycophenolic acid (MPA) trough and abbreviated AUC levels in renal allograft recipients in the Opticept Trial [abstract no: SU‐FC128]. Journal of the American Society of Nephrology 2007;18(Abstracts):96A‐7A. [CENTRAL: CN‐00724911] [Google Scholar]
ORION Study 2011 {published data only}
- Flechner S, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC)+ mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year safety results from the Orion Trial [abstract no: 444]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740477] [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the ORION study [abstract no: 1141]. American Journal of Transplantation 2007;7(Suppl 2):440. [CENTRAL: CN‐00740477] [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from the ORION study [abstract no: 52]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653711] [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB, ORION Trial Investigators. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from The ORION Study [abstract no: P477]. Transplant International 2007;20(Suppl 2):209. [Google Scholar]
- Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the efficacy and safety of two different sirolimus (SRL) regimens with tacrolimus (TAC) + mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year efficacy results from the ORION trial [abstract no: 287]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00653710] [Google Scholar]
- Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. Preliminary two‐year efficacy results from a randomized, open‐label, comparative study of two different sirolimus (SRL) regimens versus a tacrolimus (TAC) + mycophenolate regimen (MMF) in de novo renal allograft recipients [abstract no: 531]. Transplantation 2008;86(Suppl 2):186. [CENTRAL: CN‐00740478] [Google Scholar]
- Flechner SM, Glyda M, Cockfield S, Grinyo J, Legendre C, Russ G, et al. The ORION Study: comparison of two sirolimus‐based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients.[Erratum appears in Am J Transplant. 2012 Jan;12(1):268]. American Journal of Transplantation 2011;11(8):1633‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Glyda M, See Tai S, ORION Trial Investigators. Delayed graft function (DGF) in two sirolimus (SRL)‐based regimens compared with tacrolimus (TAC) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: 299]. American Journal of Transplantation 2009;9(Suppl 2):277. [CENTRAL: CN‐00774294] [Google Scholar]
- Flechner SM, Glyda M, Steinberg S, Copley JB, ORION Trial Investigators. The efficacy of sirolimus (SRL) and tacrolimus (TAC) withdrawal versus SRL and mycophenolate mofetil (MMF) compared with TAC and MMF in de novo renal allograft recipients: interim results from the Orion Study [abstract no: SU‐FC124]. Journal of the American Society of Nephrology 2007;18(Abstracts):95A. [CENTRAL: CN‐00716077] [Google Scholar]
- Russ G, Flechner SM, Cockfield S, Grinyo J, Wissing KM, Legendre C, et al. Cardiovascular risk profile of renal transplant recipients enrolled in an open‐label, randomized study comparing 2 sirolimus (SRL) regimens with a tacrolimus (TAC)+ mycophenolate regimen (MMF): preliminary results from the Orion Trial [abstract no: 445]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740477] [Google Scholar]
Pacheco‐Silva 2013 {published data only}
- Pacheco‐Silva A, Tonato E, Durao M Jr, Requiao‐Moura L, Arruda E, Chinen R, et al. A randomized clinical trial of early conversion from tacrolimus to everolimus in deceased donor kidney transplantation [abstract no: P460]. Transplant International 2013;26(Suppl 2):277‐8. [EMBASE: 71360073] [Google Scholar]
Paoletti 2012 {published data only}
- Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93(5):503‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pascual 2003 {published data only}
- Pascual M, Curtis J, Delmonico FL, Farrell ML, Williams WW, Kalil R, et al. A prospective, randomized clinical trial of cyclosporine reduction in stable patients greater than 12 months after renal transplantation. Transplantation 2003;75(9):1501‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Cardarelli F, Saidman SL, Farrell ML, et al. Analysis of the cardiovascular risk profile in stable kidney transplant recipients after 50% cyclosporine reduction. Clinical Transplantation 2004;18(4):341‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Farell ML, Shih V, Williams W, et al. Analysis of the cardiovascular risk profile in stable renal transplant recipients after 50% cyclosporine reduction [abstract]. American Journal of Transplantation 2003;3(Suppl 5):213. [CENTRAL: CN‐00448411] [Google Scholar]
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Farrell ML, Shih V, Cosimi AB, et al. A randomized clinical trial to analyze the effect of 50% cyclosporine reduction on the cardiovascular risk profile in stable kidney transplant recipients [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):186A. [CENTRAL: CN‐00550675] [Google Scholar]
Pascual 2008 {published data only}
- Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T‐regulatory cells. American Journal of Transplantation 2008;8(7):1529‐36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1991 {published data only}
- Jensen JD, Hansen HE, Pedersen EB. Increased serum erythropoietin level during azathioprine treatment in renal transplant recipients. Nephron 1994;67(3):297‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporin to azathioprine 1 year after renal transplantation. A prospective, randomized study from 1 to 6 years after transplantation. Nephrology Dialysis Transplantation 1993;8(3):250‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporin to azathioprine 1 year after renal transplantation: a prospective randomized study from 1 to 6 years after transplantation [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1047. [PubMed] [Google Scholar]
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporine to azathioprine one year after renal transplantation. A prospective, randomized study from one to six years after transplantation [abstract]. 12th International Congress of Nephrology; 1993 June 13‐18; Jerusalem, Israel. 1993:194. [CENTRAL: CN‐00644352] [PubMed]
- Pedersen EB, Hansen HE, Olsen S. The effect of cyclosporine and azathioprine on GFR and renal histopathology 12 to 24 months after renal transplantation. Transplantation Proceedings 1992;24(1):309‐10. [MEDLINE: ] [PubMed] [Google Scholar]
- Pedersen EB, Hansen HE, Olsen S, Danielsen H, Eiskjaer AW, Jespersen B, et al. Comparison between the effect of cyclosporin and azathioprine on functional and morphological renal parameters in a randomised study from one to two years after renal transplantation [abstract]. Nephrology Dialysis Transplantation 1991;6(10):832‐3. [Google Scholar]
- Pedersen EB, Hansen HE, Olsen S, Danielsen H, Eiskjaer H, Jespersen B, et al. Comparison between the effects of cyclosporine and azathioprine on functional and morphologic renal parameters in a randomized study from 12 to 24 months after renal transplantation. Clinical Transplantation 1991;5(6 I):456‐66. [EMBASE: 22024462] [Google Scholar]
Pontrelli 2008 {published data only}
- Pontrelli P, Rossini M, Infante B, Stallone G, Schena A, Loverre A, et al. Rapamycin inhibits PAI‐1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation 2008;85(1):125‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossini M, Pontrelli P, Stallone G, Infante B, Schena A, Loverre A, et al. Regression of glomerulosclerosis in chronic allograft nephropathy (CAN) following calcineurin inhibitors (CNI) withdrawal and conversion to sirolimus (SRL) [abstract no: TH‐PO554]. Journal of the American Society of Nephrology 2006;17(Abstracts):225A. [CENTRAL: CN‐00725009] [Google Scholar]
- Stallone G, Pontrelli P, Infante B, Schena A, Ursi M, Ranieri E, et al. Rapamycin (RAPA) inhibits PAI‐1 expression and reduce interstitial fibrosis in chronic allograft nephropathy (CAN) [abstract no: SO04]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v4. [CENTRAL: CN‐00716118] [DOI] [PubMed] [Google Scholar]
Qazi 2014 {published data only}
- Peddi V, Qazi Y, Shaffer D, Luan F, Shihab F, Tomlanovich S, et al. Effect of everolimus with low dose tacrolimus vs mycophenolate with standard tacrolimus regimen in African‐American de novo renal transplant recipients [abstract no: B955]. Transplantation 2014;98(Suppl 1):536. [EMBASE: 71545337] [Google Scholar]
- Qazi Y, Shaffer D, Kaplan B, Kim D, Luan F, Peddi V, et al. Efficacy and safety of everolimus with low‐dose tacrolimus in de novo renal transplant recipients: 12‐month randomized study [abstract no: 713]. Transplantation 2014;98(Suppl 1):80. [EMBASE: 71543789] [DOI] [PubMed] [Google Scholar]
- Shihab F, Qazi Y, Kaplan B, Kim D, Mulgaonkar S, Peddi V, et al. Everolimus‐facilitated tacrolimus minimization preserves renal function in de novo renal transplant recipients [abstract no: B962]. Transplantation 2014;98(Suppl 1):538‐9. [EMBASE: 71545344] [Google Scholar]
REFERENCE Study 2006 {published data only}
- Frimat L, Cassuto‐Viguier E, Charpentier B, Noel C, Provot F, Rostaing L, et al. Impact of cyclosporine reduction with MMF: a randomized trial in chronic allograft dysfunction. The 'REFERENCE' study. American Journal of Transplantation 2006;6(11):2725‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kessler M. Renal function evaluation after half dose reduction of Neoral in combination with Cellcept in renal transplant patients with altered renal function: preliminary 12 months results: randomized, open, multicentric, prospective, controlled study [abstract no: 773]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446065] [Google Scholar]
- Kessler M, Frimat L. Renal function evaluation after half dose reduction of neoral® in combination with Cellcept® in renal transplant patients with altered renal function: preliminary 2 year safety and efficacy results of the MMF‐REFERENCE study: a randomised [abstract no: 013]. Transplantation 2004;78(2 Suppl):5. [CENTRAL: CN‐00509272] [Google Scholar]
- Kessler M, Frimat L, Cassuto‐Viguier E, Charpentier B, Djeffal R, Noel C, et al. Impact of cyclosporine reduction with MMF in chronic allograft dysfunction: 4‐year results of a multicenter randomized controlled study. The "Reference" study [abstract no: 850]. American Journal of Transplantation 2006;6(Suppl 2):353. [CENTRAL: CN‐00765336] [DOI] [PubMed] [Google Scholar]
- Kessler M, Frimat L, Charpentier B, Durrbach A, Noel C, Pruvot F, et al. Renal function evaluation after half dose reduction of neoral® in combination with cellcept® in renal transplant patients with altered renal function: preliminary 2 year safety and efficacy results of the MMF ‐ REFERENCE study: a randomised, open, multicentre, prospective, controlled study [abstract no: 462]. American Journal of Transplantation 2004;4(Suppl 8):285. [CENTRAL: CN‐00602100] [Google Scholar]
Rivelli 2015 {published data only}
- Rivelli RF, Goncalves RT, Leite M Jr, Santos MA, Delgado AG, Cardoso LR, et al. Early withdrawal of calcineurin inhibitor from a sirolimus‐based immunosuppression stabilizes fibrosis and the transforming growth factor‐beta signalling pathway in kidney transplant. Nephrology 2015;20(3):168‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RMR Study 2001 {published data only}
- Balshaw R, Marra C, Nashan B, Hagenmeyer EG, Keown P. First year cost‐implications of early cyclosporine withdrawal in renal transplantation [abstract no: 2378]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415221]
- Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. Journal of the American Society of Nephrology 2006;17(2):581‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Campistol JM, Kreis H, Oberbauer R, Mota A, Riad H, Chapman J, et al. Sirolimus‐based therapy following early cyclosporin withdrawal resulted in superior renal allograft survival at 48 months compared with continuous combined sirolimus and cyclosporine [abstract]. American Journal of Transplantation 2004;4(Suppl 8):344. [CENTRAL: CN‐00509115] [Google Scholar]
- Campistol JM, Legendre C, Friend P, Riad H, Castagneto M. Evaluating cardiovascular risk factors associated with long‐term sirolimus (Rapamune®) immunotherapy used with or without cyclosporine [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):311. [CENTRAL: CN‐00550754] [Google Scholar]
- Campistol JM, Oberbauer R, Hartmann A, Kreis H, Mota A, Arias M, et al. Long‐term graft survival is significantly better in patients receiving sirolimus‐based therapy after early cyclosporine withdrawal [abstract no: W736]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):784. [CENTRAL: CN‐00444661] [Google Scholar]
- Campistol JM, Oberbauer R, Kreis H, Brault Y, Burke JT, Rapamune Maintenance Regimen Study Group. Predicting 10‐year graft survival with sirolimus used either as base therapy or in association with cyclosporine [abstract no: 1458]. American Journal of Transplantation 2003;3(Suppl 5):474. [CENTRAL: CN‐00444662] [Google Scholar]
- Campistol JM, Oberbauer R, Kreis H, Lawen J, Morales JM, Chapman J, et al. Elimination of cyclosporine with sirolimus‐based immunotherapy: 2‐year results confirm improved renal function [abstract no: SA‐P0489]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):363A. [CENTRAL: CN‐00444663] [Google Scholar]
- Chapman J, Johnson RW, Kreis H, Oberbauer R, Vanrenterghem Y, Claesson K, et al. Improved renal function using sirolimus (Rapamune®) maintenance therapy and cyclosporine elimination in renal allograft recipients: 12‐month results of the tri‐continental trial [abstract no: A4615]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):882A. [CENTRAL: CN‐00550709] [Google Scholar]
- Chapman J, Oberbauer R, Campistol JM, Grinyo JM, Morales JM, Mota A, et al. Renal transplant patients receiving sirolimus‐based therapy after early cyclosporine withdrawal demonstrate superior renal allograft survival and improved renal function at 48 months after transplantation [abstract no: SU‐FC103]. Journal of the American Society of Nephrology 2004;15(Oct):66A. [CENTRAL: CN‐00583160] [Google Scholar]
- Eris J, Grinyo JM, Brault Y, Hutchison B. Sirolimus therapy after early cyclosporine withdrawal in renal transplantation significantly reduces the rate of skin cancer compared with continuous combined therapy with cyclosporine [abstract no: OR‐040]. Transplant International 2005;18(Suppl):10. [Google Scholar]
- Eris J, Russ G, Hutchinson B, Walker R, Chapman J, Pussell B, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk of malignancy at 5 years after renal transplantation [abstract no: 105]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand (TSANZ); 2006 Mar 29‐31; Canberra, Australia. 2006:91. [CENTRAL: CN‐00644323]
- Eris J, Russ G, Hutchison B, Walker R, Chapman J, Pussell B, et al. Conversion to sirolimus therapy after early cyclosporin withdrawal reduces the risk of malignancy at 5 years post renal transplantation [abstract no: 1581]. Nephrology 2006;11(Suppl 2):A24. [CENTRAL: CN‐00644325] [Google Scholar]
- Friend P, Mota A, Kreis H, Segoloni G, Legendre C, Prats D, et al. Cyclosporine withdrawal followed by sirolimus (Rapamune) maintenance therapy is beneficial to patients regardless of baseline renal function [abstract no: 0553]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415680]
- Friend P, Russ G, Oberbauer R, Murgia MG, Tufveson G, Chapman J, et al. Incidence of anemia in sirolimus‐treated renal transplant recipients: the importance of preserving renal function. Transplant International 2007;20(9):754‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo JM, Vanrentergheim Y, Hutchison B, Segoloni G, Durand D, Sorba G, et al. Sirolimus (Rapamune) in patients receiving kidney from older donors: 2‐year results from the Rapamune Maintenance Regimen trial [abstract no: 534]. American Journal of Transplantation 2002;2(Suppl 3):272. [CENTRAL: CN‐00415776] [Google Scholar]
- Grinyo JM, Vanrenterghem Y, Hutchison B, Segoloni G, Durand D, Sorba G, et al. The effects of sirolimus (Rapamune) in patients receiving kidneys from older donors: 2‐year results from the Rapamune Maintenance Regimen trial [abstract no: 0435]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00420830]
- Hartmann A, Colon J, Pena J, Martinez J, Walker R, Arns W, et al. Favourable cardiovascular risk profile in renal transplant patients receiving sirolimus maintenance therapy following cyclosporine withdrawal: 3 year results of the Rapamune Maintenance Regimen trial [abstract no: W756]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):791‐2. [CENTRAL: CN‐00445657] [Google Scholar]
- Hartmann A, Mota A, Legendre C, Segoloni G, Grinyo JM, Hutchison B, et al. Patients receiving sirolimus maintenance therapy have better renal function after early cyclosporine withdrawal irrespective of baseline renal function: intent‐to‐treat results at 3 years [abstract no: W743]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):787. [CENTRAL: CN‐00445658] [Google Scholar]
- Hartmann A, Oberbauer R, Kreis H, Johnson RW, Mota A, Schena FP, et al. Long‐term results of cyclosporine elimination with sirolimus‐based therapy show improved renal function [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):19. [CENTRAL: CN‐00550651] [Google Scholar]
- Hutchison B, Claesson K, Mota A, Eris JM, Kreis H, Oberbauer R, et al. Quality of life in sirolimus‐treated kidney transplant patients after cyclosporine elimination: 2‐year results [abstract no: 499]. American Journal of Transplantation 2002;2(Suppl 3):263. [CENTRAL: CN‐00415909] [DOI] [PubMed] [Google Scholar]
- Johnson R, Oberbauer R, Kreis H, Brattstrom B, Claesson K, Eris J, et al. Sirolimus allows early cyclosporine free immunosuppression in renal transplantation: an international trial with the tablet formulation [abstract no: 0429]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445947]
- Johnson RW, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 2001;72(5):777‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keown P, Chen J, Kreis H, Oberbauer R, Claesson K, Mota A, et al. Improved quality of life after cyclosporine elimination in sirolimus‐treated renal transplant patients [abstract no: A4711]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):901A. [CENTRAL: CN‐00550607] [Google Scholar]
- Kreis H, Johnson R, Mayer G, Brattstrom C, Claesson K, Eris J, et al. Sirolimus allows early cyclosporine‐free immunosuppression in renal transplantation resulting in lower blood pressure and improved renal function [abstract no: A3662]. Journal of the American Society of Nephrology 2000;11(Sept):696A‐7A. [CENTRAL: CN‐00550424] [Google Scholar]
- Kreis H, Oberbauer R, Campistol JM, Mathew T, Daloze P, Schena FP, et al. Long‐term benefits with sirolimus‐based therapy after early cyclosporine withdrawal. Journal of the American Society of Nephrology 2004;15(3):809‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kreis H, Oberbauer R, Claesson K, Mota A, Arias M, Durand D, et al. Quality‐of‐life assessments in sirolimus‐treated renal transplant patients after cyclosporine elimination [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00446200] [Google Scholar]
- Kreis H, Oberbauer R, Mota A, Claesson K, Riad H, Campistol JM, et al. Sirolimus‐based therapy following early cyclosporine withdrawal results in superior renal allograft survival compared with a continuous combination of sirolimus and cyclosporine [abstract]. American Journal of Transplantation 2003;3(Suppl 5):460. [CENTRAL: CN‐00446201] [Google Scholar]
- Legendre C, Brault Y, Morales JM, Oberbauer R, Altieri P, Riad H, et al. Factors influencing glomerular filtration rate in renal transplantation after cyclosporine withdrawal using sirolimus‐based therapy: a multivariate analysis of results at five years. Clinical Transplantation 2007;21(3):330‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Daloze P, Colon J, Chapman J, Pena J, Martinez J, et al. Sirolimus (Rapamune) used with or without cyclosporine for long term therapy: a comparison of cardiovascular risk factors. [abstract no: 1015]. American Journal of Transplantation 2002;2(Suppl 3):393. [CENTRAL: CN‐00416111] [Google Scholar]
- Legendre C, Mota A, Friend P, Segoloni G, Grinyo JM, Hutchison B, et al. Superior renal function after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function: intent‐to‐treat results at 3 years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):466. [CENTRAL: CN‐00446313] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Malignancy in renal transplant recipients receiving sirolimus (rapamune) maintenance therapy: 2‐year follow up from 5 multicenter studies [abstract no: 58]. American Journal of Transplantation 2002;2(Suppl 3):152. [CENTRAL: CN‐00416243] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Two‐year incidence of malignancy in sirolimus (Rapamune)‐treated renal transplant recipients: follow‐up results from 5 multicenter studies [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416244] [DOI] [PubMed]
- Mathew T, Kreis H, Friend P. Two‐year incidence of malignancy in sirolimus‐treated renal transplant recipients: results from five multicenter studies. Clinical Transplantation 2004;18(4):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Hartmann A, Walker R, Arns W, Senatorski G, Grinyo JM, et al. Similar lipid profile but improved long‐term outcomes with sirolimus after cyclosporine withdrawal compared to sirolimus with continuous cyclosporine. Transplantation Proceedings 2009;41(6):2339‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Kreis H, Oberbauer R, Campistol JM, Mota A, Henriques AC, et al. 48‐month results of sirolimus‐based therapy following early cyclosporine withdrawal demonstrate superior renal allograft survival [abstract no: MO05]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:214. [CENTRAL: CN‐00509360]
- Morales JM, Oberbauer R, Legendre C, Ruiz JC, Mota A, Chapman J, et al. Analysis of risk factors for reduced renal function in renal transplant patients receiving sirolimus maintenance therapy after early cyclosporine withdrawal: 5‐year results from the Rapamune Maintenance Regimen (RMR) trial [abstract no: F‐FC156]. Journal of the American Society of Nephrology 2005;16:72A. [CENTRAL: CN‐00644316] [Google Scholar]
- Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Legendre C, et al. Sirolimus‐based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. American Journal of Transplantation 2004;4(6):953‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mota A, Arias M, Taskinen EI, Paavonen T, Legendre C, Claesson K, et al. Sirolimus‐based therapy after early cyclosporine withdrawal results in significantly better renal histology and function at 3 years following kidney transplantation [abstract no: 1486]. American Journal of Transplantation 2004;4(Suppl 8):566. [CENTRAL: CN‐00509364] [DOI] [PubMed] [Google Scholar]
- Mota A, Segoloni G, Legendre C, Prats D, Hutchison B, Morales JM, et al. Patients benefit from cyclosporine withdrawal followed by sirolimus (Rapamune) maintenance therapy irrespective of baseline renal function [abstract no: 395]. American Journal of Transplantation 2002;2(Suppl 3):237. [CENTRAL: CN‐00420843] [Google Scholar]
- Oberbauer R, Arias M, Claesson K, Mota A, Eris JM, Hutchison B, et al. Two‐year assessment of health‐related quality of life in sirolimus‐treated kidney transplant patients after cyclosporine elimination [abstract no: 0257]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416378]
- Oberbauer R, Hutchison B, Eris J, Arias M, Claesson K, Mota A, et al. Health‐related quality‐of‐life outcomes of sirolimus‐treated kidney transplant patients after elimination of cyclosporine A: results of a 2‐year randomized clinical trial. Transplantation 2003;75(8):1277‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oberbauer R, Hutchison B, Eris J, Arias M, Mota A, the Rapamune Maintenance Regimen Study Group. Health‐related quality‐of‐life assessment in kidney transplant patients receiving sirolimus maintenance therapy with early elimination of cyclosporine: 3‐year results [abstract no: W758]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):243. [CENTRAL: CN‐00446999] [Google Scholar]
- Oberbauer R, Hutchison B, Eris J, Riad H, Claesson K, Lawen J, et al. Three‐year assessment of health‐related quality of life in sirolimus‐treated kidney transplant patients after cyclosporine elimination [abstract no: 249]. American Journal of Transplantation 2003;3(Suppl 5):215. [CENTRAL: CN‐00447000] [Google Scholar]
- Oberbauer R, Kreis H, Campistol JM, Mota A, Daloze P, Ruiz JC, et al. Renal function improves significantly after early cyclosporine withdrawal in sirolimus‐treated renal transplant recipients: 3‐year results of the Rapamune Maintenance Regimen (RMR) trial [abstract no: F‐FC041]. Journal of the American Society of Nephrology 2003;14(Nov):10A. [CENTRAL: CN‐00550606] [Google Scholar]
- Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, et al. Long‐term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2‐year results of the Rapamune Maintenance Regimen Study. Transplantation 2003;76(2):364‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oberbauer R, Kreis H, Johnson RWG, Mota A, Claesson K, Arias M, et al. Long‐term improvement in renal function is shown in patients treated with sirolimus (Rapamune) and cyclosporine withdrawal: 2‐year results of the Rapamune Maintenance Regimen trial [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416379]
- Oberbauer R, Mota A, Prats D, Mathew T, Morales JM, Daloze P, et al. Renal transplant patients with risk factors for reduced renal function benefit from cyclosporine withdrawal and sirolimus maintenance therapy [abstract no: W742]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786‐7. [CENTRAL: CN‐00447001] [Google Scholar]
- Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, et al. Early cyclosporine withdrawal from a sirolimus‐based regimen results in better renal allograft survival and renal function at 48 months after transplantation [abstract no: O80]. Transplantation 2004;78(2 Suppl):30. [CENTRAL: CN‐00653813] [DOI] [PubMed] [Google Scholar]
- Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, et al. Early cyclosporine withdrawal from a sirolimus‐based regimen results in better renal allograft survival and renal function at 48 months after transplantation.[erratum appears in Transpl Int. 2005 Mar;18(3):369]. Transplant International 2005;18(1):22‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paczek L, Mota A, Schena FP, Johnson RW, Prats D, Mathew T, et al. Renal function benefit from sirolimus maintenance therapy after early cyclosporine withdrawal in the presence of risk factors [abstract no: 180]. American Journal of Transplantation 2003;3(Suppl 5):197. [CENTRAL: CN‐00447075] [Google Scholar]
- Ruiz JC, Campistol JM, Grinyo JM, Mota A, Prats D, Gutierrez JA, et al. Early cyclosporine a withdrawal in kidney‐transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation 2004;78(9):1312‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Campistol JM, Mota A, Prats D, Gutierrez JA, Castro A, et al. Early cyclosporine A withdrawal in kidney transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions [abstract no: SU‐FC0235]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):48A. [CENTRAL: CN‐00447509] [Google Scholar]
- Ruiz JC, Campistol JM, Mota A, Prats D, Gutierrez JA, Castro A, et al. Early elimination of cyclosporine in kidney transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation Proceedings 2003;35(5):1669‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Campistol JM, Sanchez‐Fructuoso A, Mota A, Grinyo JM, Paul J, et al. Early sirolimus use with cyclosporine elimination does not induce progressive proteinuria. Transplantation Proceedings 2007;39(7):2151‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Campstol JM, Sanchez‐Fructuoso A, Mota A, Grinyo JM, Paul J, et al. The early use of sirolimus with CsA elimination is not associated with progressive proteinuria [abstract no: P113]. Transplant International 2007;20(Suppl 2):122. [CENTRAL: CN‐00740556] [Google Scholar]
- Russ G, Eris J, Hutchison B, Walker R, Chapman J, Pussell B, et al. Anaemia in sirolimus‐treated renal transplant recipients: the importance of preserving renal function [abstract no: 2504]. Nephrology 2006;11(Suppl 2):A37. [CENTRAL: CN‐00644362] [Google Scholar]
- Russ G, Eris J, Hutchison B, Walker R, Chapman J, Pussell B, et al. Sirolimus (SRL) maintenance therapy after early cyclosporine (CsA) withdrawal benefits renal transplant recipients with risk factors for reduced renal function: 5‐year results [abstract no: 103]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand (TSANZ); 2006 Mar 29‐31; Canberra, Australia. 2006:90. [CENTRAL: CN‐00644320]
- Russ G, Eris J, Hutchison B, Walker R, Chapman J, Pussell B, et al. Sirolimus‐based therapy following early cyclosporine withdrawal resulted in superior renal allograft survival at 48 months compared with continuous combined sirolimus and cyclosporine [abstract no: P150]. Nephrology 2004;9(Suppl 1):A38. [CENTRAL: CN‐00509449] [Google Scholar]
- Russ G, Eris J, Hutchison B, Walker R, Chapman J, Pussell B, et al. Superior outcomes after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function: intent‐to‐treat results at 4 years [abstract no: 159]. Nephrology 2005;10(Suppl 3):A422. [CENTRAL: CN‐00644317] [Google Scholar]
- Russ G, Jamieson N, Oberbauer R, Arias M, Murgia MG, Blancho G, et al. Three‐year health‐related quality‐of‐life outcomes for sirolimus‐treated kidney transplant patients after elimination of cyclosporine. Transplant International 2007;20(10):875‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Russ G, Oberbauer R, Friend P, Campistol JM, Burke JT. The impact of renal function on the incidence of anemia in sirolimus‐treated renal transplant recipients [abstract no: TH‐PO563]. Journal of the American Society of Nephrology 2006;17(Abstracts):227A. [CENTRAL: CN‐00615898] [Google Scholar]
- Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Long‐term impact of baseline renal function in kidney transplant patients receiving sirolimus maintenance therapy after early cyclosporine withdrawal [abstract no: FC‐50004]. Nephrology 2005;10(Suppl 1):A2. [CENTRAL: CN‐00644319] [DOI] [PubMed] [Google Scholar]
- Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Superior outcomes after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function: intent‐to‐treat results at 4 years [abstract no: 1009]. American Journal of Transplantation 2005;5(Suppl 11):413. [DOI] [PubMed] [Google Scholar]
- Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Superior outcomes in renal transplantation after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function. Transplantation 2005;80(9):1204‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schena FP, Jamieson N, Colon J, Shoker A, Pena J, Martinez M, et al. Favorable cardiovascular risk profile when sirolimus is used without cyclosporine compared to combination therapy: 3‐year results of the Rapamune Maintenance Regimen trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):479. [CENTRAL: CN‐00447616] [Google Scholar]
- Vanrenterghem Y, Kreis H, Oberbauer R, Mota A, Johnson WG, Sirolimus Tri‐contienental Renal Transplant Study Group. Improved renal function in renal allograft recipients with sirolimus (Rapamune) maintenance therapy and early cyclosporine withdrawal: 12‐month results [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A209. [CENTRAL: CN‐00448163] [Google Scholar]
- Velosa JA, Larson TS, Gloor JM, Stegall MD. Cyclosporine elimination in the presence of TOR inhibitors: effects on renal function, acute rejection, and safety. American Journal of Kidney Diseases 2001;38(4 Suppl 2):S3‐S10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walker R, Russ G, Eris J, Hutchinson B, Chapman J, Pussell B, et al. Treatment of hyperlipidemia in sirolimus (SRL)‐treated renal transplant recipients: long‐term findings from the Rapamune Maintenance Regimen (RMR) Trial [abstract no 27]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand [TSANZ]; 2006 Mar 29‐31; Canberra, Australia. 2006:49. [CENTRAL: CN‐00644318]
Rossini 2007 {published data only}
- Rossini M, Loverre A, Stallone G, Infante B, Schena A, Maiorano A, et al. Proteinuria and vascular endothelial growth factor (VEGF) expression following calcineurin inhibitors (CNI) withdrawal and conversion to rapamycin [abstract no: 1149]. American Journal of Transplantation 2007;7(Suppl 2):442. [CENTRAL: CN‐00725010] [Google Scholar]
- Rossini M, Stallone G, Infante B, Capobianco C, Loverre A, Schena A, et al. Proteinuria and vascular endothelial growth factor (VEGF) expression following calcineurin inhibitors (CNI) withdrawal and conversion to rapamycin [abstract no: 548]. Transplant International 2007;20(Suppl 2):226. [Google Scholar]
Russ 2003 {published data only}
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Comparison of reduced‐and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results [abstract]. Transplantation Society of Australia and New Zealand. 21st Annual Meeting; 2003 Apr 9‐11; Canberra, ACT. 2003:64. [CENTRAL: CN‐00447521]
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. The safety and efficacy of reduced‐ and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results from Australia [abstract no: SA‐PO496]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):364A. [CENTRAL: CN‐00447522] [Google Scholar]
- Russ GR, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Reduced and standard target concentration tacrolimus with sirolimus in renal allograft recipients. Transplantation Proceedings 2003;35(3 Suppl):115S‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351] [Google Scholar]
Salvadori 2007 {published data only}
- Salvadori M, Scolari MP, Stefoni S, Bertoni E, Sandrini S, Rigotti P, et al. Randomized trial of sodium mycophenolate (MPS) and basiliximab in combination with reduced or standard cyclosporine (CsA) in old recipients of kidney transplant (KTx) [abstract no: O069]. Transplant International 2007;20(Suppl 2):20. [Google Scholar]
- Salvadori M, Scolari MP, Stefoni S, Bertoni E, Sandrini S, Rigotti P, et al. Randomized trial of sodium mycophenolate and basiliximab in combination with reduced or standard cyclosporine exposure in old recipients of kidney transplants from deceased donors [abstract no: 108]. Transplantation 2008;86(2 Suppl):39. [CENTRAL: CN‐00679020] [Google Scholar]
Schaefer 2006 {published data only}
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short‐term results under three different immunosuppressive regimens at one center. Transplantation Proceedings 2006;38(10):3466‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smak Gregoor 1999 {published data only}
- Roodnat J, Hilbrands LB, Hene RJ, Sevaux RG, Gregoor PJ, Kal‐van Gestel JA, et al. 15 year follow‐up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation [abstract no: BO156]. Transplant International 2013;26(Suppl 2):83‐4. [EMBASE: 71359271] [Google Scholar]
- Roodnat JI, Hilbrands LB, Hene RJ, Sevaux RG, Smak Gregoor PJ, Kal‐van Gestel JA, et al. 15‐year follow‐up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014;98(1):47‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hesse CJ, Hilbrands LB, Vos P, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999;68(10):1603‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. A prospective randomised study of withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: 18 months follow‐up data [abstract no: 441]. American Journal of Transplantation 2001;1(Suppl 1):246. [CENTRAL: CN‐00763745] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. Journal of the American Society of Nephrology 2002;13(5):1365‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Gelder T, IJzermans JN, Weimar W. Long‐term results of a randomized, prospective study after withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone [abstract]. American Journal of Transplantation 2003;3(Suppl 5):217. [CENTRAL: CN‐00447777] [Google Scholar]
- Gelder T, Sevaux R, Hene R, Weimar W, Hoitsma A, Ligtenberg G, et al. Discontinuation of cyclosporine or prednisone 6 months after kidney transplantation: a randomized trial [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):920A. [CENTRAL: CN‐00583812] [Google Scholar]
- Sevaux RG, Gregoor P, Smak JH, Hene RJ, Weimar W, Hoitsma AJ, et al. Withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: a randomized study [abstract no: 935]. Transplantation 1999;67(7):S240. [CENTRAL: CN‐00767038] [Google Scholar]
SMART TX Study 2010 {published data only}
- Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J, et al. A randomized multicenter trial of early conversion to sirolimus/mycophenolate/steroids versus cyclosporine/mycophenolate/steroids in renal transplantation: one‐year analysis (SMART‐Study) [abstract no: 1088]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00763842] [Google Scholar]
- Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J, et al. Comparison of a delayed low‐dose sirolimus (Siro)/mycophenolate mofetil (MMF)‐based therapy with standard cyclosporine (CsA/MMF‐based immunosuppression in patients after kidney transplantation (SMART study) [abstract no: P188]. Transplant International 2007;20(Suppl 2):141. [CENTRAL: CN‐00724890] [Google Scholar]
- Guba M, Pratschke J, Hugo C, Kramer B, Nohr‐Westphal C, Brockmann J. Renal function, efficacy and safety of sirolimus and mycophenolate mofetil therapy after early calcineurin‐inhibitor withdrawal in de novo renal transplant patients: one‐year analysis of a randomized multicenter trial [abstract no: O‐296]. Transplant International 2009;22(Suppl 2):78. [DOI] [PubMed] [Google Scholar]
- Guba M, Pratschke J, Hugo C, Kramer BK, Pascher A, Pressmar K, et al. Early conversion to a sirolimus‐based, calcineurin‐inhibitor‐free immunosuppression in the SMART trial: observational results at 24 and 36months after transplantation. Transplant International 2012;25(4):416‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guba M, Pratschke J, Hugo C, Krämer BK, Nohr‐Westphal C, Brockmann J, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short‐term calcineurin inhibitor‐based quadruple therapy in de novo renal transplant patients: one‐year analysis of a randomized multicenter trial. Transplantation 2010;90(2):175‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jauch KW, Pratschke J, Hugo C, Kramer B, Hakenberg O, Brockmann J, et al. Delayed introduction of a calcineurin‐inhibitor (CNI)‐free, sirolimus (SRL)‐based immunosuppression in renal transplantation is safe. Preliminary results of the randomised, multicenter SMART Trial [abstract no: 535]. Transplantation 2008;86(Suppl 2):187. [CENTRAL: CN‐00740484] [Google Scholar]
Spare‐the‐Nephron Study 2011 {published data only}
- Kalil R, Pearson TC, Mulgaonkar S, Patel A, Shidban H, Weir M, et al. Final 1‐year outcomes of the spare‐the‐nephron (STN) trial: mycophenolate mofetil (MMF) ‐ based regimen combined with sirolimus (SRL) to preserve renal function in renal transplantation [abstract no: TH‐FC042]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):10A. [CENTRAL: CN‐00716075] [Google Scholar]
- Mulgaonkar S, Pearson TC, Patel A, Scandling J, Shidban H, Weir M, et al. Final renal function outcomes from the Spare‐the‐Nephron (STN) trial: mycophenolate mofetil (MMF)/sirolimus (SRL) maintenance therapy and CNI withdrawal in renal transplant recipients [abstract no: 530]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00690361] [Google Scholar]
- Patel A, Weir MR, Wali R, Pearson T, Mulgaonkar S, Shidban H, et al. Spare‐the‐Nephron (STN) Trial: Updated analysis of renal function after one year of mycophenolate mofetil/sirolimus maintenance therapy and calcineurin inhibitor withdrawal in renal transplant recipients [abstract no: 1138]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615833] [Google Scholar]
- Pearson T, Mulgaonkar S, Kalil R, Patel A, Scandling J, Patel D, et al. CNI withdrawal in African Americans ‐ 1 year outcomes of African American renal transplant recipients in the Spare‐the‐Nephron (STN) trial [abstract no: 1083]. American Journal of Transplantation 2009;9(Suppl 2):496. [Google Scholar]
- Pearson TC, Mulgaonkar S, Patel A, Scandling J, Shidban H, Weir M, et al. Efficacy and safety of mycophenolate mofetil (MMF)/sirolimus (SRL) maintenance therapy after calcineurin inhibitor (CNI) withdrawal in renal transplant recipients: final results of the Spare‐the‐Nephron (STN) trial [abstract no: 129]. American Journal of Transplantation 2008;8(Suppl 2):213. [CENTRAL: CN‐00690150] [Google Scholar]
- Pearson TC, Patel A, Scandling J, Shidban H, Weir M, Patel D, et al. Final results of the Spare the Nephron trial (CNI withdrawal trial) [abstract no: 629]. Transplantation 2008;86(2 Suppl):220‐1. [Google Scholar]
- Scandling J, Mulgaonkar S, Shidban H, Waid T, Peddi VR, Patel D. Effect of conversion from a calcineurin inhibitor (CNI)‐based regimen to sirolimus on proteinuria: 1‐year results of the spare‐the‐nephron trial in renal transplant recipients [abstract no: F‐PO594]. Journal of the American Society of Nephrology 2007;18(Abstracts):230A. [CENTRAL: CN‐00716076] [Google Scholar]
- Wali R, Pearson TC, Shidban H, Patel A, Chan L, Patel D. The Spare‐the‐Nephron Trial: Evaluating mycophenolate mofetil (MMF)/Sirolimus (SRL) maintenance therapy after calcineurin inhibitor (CNI) withdrawal, 1 year interim results [abstract no: F‐PO1087]. Journal of the American Society of Nephrology 2006;17(Abstracts):565A. [CENTRAL: CN‐00615895] [Google Scholar]
- Weir M, Mulgaonkar S, Pearson T, Patel A, Patel D, Shidban H, et al. Mycophenolate mofetil/sirolimus maintenance therapy after calcineurin inhibitor withdrawal in renal transplant recipients: 2‐year outcomes of the SPARE‐THE‐NEPHRON (STN) trial [abstract no: 33]. American Journal of Transplantation 2009;9(Suppl 2):200‐1. [Google Scholar]
- Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, et al. Mycophenolate mofetil‐based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare‐the‐Nephron trial. Kidney International 2011;79(8):897‐907. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Pearson TC, Patel A, Peddi VR, Kalil R, Scandling J, et al. Long‐term follow‐up of kidney transplant recipients in the Spare‐the‐Nephron‐Trial. Transplantation 2016;101(1):157‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Wali R, Pearson TC, Patel A, Mulgaonkar S, Shidban H, et al. Spare‐the‐Nephron (STN) Trial: Updated 1 year efficacy and safety of mycophenolate mofetil/Sirolimus maintenance therapy after calcineurin inhibitor withdrawal in renal transplant recipients [abstract no: 51]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615832] [Google Scholar]
Stallone 2003 {published data only}
- Stallone G, Paola S, Schena A, Infante B, Grandaliano G, Battaglia M, et al. Early withdrawal of cyclosporine A improves 1‐year kidney graft structure and function in sirolimus‐treated patients. Transplantation 2003;75(7):998‐1003. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Grandaliano G, Gesualdo L, Schena PF, et al. Early withdrawal of cyclosporine (CSA) ameliorates 1‐year kidney graft function and structure in sirolimus (SRL)‐treated patients. [abstract no: 1014]. American Journal of Transplantation 2002;2(Suppl 3):393. [CENTRAL: CN‐00527169] [Google Scholar]
Stallone 2004 {published data only}
- Stallone G, Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1‐year graft function. Journal of the American Society of Nephrology 2004;15(1):228‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Schena A, Paolo S, Gesualdo L, Ditonno P, et al. Sirolimus prolongs delayed graft function in recipients of sub‐optimal cadaveric kidney donors [abstract]. American Journal of Transplantation 2003;3(Suppl 5):563. [CENTRAL: CN‐00447829] [Google Scholar]
Stegall 2003 {published data only}
- Dean PG, Grande JP, Sethi S, Park WD, Griffin MD, Cosio FG, et al. Kidney transplant histology after one year of continuous therapy with sirolimus compared with tacrolimus. Transplantation 2008;85(8):1212‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effect of immunosuppression on renal function and graft histology: a comparison of tacrolimus and sirolimus [abstract no: 379]. American Journal of Transplantation 2005;5(Suppl 11):252. [CENTRAL: CN‐00725015] [Google Scholar]
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effects of calcineurin inhibitor avoidance on renal function and graft histology after kidney transplantation: a prospective, randomized comparison of tacrolimus and sirolimus [abstract no: 259]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509154] [Google Scholar]
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus [abstract]. American Journal of Transplantation 2004;4(Suppl 8):229. [DOI] [PubMed] [Google Scholar]
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound‐healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77(10):1555‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hamad A, Buehrig CK, Kreps MA, Lager DJ, Fidler ME, Gloor JM, et al. Frequency of polyomavirus‐associated nephropathy in a randomized controlled trial of tacrolimus versus sirolimus‐based immunosuppression [abstract no: 147]. American Journal of Transplantation 2003;3(Suppl 5):189. [CENTRAL: CN‐00445626] [Google Scholar]
- Kudva YC, Basu A, Larson TS, Stegall MD. Metabolic complications after renal transplantation: a comparison of sirolimus vs tacrolimus‐based immunosuppression [abstract no: 256]. American Journal of Transplantation 2003;3(Suppl 5):217. [CENTRAL: CN‐00446214] [Google Scholar]
- Larson TS, Dean PG, Griffin MD, Prieto M, Schwab TR, Cosio FG, et al. Preserving renal function by avoiding calcineurin‐inhibitors in renal transplantation: a randomized trial of sirolimus vs. tacrolimus [abstract no: 461]. American Journal of Transplantation 2004;4(Suppl 8):285. [CENTRAL: CN‐00509307] [Google Scholar]
- Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. American Journal of Transplantation 2006;6(3):514‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Larson TS, Griffin MD, Prieto M, Schwab TR, Lund WJ, Nyberg SL, et al. Avoiding calcineurin‐inhibitors in renal transplantation: a randomized trial of sirolimus vs tacrolimus [abstract no: 1261]. American Journal of Transplantation 2003;3(Suppl 5):475. [CENTRAL: CN‐00446279] [DOI] [PubMed] [Google Scholar]
- Larson TS, Velosa JA, Prieto M, Lund WJ, Griffin MD, Gloor JM, et al. Kidney transplantation without calcineurin‐inhibitors: a randomized trial of sirolimus vs tacrolimus [abstract no: 396]. American Journal of Transplantation 2002;2(Suppl 3):237. [Google Scholar]
- Larson TS, Velosa JA, Prieto M, Lund WJ, Griffin MD, Gloor JM, et al. Kidney transplantation without calcineurin‐inhibitors: a randomized trial of sirolimus vs tacrolimus [abstract no: O552]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416102]
- Lund WJ, Larson TS, Stegall MD, Prieto M, Kremers WK. Obesity increases wound complications in sirolimus‐treated renal allograft recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):214. [CENTRAL: CN‐00446497] [Google Scholar]
- Qian Q, Du H, King BF, Kumar S, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients after renal transplantation [abstract no: SA‐PO094]. Journal of the American Society of Nephrology 2007;18(Abstracts):365A. [CENTRAL: CN‐00716079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. Journal of the American Society of Nephrology 2008;19(3):631‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegall MD, Larson TS, Prieto M, Gloor J, Textor S, Nyberg S, et al. Kidney transplantation without calcineurin inhibitors using sirolimus. Transplantation Proceedings 2003;35(3 Suppl):125S‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suwelack 2002 {published data only}
- Hillebrand U, Suwelack B, Gerhardt U, Kobelt V, Kraemer J, Hohage H. Impact of calcineurin inhibitor withdrawal and mycophenolate mofetil substitution on hematologic toxicity [abstract no: 0014]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415864]
- Suwelack B, Diet KH, Kobelt V, Hohage H, Gerhardt U. Impact of calcineurin‐inhibitor withdrawal on arterial distensibility and endothelial function in chronic allograft nephropathy [abstract no: 1048]. American Journal of Transplantation 2002;2(Suppl 3):402. [CENTRAL: CN‐00416722] [Google Scholar]
- Suwelack B, Gerhardt U, Hohage H. Withdrawal of cyclosporine or tacrolimus after addition of mycophenolate mofetil in patients with chronic allograft nephropathy. American Journal of Transplantation 2004;4(4):655‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suwelack B, Gerhardt U, Kobelt V, Hillebrand U, Matzkies F, Hohage H. Design and preliminary results of a randomized study on the conversion of treatment with calcineurin inhibitors to mycophenolate mofetil in chronic renal graft failure: effect, on serum cholesterol levels. Transplantation Proceedings 2002;34(5):1803‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suwelack B, Hausberg M, Hillebrand U, Rahn KH, Hohage H. Study on effects of calcineurin inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients [abstract no: 0637]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416723] [DOI] [PubMed]
- Suwelack B, Kobelt V, Gerhardt U, Volmer S, Hohage H. Course of intima thickness in long term renal transplant recipients treated with or without calcineurin‐inhibitors [abstract no: P04.21]. Kidney & Blood Pressure Research 2004;27(5‐6):335. [CENTRAL: CN‐00615862] [Google Scholar]
- Suwelack B, Kobelt V, Hillebrand U, Gerhardt U, Matzkies F, Hohage H. Replacement of calcineurin inhibitors by mycophenolate mofetil: effect on hemoglobine levels. Transplantation Proceedings 2002;34(5):1808‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suwelack B, Kobelt V, Hohage H, Gerhardt U. Studies on effects of calcineurin‐inhibitor withdrawal on arterial wall structure and intima media thickness in longterm transplant recipients [abstract no: P290]. Transplantation 2004;78(2 Suppl):295. [CENTRAL: CN‐00509495] [Google Scholar]
- Suwelack B, Malyar V, Waldschmidt G, Hohage H. 5 year follow‐up of a randomized study on calcineurin‐inhibitor‐withdrawal with addition of MMF in chronic allograft nephropathy [abstract no: F‐PO600]. Journal of the American Society of Nephrology 2007;18(Abstracts):231A. [Google Scholar]
- Suwelack BM, Gerhardt UW, Hohage H. Influence of calcineurin‐inhibitor free immunosuppression on carotid intima media thickness in chronic allograft nephropathy [abstract no: 659]. American Journal of Transplantation 2003;3(Suppl 5):320. [CENTRAL: CN‐00447914] [Google Scholar]
SYMPHONY Study 2007 {published data only}
- Bagul A, Nicholson M, Chavez R, Grinyo J, Frei U, Vanrenterghem Y, et al. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: The experience of the SYMPHONY study [abstract no: P23]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
- Chavez R, Nicholson M, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, et al. SYMPHONY ‐ comparing efficacy of standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation. Sub analysis of GFR in cases that completed one year within an intended regime [abstract no: O06]. British Transplantation Society (BTS). 10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
- Claes K, Meier‐Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrology Dialysis Transplantation 2012;27(2):850‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981] [Google Scholar]
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [Google Scholar]
- Demirbas A, Hugo C, Grinyo J, Frei U, Gurkan A, Marcen R, et al. Low toxicity regimens in renal transplantation: a country subset analysis of the Symphony study. Transplant International 2009;22(12):1172‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Demirbas A, Hugo C, Grinyo J, Frei U, Gurkan A, Marcen R, et al. Results of the Symphony Study are applicable across transplant populations; experience from Spain, Turkey and Germany [abstract no: P581]. Transplant International 2007;20(Suppl 2):234. [Google Scholar]
- Ekberg H, Bernasconi C, Halloran P. CNI minimisation with 2 G mycophenolate mofetil ‐ what have we learned from the Symphony Study [abstract no: 964]. Transplantation 2008;86(2 Suppl):334. [CENTRAL: CN‐00671785] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrology Dialysis Transplantation 2010;25(6):2004‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retained their distinct toxicity profiles despite low doses in the Symphony Study [abstract no: 55]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653721] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Halloran P, Vanrenterghem Y, Schold J, Meier‐Kriesche H. Renal function progression following DGF and early AR is not impaired if function is restored: evidence from the Symphony Study [abstract no: P583]. Transplant International 2007;20(Suppl 2):235. [Google Scholar]
- Ekberg H, Mamelok R, Bernasconi C, Vincenti F, Tedesco‐Silva H, Daloze P, et al. The challenge of meeting target drug concentrations: experience from the Symphony study [abstract no: 58]. American Journal of Transplantation 2007;7(Suppl 2):161. [CENTRAL: CN‐00615834] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Bitko S, Klempnauer J, Gurkan A, et al. Improved outcomes after de novo renal transplantation: 2‐year results from the Symphony Study [abstract no: 531]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00653754] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: LB04]. American Journal of Transplantation 2008;8(Suppl 2):336. [CENTRAL: CN‐00653755] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing with mycophenolate mofetil in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: 623]. Transplantation 2008;86(2 Suppl):218. [CENTRAL: CN‐00676055] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. New England Journal of Medicine 2007;357(25):2562‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Symphony ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: 49]. American Journal of Transplantation 2006;6(Suppl 2):83. [Google Scholar]
- Ekberg H, Vincenti F, Tedesco da Silva H, Daloze P, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the Symphony Study [abstract no: 691]. American Journal of Transplantation 2006;6(Suppl 2):300. [CENTRAL: CN‐00602015] [Google Scholar]
- Frei U, Daloze P, Vitko S, Klempnauer J, Reyes‐Acevedo R, Titiz I, et al. Acute rejection in low‐toxicity regimens: clinical impact and risk factors in the Symphony study. Clinical Transplantation 2010;24(4):500‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frei U, Daloze P, Vitko S, Klempnauer J, Reyes‐Acevedo R, Titiz I, et al. Characterization of acute rejections and associated relative risk factors in the Symphony Study [abstract no: 245]. American Journal of Transplantation 2007;7(Suppl 2):210. [CENTRAL: CN‐00653713] [Google Scholar]
- Frei U, Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, et al. SYMPHONY ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: F‐FC152]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00602018] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Gentil MA, Hernandez D, Plumed JS, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study [abstract no: P342]. Transplant International 2007;20(Suppl 2):177. [CENTRAL: CN‐00653737] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the Symphony PK substudy [abstract no: 824]. American Journal of Transplantation 2006;6(Suppl 2):345. [CENTRAL: CN‐00602016] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study [abstract no: 1151]. American Journal of Transplantation 2007;7(Suppl 2):443. [Google Scholar]
- Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard‐dose or low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony pharmacokinetic substudy. Nephrology Dialysis Transplantation 2009;24(7):2269‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Halloran P, Meier‐Kriesche HU, Schold J, Vanrenterghem Y. Blood pressure in the first year following renal transplantation is associated with immunosuppressive regimens: evidence from the Symphony study [abstract no: 1137]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615835] [Google Scholar]
- Hugo C, Frei U, Margreiter R, Peeters P, Ok E, Viebahn R, et al. Elderly kidney transplant recipients are a high‐risk group for death, infections and PTx diabetes: evidence from the Symphony study [abstract no: 154]. American Journal of Transplantation 2007;7(Suppl 2):186. [CENTRAL: CN‐00724927] [Google Scholar]
- Hugo C, Frei U, Margreiter R, Peeters P, Toz H, Viebahn R, et al. Marginal donor quality transmits a high risk profile in elderly but not young renal transplant patients ‐ a subgroup analysis of the Symphony Study [abstract no: F‐PO1471]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):436A. [CENTRAL: CN‐00724891] [Google Scholar]
- Kuypers D, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the SYMPHONY PK substudy [abstract no: TH‐PO564]. Journal of the American Society of Nephrology 2006;17(Abstracts):227A. [Google Scholar]
- Llaudo I, Colom H, Gimenez‐Bonafe P, Torras J, Caldes A, Sarrias M, et al. Do drug transporter (ABCB1) SNPs and P‐glycoprotein function influence cyclosporine and macrolides exposure in renal transplant patients? Results of the pharmacogenomic substudy within the SYMPHONY study. Transplant International 2013;26(2):177‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Brunet M, Torras J, Cruzado JM, Oppenheimer F, Sanchez‐Plumed J, et al. Influence of MRP2 and UGT1A9 polymorphisms in the MPA pharmacokinetics in renal transplant recipients. Results of the pharmacogenomic substudy within the Symphony Study [abstract no: TH‐PO508]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):220A. [CENTRAL: CN‐00724892] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Brunet M, Torras J, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. Influence of MRP2 and UGT1A9 polymorphisms in the MPA pharmacokinetics in renal transplant substudy within the Symphony Study [abstract no: 2243]. Transplantation 2008;86(2 Suppl):733. [CENTRAL: CN‐00671787] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Llaudo I, Torras J, Caldes A, Cruzado JM. Polymorphisms of the MDR1 gene in renal transplant recipients affect the PGP activity. Results of the pharmacogenetic substudy within the Symphony Study [abstract no: 2244]. Transplantation 2008;86(2 Suppl):734. [CENTRAL: CN‐00671786] [Google Scholar]
- Lloberas N, Llaudo I, Torras J, Cruzado JM, Caldes A, Oppenheimer F, et al. Polymorphisms of the MDR1 gene in renal transplant recipients affect the PGP activity. Results of the pharmacogenomic substudy (Symphony Study) [abstract no: TH‐PO507]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):220A. [CENTRAL: CN‐00724893] [Google Scholar]
- Lloberas N, Torras J, Cruzado JM, Andreu F, Oppenheimer F, Sanchez‐Plumed J, et al. Influence of MRP2 on MPA pharmacokinetics in renal transplant recipients‐results of the pharmacogenomic substudy within the Symphony Study. Nephrology Dialysis Transplantation 2011;26(11):3784‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meier‐Kriesche H, Halloran P, Vanrenterghem Y, Schold J. Early immunosuppression exposure and incidence of acute rejection and renal function progression: evidence from the Symphony Study [abstract no: 244]. American Journal of Transplantation 2007;7(Suppl 2):210. [CENTRAL: CN‐00724894] [Google Scholar]
- Meier‐Kriesche H, Schold J, Vanrenterghem Y, Halloran P, Ekberg H. Factors associated with BMI increases early after renal transplantation: evidence from the Symphony Study [abstract no: P582]. Transplant International 2007;20(Suppl 2):234. [Google Scholar]
- O'Connell PJ, Walker RG, Ierino F, Eris J, Russ G, Grinyo J, et al. Results of the SYMPHONY trial in renal transplantation ‐ comparing standard immunosuppression to low dose cyclosporine (low‐CSA), tacrolimus (low‐TAC) or sirolimus (low‐SRL with MMF, daclizumab and corticosteroids [abstract]. Immunology and Cell Biology 2007;85(4):A33. [Google Scholar]
- Oppenheimer F, Rebello P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Health‐related quality of life of patients receiving low toxicity immunosuppressive regimens. Preliminary results of the quality of life substudy of the Elite Symphony Study [abstract no: TH‐PO566]. Journal of the American Society of Nephrology 2006;17(Abstracts):228A. [CENTRAL: CN‐00747305] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez J, Gonzalez M, et al. Health care resource consumption (HCRC) of patients receiving low toxicity immunosuppressive regimens. One year follow up results of the quality of life substudy within the Symphony study [abstract no: P108]. Transplant International 2007;20(Suppl 2):121. [CENTRAL: CN‐00724895] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Cost‐effectiveness analysis of adverse events in low toxicity immunosuppressive regimens, preliminary results of the quality of life substudy within the Symphony Study [abstract no: 107]. Transplantation 2008;86(2 Suppl):38. [CENTRAL: CN‐00677744] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Health‐related quality of life of patients receiving low‐toxicity immunosuppressive regimens: a substudy of the Symphony study. Transplantation 2009;87(8):1210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Meier‐Kriesche H, Schold J, Halloran P, Ekberg H. Levels and progression of parameters associated with metabolic syndrome by immunosuppressive regimen: evidence from the Symphony Study [abstract no: P580]. Transplant International 2007;20(Suppl 2):234. [Google Scholar]
Takahashi 2013a {published data only}
- Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu‐Artis C, et al. Efficacy of everolimus with reduced cyclosporine in Japanese de novo renal transplant recipients: 24‐month, randomized, multicenter study [abstract no: B944]. American Journal of Transplantation 2013;13(Suppl S5):314. [EMBASE: 71057520] [Google Scholar]
- Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in Japanese adult de‐novo renal transplant patients: 12 month results [abstract no: 935]. American Journal of Transplantation 2012;12(Suppl S3):300. [EMBASE: 70746888] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration‐controlled everolimus with reduced‐dose cyclosporine in Japanese de novo renal transplant patients: 12‐month results. Transplantation Research 2013;2(1):14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Hoshinaga K, Watarai Y, Goto N, Kusaka M, Sasaki H, et al. Pharmacokinetics of everolimus when combined with cyclosporine in Japanese de novo renal transplant recipients. Transplantation Proceedings 2014;46(5):1314‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Watarai Y, Akutsu N, Saito K, Nakagawa Y, Kamisawa O, Kenmochi T. Everolimus plus reduced‐exposure calcineurin inhibitor versus mycophenolate mofetil plus standard‐exposure calcineurin inhibitor: 2‐year results in living donor kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953410] [Google Scholar]
Tedesco‐Silva 2010 {published data only}
- Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 43]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:64.
- Carmellini M, Garcia V, Wang Z, Vergara M, Russ G. Efficacy of everolimus with reduced‐exposure cyclosporine in de novo kidney transplant patients at increased risk for efficacy events: analysis of a randomized trial. Journal of Nephrology 2015;28(5):633‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carmellini M, Garcia V, Wong Z, Vergara M, Escrig C, Russ G. Treatment with everolimus and reduced‐exposure cyclosporine is efficacious in de novo renal transplant recipients at increased risk for efficacy failure: Post‐Hoc analysis from the A2309 study [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71954404] [Google Scholar]
- Chadban S, Pilmore H, Russ G, Kanellis J, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: seven year follow‐up of ANZ patients from a randomised controlled trial [abstract no: 079]. Nephrology 2015;30(Suppl 3):39. [EMBASE: 71995868] [Google Scholar]
- Cibrik D, Johnston T, Kim YS, Walker R, Zibari G, Mange K, et al. Everolimus allows for around 60% reduction in CsA exposure over 12 months: results from a multicenter, prospective study in renal transplantation [abstract no: 1667]. American Journal of Transplantation 2010;10(Suppl 4):511. [EMBASE: 70465042] [Google Scholar]
- Cibrik D, Johnston T, Kim YS, Walker RG, Zibari G, Cornu‐Artis C, et al. Everolimus exposure and relationship to efficacy and safety: results from a multicenter study in renal transplantation using reduced CsA exposure [abstract no: LB25]. American Journal of Transplantation 2010;10(S4):567‐8. [EMBASE: 70465240] [Google Scholar]
- Cibrik D, Kim YS, Johnston T, Walker R, Zibari G, et al. Benefits of everolimus with reduced CsA exposure on renal function: a multicenter, prospective study in renal transplantation [abstract no: 378]. American Journal of Transplantation 2010;10(Suppl 4):151‐2. [EMBASE: 70463739] [Google Scholar]
- Cibrik D, Silva HT, Vathsala A, Lackova E, Cornu‐Artis C, Walker RG, et al. Randomized trial of everolimus‐facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 2013;95(7):933‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colussi G, Vergara M, Wang Z, Roland R. Risk factors associated with efficacy outcomes in kidney transplantation: analysis of a contemporary cohort of patients from the A2309 trial. Clinical Nephrology 2015;83(6):338‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kanellis J, Walker R, Pilmore H, Russ G, Hutchison B, Chadban S, et al. Benefits of everolimus with reduced CSA exposure on renal function: a multicenter, prospective study in renal transplantation [abstract no: 6]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:40.
- Kim YS, Tedesco‐Silva H, Johnston T, Lee P, Zibari G, Walker R, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in de novo renal transplant patients: results from a multicenter, prospective study [abstract no: 1615]. Transplantation 2010;90(Suppl):256. [EMBASE: 71531587] [Google Scholar]
- Pattison J, Riad H, Yaqoob M, Chan L. Correlation of everolimus exposure with reduced CsA exposure to efficacy and safety outcomes: results from a multicentre study in renal transplantation [abstract no: 19]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Pattison J, Riad H, Yaqoob M, Tedesco‐Silva H. Preserved efficacy and renal function from 12 to 24 months with everolimus facilitated CsA reduction [abstract no: 47]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Pattison J, Riad H, Yaqoob M, Tedesco‐Silva H. Renal function in de novo kidney transplant patients receiving everolimus with reduced dose cyclosporin or enteric‐coated mycophenolic acid with standard‐dose cyclosporin: results from a large‐scale, randomised, international trial [abstract no: O36]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Riad H, Pattison J, Yaqoob M, Chan L. 12 month analysis of effects of graft type, donor criteria and gender on improved renal allograft function with everolimus facilitated CNI reduction [abstract no: 21]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Riad H, Pattison J, Yaqoob M, Kim YS. Lower incidence of CMV and BK virus with everolimus vs. mycophenolate in de novo renal transplant patients at 12 months [abstract no: 20]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Riad H, Pattison J, Yaqoob M, Tedesco‐Silva H. Everolimus with reduced‐dose cyclosporin as a strategy to optimise long‐term renal function: results from a randomised study in 833 de novo kidney transplant recipients [abstract no: P43]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Russ G, Chadban S, Campbell S, Hutchison B, Kanellis J, O'Connell P, et al. Everolimus plus reduced‐dose cyclosporine: results from a randomized, phase III study in 833 de‐novo renal transplant recipients [abstract no: 70]. Immunology & Cell Biology 2010;88(6):A22. [EMBASE: 70313681] [Google Scholar]
- Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Everolimus plus reduced CSA exposure: efficacy results from a multicenter, randomized prospective study in renal transplantation [abstract no: 1]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 June 29‐Jul 1; Canberra, ACT. 2011:36.
- Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in de novo renal transplant patients: results from a multicenter, prospective study [abstract no: 70]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 June 29‐Jul 1; Canberra, ACT. 2011:82.
- Shihab F, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, et al. Exposure‐response analysis of everolimus with reduced cyclosporine in renal transplant recipients at 24 months in a randomized trial [abstract no: 929]. American Journal of Transplantation 2012;12(Suppl S3):298‐9. [EMBASE: 70746882] [Google Scholar]
- Shihab F, Cibrik D, Kim YS, Johnston T, Walker R, Roland R, et al. De novo everolimus combined with reduced cyclosporine exposure ‐ analysis of renal function at 12 months in renal transplant recipients [abstract no: 2023]. Transplantation 2010;90(Suppl 2S):110. [Google Scholar]
- Shihab FS, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving reduced cyclosporine. Clinical Transplantation 2013;27(2):217‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco Silva H Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced‐exposure CsA versus mycophenolic acid plus standard‐exposure CsA in renal‐transplant recipients. American Journal of Transplantation 2010;10(6):1401‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H, Johnston TS, Kim Y, Zibari G, Walker R, Mange K, et al. Everolimus‐treated renal transplant patients have a lower incidence of CMV and BKV: results from a multicenter, prospective study [abstract: 1659]. American Journal of Transplantation 2010;10(Suppl 4):509. [EMBASE: 70465034] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Johnston T, Walker R, Tufvetson G, Zibari G, et al. Everolimus with reduced exposure of cyclosporine: efficacy results from a randomized prospective study in 833 de novo renal transplant recipients [abstract no: 427]. Transplantation 2010;90(Suppl):110. [EMBASE: 71531315] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Johnston T, Walker R, Zibari G, Mange K, et al. Everolimus plus reduced CsA exposure: efficacy results from a multicenter, randomized prospective study in renal transplantation [abstract no: 374]. American Journal of Transplantation 2010;10(Suppl 4):150. [EMBASE: 70463735] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Lackova E, Johnston T, Zibari G, Panis C, et al. Everolimus with reduced‐dose cyclosporine as a strategy for optimizing long‐term renal function: results from a randomized study in 833 de‐novo renal‐transplant recipients [abstract no: P‐371]. Transplant International 2009;22(Suppl 2):186‐7. [Google Scholar]
- Vathsala A, Zibari G, Kim YS, Cibrik D, Johnston T, Walker R, et al. Dose related incidences of wound healing events in renal transplant recipients treated with everolimus and cyclosporine [abstract no: 2060]. Transplantation 2012;90(Suppl):615. [EMBASE: 71532274] [Google Scholar]
- Walker R, Cibrik D, Tedesco‐Silva H, Johnston T, Kim YS, Zibari G, et al. Everolimus allows for a 60% reduction in cyclosporine exposure in subject cohorts defined by geography [abstract no: 1616]. Transplantation 2010;90(Suppl 2S):610. [EMBASE: 71532265] [Google Scholar]
- Wiseman AC, McCague K, Kim Y, Geissler F, Cooper M. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. American Journal of Transplantation 2013;13(2):442‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zibari G, Kim YS, Cibrik D, Johnston T, Walker R, Mange K, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 1661]. American Journal of Transplantation 2010;10(Suppl 4):510. [EMBASE: 70465036] [Google Scholar]
Velosa‐212 Study 2001 {published data only}
- Campistol JM, Alveranga D, Hricik DE, Velosa J, Grinyo JM, Mourad G, et al. Sirolimus‐treated renal transplant recipients experience improved renal function with cyclosporine elimination: one‐year results from a phase II trial [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00487674] [Google Scholar]
- Gonwa T, Alveranga D, Ancona G, Brinker K, Cambi V, Campistol J, et al. Sirolimus (Rapamune) permits early elimination of cyclosporine in recipients of cadaveric renal allografts [abstract]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00445514] [Google Scholar]
- Gonwa TA. Sirolimus (SRL) treated patients demonstrate improved renal function after withdrawal of cyclosporine (CSA) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):891A. [CENTRAL: CN‐00550647] [Google Scholar]
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hricik DE, Rapamune Renal Function Study Group. Sirolimus improves renal function without increasing acute rejection after cyclosporine elimination in renal transplant patients [abstract no: 0430]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00583304]
- Velosa JA, Larson TS, Gloor JM, Stegall MD. Cyclosporine elimination in the presence of TOR inhibitors: effects on renal function, acute rejection, and safety. American Journal of Kidney Diseases 2001;38(4 Suppl 2):S3‐S10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watson 2005 {published data only}
- Clatworthy M, Bradley V, Wallin E, Camilleri B, Williams P, Bradley JA, et al. Sirolimus conversion post‐renal transplantation. 5 year follow up data [abstract no: P246]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
- Clatworthy MR, Bradley V, Bradley JA, Watson CJ. Sirolimus conversion post‐renal transplantation ‐ 5 year follow‐up data [abstract no: 1096]. American Journal of Transplantation 2009;9(Suppl 2):499. [CENTRAL: CN‐00766450] [Google Scholar]
- Watson CJ, Firth J, Bradley J, Williams PF, Smith JC, Palmer CR, et al. Superior renal function following conversion to sirolimus after renal transplantation ‐ preliminary results from a randomised trial [abstract]. American Journal of Transplantation 2004;4(Suppl 8):220‐1. [CENTRAL: CN‐00509555] [Google Scholar]
- Watson CJ, Firth J, Williams PF, Bradley JR, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI‐based to sirolimus‐based immunosuppression following renal transplantation. American Journal of Transplantation 2005;5(10):2496‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, Watson CJ, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. American Journal of Transplantation 2007;7(8):2006‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ZEUS Study 2011 {published data only}
- Arns W, Becker T, Budde K, Eisenberger U, Fischer W, Kramer S, et al. Conversion to an everolimus/enteric‐coated mycophenolate sodium regimen in de novo renal transplant improves 2 year renal function [abstract no: 15]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Arns W, Budde K, Becker T, Sommerer C, Reinke P, Eisenberger U, et al. Analysis of renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: The ZEUS Study [abstract no: SU656]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00763609]
- Becker T, Arns W, Budde K, Eisenberger U, Fischer W, Kramer S, et al. Renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the Zeus Study [abstract no: O‐297]. Transplant International 2009;22(Suppl 2):78‐9. [Google Scholar]
- Becker T, Arns W, Budde K, Pietruck F, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow‐up of the ZEUS trial [abstract no: 1757]. Transplantation 2010;90(Suppl 2S):109. [EMBASE: 71531312] [Google Scholar]
- Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow‐up of the ZEUS trial [abstract no: 1638]. American Journal of Transplantation 2010;10(Suppl 4):503. [Google Scholar]
- Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Analysis of renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the ZEUS Study [abstract no: 237]. American Journal of Transplantation 2009;9(Suppl 2):259. [EMBASE: 70010110] [Google Scholar]
- Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus‐based, calcineurin‐inhibitor‐free regimen in recipients of de‐novo kidney transplants: an open‐label, randomised, controlled trial.[Erratum appears in Lancet. 2012 Dec 8;380(9858):1994], [Erratum appears in Lancet. 2011 Jun 11;377(9782):2006 Note: Wuthrich, Rudolf P [added]]. Lancet 2011;377(9768):837‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Klempnauer J, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Multi‐center, open‐label, prospective, randomized, parallel group study investigating an everolimus‐based CNI‐free regimen in comparison to a cyclosporine‐based standard therapy in de novo renal transplant patients (Zeus Study) [abstract no: 1640]. Transplantation 2008;86(Suppl 2):544. [CENTRAL: CN‐00740480] [Google Scholar]
- Budde K, Klempnauer J, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Renal function, efficacy and safety of everolimus (RAD)/enteric‐coated mycophenolate sodium (EC‐MPS) therapy after calcineurin inhibitor (CNI) withdrawal in de novo renal transplant patients: final outcomes of the Zeus Study [abstract no: 264]. Transplantation 2008;86(Suppl 2):93. [CENTRAL: CN‐00740481] [Google Scholar]
- Budde K, Lehner F, Arns W, Reinke P, Eisenberger U, Paulus EM, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 4 years follow‐up of the ZEUS trial [abstract no: 927]. American Journal of Transplantation 2012;12(Suppl S3):298. [EMBASE: 70746880] [Google Scholar]
- Budde K, Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, et al. Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3‐year results from the randomized ZEUS study. [Erratum appears in Am J Transplant. 2012 Nov;12(11):3165]. American Journal of Transplantation 2012;12(6):1528‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, et al. Five‐year outcomes in kidney transplant patients converted from cyclosporine to everolimus: the randomized ZEUS study. American Journal of Transplantation 2015;15(1):119‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Witzke O, Sommerer C, Reinke P, Eisenberger U, Paulus E, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 5 years follow‐up of the ZEUS trial [abstract no: 18]. American Journal of Transplantation 2013;13(Suppl S5):35‐6. [EMBASE: 71056595] [Google Scholar]
- Eisenberger U, Budde K, Witzke O, Lehner F, Sommerer C, Wuethrich R, et al. Explorative analysis of ZEUS after 5 years: Histological assessment from biopsy analyses [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953380] [Google Scholar]
- Eisenberger U, Pietruck F, Becker T, Arns W, Reinke P, Kramer S, et al. Efficacy and safety of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: results of the ZEUS Study [abstract no: SU667]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00774728]
- Eisenberger U, Pietruck F, Klempnauer J, Arns W, Fehr T, Sommerer C, et al. Everolimus (RAD)/enteric‐coated mycophenolate sodium (ECMPS) therapy after calcineurin inhibitor (CNI) withdrawal in de novo renal transplant patients: final outcomes of the ZEUS study [abstract no: 3.1]. Swiss Medical Weekly 2008;138(Suppl 167):5S. [Google Scholar]
- Eisenberger U, Witzke O, Hauser I, Lehner F, Sommerer C, Wuethrich R, et al. Explorative analysis of ZEUS after 5 years: histological assessment from biopsy analyses [abstract no: O220]. Transplant International 2015;28(Suppl 4):83. [EMBASE: 72111464] [Google Scholar]
- Lehner F, Arns W, Reinke P, Eisenberger U, Paulus E, Scheidl S, et al. Renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo living renal transplant recipients after calcineurin inhibitor withdrawal: subgroup analysis of the ZEUS study [abstract no: P142]. Transplant International 2011;24(Suppl 3):50‐1. [EMBASE: 70537442] [Google Scholar]
- Lehner F, Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 3 year follow‐up of the ZEUS trial [abstract no: O‐199]. Transplant International 2011;24(Suppl 3):57. [EMBASE: 70527276] [Google Scholar]
- Lehner F, Budde K, Wuethrich R, Reinke P, Arns W, Muehlfeld A, et al. Post Hoc subgroup analysis of ZEUS: Outcome on renal function, efficacy and safety in living‐donor kidney transplant recipients after conversion from a calcineurin inhibitor to an everolimus based regimen: 5 year follow‐up data [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71954049] [Google Scholar]
- Lehner F, Budde K, Zeier M, Wuthrich RP, Reinke P, Eisenberger U, et al. Efficacy and safety of conversion from cyclosporine to everolimus in living‐donor kidney transplant recipients: an analysis from the ZEUS study. Transplant International 2014;27(11):1192‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lehner F, Sommerer C, Arns W, Budde K, Wuethrich R, Reinke P, et al. Outcome on renal function, efficacy and safety in living‐donor kidney transplant recipients after conversion from CNI to everolimus‐based regimen: 5 year data post hoc analysis from the ZEUS study [abstract no: BO333]. Transplant International 2015;28(Suppl 4):242‐3. [EMBASE: 72111925] [Google Scholar]
- Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wuthrich R, et al. Post hoc subgroup analysis of ZEUS: Outcome on renal function, efficacy and safety in living‐donor kidney transplant recipients after conversion from a calcineurin inhibitor to an everolimus based regimen [abstract]. Transplantation 2014;98(Suppl 1):613. [EMBASE: 71545612] [Google Scholar]
- Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wuthrich RP, et al. Post Hoc subgroup analysis from ZEUS: outcome on renal function, efficacy and safety in living‐donor kidney transplant recipients after conversion from a calcineurin inhibitor to an everolimus based regimen [abstract no: V22]. Transplant International 2013;26(Suppl 1):8. [EMBASE: 71356217] [Google Scholar]
- Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Paulus E, et al. 5‐year follow‐up on the ZEUS KTX trial: everolimus conversion after CNI withdrawal [abstract no: BO142]. Transplant International 2013;26(Suppl 2):81. [EMBASE: 71359257] [Google Scholar]
- Pietruck F, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Efficacy and safety of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: results of the ZEUS trial [abstract no: 1093]. American Journal of Transplantation 2009;9(Suppl 2):499. [CENTRAL: CN‐00774729] [Google Scholar]
- Pietruck F, Budde K, Arns W, Reinke P, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow‐up of the Zeus‐trial [abstract no: OM007]. NDT Plus 2010;3(Suppl 3):iii553. [EMBASE: 70484927] [Google Scholar]
- Pietruck F, Klempnauer J, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus/enteric‐coated mycophenolate sodium (EC‐MPS) therapy after calcineurin inhibitor withdrawal in de novo renal transplant patients: final outcomes of the ZEUS study [abstract no: TH‐FC041]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):10A. [CENTRAL: CN‐00716070] [Google Scholar]
- Reinke P, Arns W, Becker T, Eisenberger U, Fischer W, Kramer S, et al. Efficacy and safety of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: results of the ZEUS trial [abstract no: O‐202]. Transplant International 2009;22(Suppl 2):53. [Google Scholar]
- Reinke P, Lehner F, Witzke O, Sommerer C, Eisenberger U, Arns W, et al. 5 Years follow‐up on renal function ‐ ZEUS trial: Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients [abstract no: V76]. Transplant International 2013;26(Suppl 1):21. [EMBASE: 71356260] [Google Scholar]
- Sommerer C, Lehner F, Arns W, Reinke P, Eisenberger U, Heller K, et al. Post hoc analysis of ZEUS and HERAKLES, two prospective, open‐label, multicenter, randomized trials: Onset and progression of diabetes in kidney transplant recipients on cyclosporine standard or converted to CNI‐free or CNI‐low everolimus regimen [abstract]. Transplantation 2014;98(Suppl 1):145‐6. [EMBASE: 71543989] [Google Scholar]
- Witzke O, Lehner F, Arns W, Reinke P, Eisenberger U, Paulus E, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 4 years follow‐up of the ZEUS trial [abstract no: 1671]. Transplantation 2012;94(Suppl 10S):993. [EMBASE: 71251765] [Google Scholar]
- Giet M, Brakemeier S, Liefeldt L, Glander P, Diekmann F, Hohne M, et al. The impact of everolimus versus CNI‐based immunosuppression on cardiovascular function and stiffness after renal transplantation [abstract no: 1646]. American Journal of Transplantation 2010;10(Suppl 4):506. [Google Scholar]
References to studies excluded from this review
Abouna 1991 {published data only}
- Abouna GM, Kumar SM, White AG, Samhan M, Kalawi M, al‐Sabawi N. Cyclosporine withdrawal in renal transplant recipients maintained on triple therapy. Transplantation Proceedings 1991 Feb;23(1 (Pt 2)):1009‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Alexander 2006 {published data only}
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transplant International 2006;19(4):295‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alpay 2013 {published data only}
- Alpay N. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF‐beta and urinary NGAL in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i500‐1. [EMBASE: 71076549] [Google Scholar]
- Alpay N, Ozkok A, Caliskan Y, Akagun T, Cinar S, Deniz G, et al. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF‐beta and urinary NGAL in renal transplant recipients [abstract no: 1744]. American Journal of Transplantation 2013;13(Suppl 5):545. [EMBASE: 71058320] [Google Scholar]
Artz 2002 {published data only}
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Hene RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved lipid profile and unchanged plasma homocysteine levels. Transplantation Proceedings 2002;34(5):1793‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Conversion from cyclosporine to tacrolimus improves quality‐of‐life indices, renal graft function and cardiovascular risk profile. American Journal of Transplantation 2004;4(6):937‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. Journal of the American Society of Nephrology 2003;14(7):1880‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Randomized conversion from cyclosporine to tacrolimus improves renal graft function, the cardiovascular risk profile, and perceived side‐effects [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550511]
- Artz MA, Ligtenberg G, Roodnat JI, Christiaans MH, Boots HM, Hene RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved parameters of lipid metabolism and unchanged plasma homocysteine levels [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):876A. [CENTRAL: CN‐00433618] [Google Scholar]
Asberg 2013 {published data only}
- Asberg A, Apeland T, Reisaeter AV, Foss A, Leivestad T, Heldal K, et al. Long‐term outcomes after cyclosporine or mycophenolate withdrawal in kidney transplantation ‐ results from an aborted trial. Clinical Transplantation 2013;27(2):E151‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baboolal 2003 {published data only}
- Baboolal K. A phase III prospective, randomized study to evaluate concentration‐controlled sirolimus (Rapamune) with cyclosporine dose minimization or elimination at six months in de novo renal allograft recipients. Transplantation 2003;75(8):1404‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baboolal 2004 {published data only}
- Baboolal K. Six month interim analysis of a phase III prospective, randomized study to compare conversion from calcineurin inhibitors to rapamycin in established renal allograft recipients with mild to moderate renal insufficiency [abstract]. American Journal of Transplantation 2004;4(Suppl 8):220. [CENTRAL: CN‐00509073] [Google Scholar]
- Baboolal K, Zaiac M, Newstead C. Development of early malignant disease in a multicentre, randomised study comparing conversion from calcineurin inhibitors (CNIs) to sirolimus in renal allograft recipients [abstract no: P302]. British Transplantation Society (BTS). 12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
- Baboolal K, Zaiac M, Zamauskaite A, Newstead C. This multicentre, randomised study comparing conversion from calcineurin inhibitors (CNRs) to sirolimus versus standard therapy in renal allograft recipients showed a lower rate of development of subsequent malignant disease in the group receiving sirolimus [abstract no: 164]. American Journal of Transplantation 2009;9(Suppl 2):238. [CENTRAL: CN‐00776739] [Google Scholar]
Baxter 1982 {published data only}
- Baxter CR, Duggin GG, Willis N, Hall BM, et al. Cyclosporin A in renal transplantation: plasma concentration and nephrotoxicity [abstract]. Australian & New Zealand Journal of Medicine 1982;12:347‐8. [CENTRAL: CN‐00319765] [Google Scholar]
Brady 1990 {published data only}
- Brady HR, Kamel KS, Harding ME, Cook GT, deVeber GA, Cardella CJ. Low dose ciclosporin from the early postoperative period yields potent immunosuppression after renal transplantation. Nephron 1990;55(4):394‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burkhalter 2012 {published data only}
- Burkhalter F, Oettl T, Descoeudres B, Bachmann A, Guerke L, Mihatsch MJ, et al. High incidence of rejection episodes and poor tolerance of sirolimus in a protocol with early steroid withdrawal and calcineurin inhibitor‐free maintenance therapy in renal transplantation: experiences of a randomized prospective single‐center study. Transplantation Proceedings 2012;44(10):2961‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oettl T, Descoeudres B, Burkhalter F, Bachmann A, Gürke L, Mihatsch MJ, et al. An open, single centre, prospective study to investigate a steroid free immunosuppressive regimen for de novo renal transplant recipients followed by a two arm randomisation to a CNI‐sparing and a CNI‐free maintenance immunosuppression after 3 months [abstract no: 38]. Swiss Medical Weekly 2008;138(Suppl 167):15S. [Google Scholar]
CAMPASIA Study 2005 {published data only}
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vathsala A, CAMPASIA Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl 1):A215. [CENTRAL: CN‐00583367] [Google Scholar]
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosprorine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract no: O145]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538] [Google Scholar]
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. CAMPASIA: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants. [abstract no: 908]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Lymphocyte recovery after depletion by alemtuzumab in renal transplant recipients: impact on outcome [abstract no: 1015]. American Journal of Transplantation 2005;5(Suppl 11):415. [CENTRAL: CN‐00644284] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Casasola CB, et al. Induction therapy with alemtuzumab together with low dose cyclosporine monotherapy permits steroid‐free immunosuppression, mitigates drug‐related, non‐immune toxicities and improves quality of life [abstract no: TH‐PO550]. Journal of the American Society of Nephrology 2006;17(Abstracts):224A. [CENTRAL: CN‐00644285] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Casasola CB, et al. Randomized trial of alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation 2005;80(6):765‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cattaneo 2005 {published data only}
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, et al. Influence of co‐medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. American Journal of Transplantation 2005;5(12):2937‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Perico N, Gotti E, et al. Effects of sirolimus on the pharmacokinetics of mycophenolic acid in kidney transplantation [abstract no: 650]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐00724878] [DOI] [PubMed] [Google Scholar]
Chapman 1985 {published data only}
- Chapman JR, Griffiths D, Harding NG, Morris PJ. Reversibility of cyclosporin nephrotoxicity after three months' treatment. Lancet 1985;325(8421):128‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chapman JR, Marcen R, Arias M, Raine AE, Dunnill MS, Morris PJ. Hypertension after renal transplantation. A comparison of cyclosporine and conventional immunosuppression. Transplantation 1987;43(6):860‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Higgins RM, Richardson AJ, Endre ZH, Frostick SP, Morris PJ. Hypophosphataemia after renal transplantation: relationship to immunosuppressive drug therapy and effects on muscle detected by 31P nuclear magnetic resonance spectroscopy. Nephrology Dialysis Transplantation 1990;5(1):62‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morris PJ, Chapman JR, Allen RD, Ting A, Thompson JF, Dunnill MS, et al. Cyclosporin conversion versus conventional immunosuppression: long‐term follow‐up and histological evaluation. Lancet 1987;1(8533):586‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CIS Trial 2014 {published data only}
- Sommerer C, Schaier M, Morath C, Schwenger V, Rauch G, Giese T, et al. The Calcineurin Inhibitor‐Sparing (CIS) Trial ‐ individualised calcineurin‐inhibitor treatment by immunomonitoring in renal allograft recipients: protocol for a randomised controlled trial. Trials [Electronic Resource] 2014;15:489. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONCERTO Study 2005 {published data only}
- Curtis JJ, Thervet E, Vincenti F, Rodriguez A, Soergel M, Barbeito R. Outcomes of 117 cases of delayed graft function from two clinical trials involving early C2 monitored neoral and antibody therapy [abstract]. Transplantation 2004;78(2 Suppl):5. [CENTRAL: CN‐00509145] [Google Scholar]
- Mendez R, Light J, Pearson TC, Wu YM, Curtis J, Vincenti F. Neoral® therapy optimized by C2 monitoring and Simulect® induction can result in low acute rejection rate in renal transplant recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):255. [CENTRAL: CN‐00509350] [Google Scholar]
- Vincenti F, Mendez R, Curtis J, Light J, Pearson T, Wu YM, et al. A multicenter, prospective study of C2‐monitored cyclosporine microemulsion in a U.S. population of de novo renal transplant recipients. Transplantation 2005;80(7):910‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
David‐Neto 2001 {published data only}
- David‐Neto E, Cristiane A, Ianhez L, Nahas W, Zita B. A randomized, open‐label, prospective study comparing two different CYA‐AUC for the prevention of rejection in renal transplanted patients with and without induction therapy [abstract no: 1398]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00444997]
de Sandes Freitas 2011 {published data only}
- Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. International Urology & Nephrology 2011;43(4):1221‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Sevaux 1998 {published data only}
- Sevaux RG, Hilbrands LB, Tiggeler RG, Koene RA, Hoitsma AJ. A randomised, prospective study on the conversion from cyclosporine‐prednisone to cyclosporine‐azathioprine at 6 months after renal transplantation. Transplant International 1998;11 Suppl 1:S322‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sévaux RG, Hoitsma AJ, Hilbrands LB, Tiggeler RG, Koene RA. A randomised study on the effects of conversion of cyslosporine/prednisone to cyclosporine/azathioprine 6 months after kidney transplantation [abstract no: 234]. Transplantation 1998;65(12):S61. [CENTRAL: CN‐00583516] [DOI] [PubMed] [Google Scholar]
EVEREST Study 2009 {published data only}
- Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the Everest Study [abstract no: P118]. Transplant International 2007;20(Suppl 2):124. [CENTRAL: CN‐00724884] [Google Scholar]
- Corbetta G, Salvadori M, Scolari MP, Citterio F, Rigotti P, Cossu M, et al. Exposure to everolimus, and not to cyclosporine, is associated with freedom from acute rejection in de novo renal recipients [abstract no: 447]. Transplantation 2008;86(Suppl 2):157. [Google Scholar]
- Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, et al. Everolimus and minimization of cyclosporine in renal transplantation: 24‐month follow‐up of the EVEREST study. Transplantation 2011;91(10):e72‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salvadori M, Scolari M, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Targeting upper everolimus blood levels with very low‐dose cyclosporine is effective and safe in de novo renal transplantation [abstract no: 448]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740453] [Google Scholar]
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M. Upper everolimus blood levels with very low‐dose cyclosporin: 12 months follow up of the Everest Study [abstract no: 1085]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00776867] [Google Scholar]
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Everolimus with very low‐exposure cyclosporine a in de novo kidney transplantation: a multicenter, randomized, controlled trial. Transplantation 2009;88(10):1194‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flechner 2004 {published data only}
- Flechner SM, Goel M, Feng J, Mastroianni B, Savaas K, Arnovitz J, et al. The effect of 2‐gram vs 1‐gram concentration controlled mycophenolate mofetil on transplant outcomes using sirolimus based calcineurin inhibitor drug‐free immunosuppression [abstract no: 214]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550626]
Fleming 2016 {published data only}
- Fleming J, Taber D, Pilch N, Meadows H, Mardis C, McGillicuddy J, et al. mTOR‐based CNI minimization vs withdrawal in African American kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953883] [Google Scholar]
- Fleming JN, Taber DJ, Pilch NA, McGillicuddy JW, Srinivas TR, Baliga PK, et al. A randomized, prospective comparison of transition to sirolimus‐based CNI‐minimization or withdrawal in African American kidney transplant recipients. Clinical Transplantation 2016;30(5):528‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forwell 1986 {published data only}
- Forwell MA, Bradley JA. Low‐dose cyclosporin or azathioprine one year after renal transplantation [abstract]. Nephrology Dialysis Transplantation 1986;1(2):138. [CENTRAL: CN‐00260295] [PubMed] [Google Scholar]
Fries 1988 {published data only}
- Fries D, Hiesse C, Charpentier B, Benoit G. Triple combination of low‐dose cyclosporin, azathioprine and steroids in first‐cadaver allografts [abstract]. Nephrology Dialysis Transplantation 1988;3(1):96. [CENTRAL: CN‐00260361] [PubMed] [Google Scholar]
- Fries D, Hiesse C, Charpentier B, Lantz O, Bensadoun H, Benoit G. A single center experience with "low‐dose" cyclosporine in cadaveric renal transplantation. Clinical Transplants 1988:115‐29. [MEDLINE: ] [PubMed] [Google Scholar]
- Fries D, Hiesse C, Santelli G, Gardin JP, Cantarovich M, Lantz O, et al. Triple therapy with low‐dose cyclosporine, azathioprine, and steroids: long‐term results of a randomized study in cadaver donor renal transplantation. Transplantation Proceedings 1988;20(3 Suppl 3):130‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Fries 1988a {published data only}
- Fries D. Optimal results in cadaver renal transplantation using prophylactic ALG, cyclosporin (CsA) and prednisone (P) [abstract]. Nephrology Dialysis Transplantation 1988;3(1):95. [CENTRAL: CN‐00260355] [PubMed] [Google Scholar]
Fruchaud 1996 {published data only}
- Fruchaud G, Buisson C, Abbou C, Desvaux D, Baron C, Benmaadi A, et al. Prospective randomized study of quadruple versus triple therapy in long‐term kidney allografts. Transplantation Proceedings 1996;28(5):2819. [MEDLINE: ] [PubMed] [Google Scholar]
Gaber 2003 {published data only}
- Gaber AO, Egidi MF, Lo A, Gaber LW, Shokouh‐Amiri MH, Grewal HP, et al. Defining the optimal doses of sirolimus and tacrolimus in high‐risk cadaveric renal transplant recipients [abstract no: 541]. American Journal of Transplantation 2002;2(Suppl 3):274. [CENTRAL: CN‐00415690] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus‐based calcineurin inhibitor‐sparing and calcineurin inhibitor‐free regimens in cadaveric renal transplantation. Transplantation 2004;77(8):1228‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Gaber AO. Observations on the use of sirolimus and tacrolimus in high‐risk renal transplant recipients. Transplantation Proceedings 2003;35(3 Suppl):105S‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Shokouh‐Amiri MH, Nazakatgoo N, Fisher JS, et al. Observations regarding the use of sirolimus and tacrolimus in high‐risk cadaveric renal transplantation. Clinical Transplantation 2004;18(1):53‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gelens 2006 {published data only}
- Gelens M, Christiaans M, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: P‐3]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
- Gelens M, Christiaans M, Hooff JV. Incidence of post transplant diabetes mellitus during calcineurin‐free and calcineurin‐based immunosuppression with limited steroid exposure [abstract no: 1210]. American Journal of Transplantation 2005;5(Suppl 11):465. [Google Scholar]
- Gelens M, Christianns M, Heurn EV, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: 865]. American Journal of Transplantation 2005;5(Suppl 11):376. [Google Scholar]
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghafari 2007 {published data only}
- Ghafari A, Makhdoomi K, Ahmadpoor P. Early low dose versus high dose cyclosporine A (CSA) induction protocols in renal transplantation [abstract no: SP742]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv265. [DOI] [PubMed] [Google Scholar]
- Ghafari A, Makhdoomi K, Ahmadpoor P, Afshari AT, Farshid B, Fallah MM, et al. Mycophenolate mofetil (MMF) plus low dose versus high dose cyclosporine A (CSA) induction protocols in renal transplantation [abstract no: T‐PO50034]. Nephrology 2005;10(Suppl):A216. [Google Scholar]
- Ghafari A, Makhdoomi K, Ahmadpour P, Afshari AT, Fallah MM, Rad PS. Low‐dose versus high‐dose cyclosporine induction protocols in renal transplantation. Transplantation Proceedings 2007;39(4):1219‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gotti 2003 {published data only}
- Gotti E, Perico N, Perna A, Gaspari F, Cattaneo D, Caruso R, et al. Renal transplantation: can we reduce calcineurin inhibitor/stop steroids? Evidence based on protocol biopsy findings. Journal of the American Society of Nephrology 2003;14(3):755‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griffin 1993 {published data only}
- Griffin PJ, Moore RH, Krishnan H, Fenn N, Salaman JR. Is there an optimal time for the first cyclosporin dose in renal transplantation?. Transplant International 1993;6(4):223‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grino 1991 {published data only}
- Gonzalez C, Grino JM, Castelao AM, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CsA) and steroids versus pre‐transplant OKT3, CsA and steroids in kidney cadaveric transplantation [abstract]. Kidney International 1990;37(6):1601. [CENTRAL: CN‐00601919] [Google Scholar]
- Grino JM, Castelao AM, Gonzalez C, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CYA) and steroids in kidney cadaveric transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:513A. [CENTRAL: CN‐00601920]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Antilymphocyte globulin versus OKT3 induction therapy in cadaveric kidney transplantation: a prospective randomized study. American Journal of Kidney Diseases 1992;20(6):603‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Prophylactic OKT3, CyA, and steroids versus antilymphoblast globulin, CyA, and steroids in cadaveric kidney transplantation. Transplantation Proceedings 1992;24(1):39‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Grino JM, Castelao AM, Seron D, Gonzalez C, Gil‐Vernet S, Andres E, et al. Antilymphocyte serum, cyclosporine and corticoids, versus OKT3, cyclosporine, and corticoids in kidney transplantation [Serum anti‐lymphocyte, ciclosporine et corticoides, versus OKT3, ciclosporine et corticoides en transplantation renale]. Presse Medicale 1991;20(40):2039‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Mestre M, Gonzalez C, Grino JM, Valls A, Bonete J, Mane E, et al. Sequential monitoring of immunoregulatory T cell subsets in renal transplantation. Transplantation Proceedings 1992;24(1):73‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Hamdy 2005 {published data only}
- Hamdy A, El‐Baz M, Bakr M, Ghoneim M. The incidence of chronic allograft nephropathy among live‐donor renal transplant recipients primarily treated with sirolimus‐based regimens [abstract no: SA734]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776624]
- Hamdy AF, Bakr MA, Ghoneim MA. Long‐term efficacy and safety of a calcineurin inhibitor‐free regimen in live‐donor renal transplant recipients. Journal of the American Society of Nephrology 2008;19(6):1225‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live‐donor renal transplant recipients' long‐term experience. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2010;8(4):283‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy el‐M, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hariran 2015 {published data only}
- Haririan A, Klassen D, Keshtkar M, Drachenberg C, Dowling T, Ramos E, et al. Delayed tacrolimus (TAC) to rapamycin (RAPA) conversion in renal transplant recipients with DGF/SGF [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953312] [Google Scholar]
Henny 1986 {published data only}
- Henny FC, Kootte AM, Bockel JH, Baldwin WM, Hermans J, Bos B, et al. A prospective randomised comparative study on the influence of cyclosporin and azathioprine on renal allograft survival and function. Nephrology Dialysis Transplantation 1986;1(1):44‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Hollander AA, Saase JL, Kootte AM, Dorp WT, Bockel HJ, Es LA, et al. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. Lancet 1995;345(8950):610‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hollander AM, Saase JL, Kootte AM, Dorp WT, Bockel HH, Es LA, et al. Conversion from cyclosporine (CYA) to azathioprine (AZA) after cadaveric kidney transplantation (KT): in the long run a better renal function, less hypertension and equal graft survival [abstract no: 8P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):941. [CENTRAL: CN‐00484382] [Google Scholar]
- Kootte AM, Lensen LM, Es LA, Paul LC. Controlled cyclosporine conversion at three months after renal transplantation. Long‐term results. Transplantation 1988;46(5):677‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kootte AM, Lensen LM, Bockel JH, Paul LC. A randomized study comparing high‐ and low‐dose regimens of cyclosporine in renal transplantation. Transplantation Proceedings 1988;20(3 Suppl 3):136‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Kootte AM, Lensen LM, Bockel JH, Es LA, Paul LC. High and low‐dose regimens of cyclosporin in renal transplantation: immunosuppressive efficacy and side‐effects. Nephrology Dialysis Transplantation 1988;3(5):666‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kootte AM, Lensen LM, Bockel JH, Es LA, Paul LC. Long‐term results of controlled cyclosporine conversion at 3 months after renal transplantation [abstract]. Kidney International 1988;34(4):559. [Google Scholar]
- Kootte AM, Es LA, Paul LS. Randomised conversion of cyclosporin to conventional therapy at 3 months after renal transplantation: 4‐year results [abstract]. Nephrology Dialysis Transplantation 1989;4(5):505. [CENTRAL: CN‐00260455] [Google Scholar]
- Kootte AM, Es LA, Paul LC. Randomized conversion of cyclosporine to conventional therapy at 3 months after renal transplantation: Four year results. [abstract]. Kidney International 1989;36(3):512. [CENTRAL: CN‐00626040] [Google Scholar]
- Dorp WT, Kootte AM, Gemert GW, Es LA, Paul LC. Infections in renal transplant patients treated with cyclosporine or azathioprine. Scandinavian Journal of Infectious Diseases 1989;21(1):75‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hernandez 2007 {published data only}
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiesse 1991 {published data only}
- Hiesse C, Neyrat N, Deglise‐Favre A, Lantz O, Bensadoun H, Benoit G, et al. Randomized prospective trial of elective cyclosporine withdrawal from triple therapy at 6 months after cadaveric renal transplantation. Transplantation Proceedings 1991;23(1 (Pt 2)):987‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hilbrands 1993 {published data only}
- Hilbrands LB, Demacker PN, Hoitsma AJ. Cyclosporin and serum lipids in renal transplant recipients. Lancet 1993;341(8847):765‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (apo)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene KA. Randomized, prospective trial of cyclosporine monotherapy versus azathioprine‐prednisone from three months after renal transplantation. Transplantation 1996;61(7):1038‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Costs of drugs used after renal transplantation. Transplant International 1996;9 Suppl 1:S399‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Effect of immunosuppressive therapy on quality of life after renal transplantation [abstract]. Journal of the American Society of Nephrology 1994;5(3):1011. [CENTRAL: CN‐00615875] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Medication compliance after renal transplantation. Transplantation 1995;60(9):914‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. The effect of immunosuppressive drugs on quality of life after renal transplantation. Transplantation 1995;59(9):1263‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Hourmant 1987 {published data only}
- Hourmant M, Buzelin F, Dubigeon P, Soulillou JP. High long‐term graft survival rates in kidney transplantation with the sequential association of antithymocyte globulin and cyclosporine A monotherapy. Transplantation Proceedings 1987;19(1 (Pt 3)):2113‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Hourmant M, Soulillou JP. Use of delayed cyclosporin A in after administration of anti‐lymphocyte serum in kidney transplantation [Utilisation de la cyclosporine A en relais du serum anti‐lymphocytaire en transplantation renale]. Biomedicine & Pharmacotherapy 1987;41(5):247‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hricik 1990 {published data only}
- Hricik DE, Mayes JT, Schulak JA. Cyclosporine (CsA) inhibits the generation of antibodies (ABs) to OKT3 [abstract]. Kidney International 1990;37:607. [CENTRAL: CN‐00626073] [Google Scholar]
- Hricik DE, Mayes JT, Schulak JA. Inhibition of anti‐OKT3 antibody generation by cyclosporine‐‐results of a prospective randomized trial. Transplantation 1990;50(2):237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Infante 2008 {published data only}
- Infante B, Stallone G, Pontrelli P, Gigante M, Ranieri E, Schena FP, et al. Role of rapamycin in the induction of operational tolerance through up‐regulation of ILT3 and ILT4 in kidney transplanted patients [abstract no: F‐FC248]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):56A. [CENTRAL: CN‐00716071] [Google Scholar]
Jain 2001 {published data only}
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil. Transplantation Proceedings 2001;33(3):2165‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Randomized trial comparing mycophenolate mofetil and azathioprine to allow cyclosporin reduction in chronic allograft nephropathy [abstract no: PO513W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445890]
- Nicholson ML, Jain S, Metcalfe M, White SA, Furness PN. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil [abstract no: 437]. Transplantation 2000;69(8 Suppl):S227. [CENTRAL: CN‐00446949] [DOI] [PubMed] [Google Scholar]
Jindal 2002 {published data only}
- Jardine A. 12 month results of a phase III prospective, randomised study to evaluate concentration controlled Rapamune with cyclosporin dose minimization or elimination in de novo renal allograft recipient [abstract no: 1206]. American Journal of Transplantation 2003;3(Suppl 5):461. [CENTRAL: CN‐00445901] [DOI] [PubMed] [Google Scholar]
- Jardine A, European and South African Rapamune Study Group. Phase III prospective, randomized study to evaluate the safety and efficacy of concentration controlled Rapamune (sirolimus) with cyclosporine dose minimisation or elimination in de novo renal allograft recipients at 12 months [abstract no: O81]. Transplantation 2004;78(2 Suppl):31. [CENTRAL: CN‐00509251] [Google Scholar]
- Jardine AG. Phase III prospective, randomized study to evaluate the safety and efficacy of concentration controlled Rapamune (sirolimus) with cyclosporin dose minimisation or elimination in de novo renal allograft recipients at 12 months [abstract]. American Journal of Transplantation 2004;4(Suppl 8):286. [CENTRAL: CN‐00520346] [DOI] [PubMed] [Google Scholar]
- Jindal RM, UK and Ireland Rapamune Study Group. A phase III prospective, randomised study to evaluate concentration controlled rapamune with cyclosporin dose minimization or elimination at six months in de novo renal allograft recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415940] [DOI] [PubMed]
John 1999 {published data only}
- John GT, Dakshinamurthy DS, Jeyaseelan L, Jacob CK. The effect of cyclosporin A on plasma lipids during the first year after renal transplantation. National Medical Journal of India 1999;12(1):14‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Kamar 2012 {published data only}
- Kamar N, Mariat C, Albano L, Villemain F, Moal MC, Ladriere M, et al. Improvement in renal function following conversion from low‐dose mycophenolate acid to higher‐dose enteric‐coated mycophenolate sodium (EC‐MPS) with concomitant tacrolimus reduction in maintenance kidney transplant recipients [abstract no: O‐295]. Transplant International 2009;22(Suppl 2):78. [Google Scholar]
- Kamar N, Rostaing L, Cassuto E, Villemain F, Moal MC, Ladriere M, et al. A multicenter, randomized trial of increased mycophenolic acid dose using enteric‐coated mycophenolate sodium with reduced tacrolimus exposure in maintenance kidney transplant recipients. Clinical Nephrology 2012;77(2):126‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kandaswamy 2005 {published data only}
- Kandaswamy R, Humar A, Dunn T, Gross E, Hughes M, Hill M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: 5‐year results [abstract no: 286]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00677752] [Google Scholar]
- Kandaswamy R, Humar A, Khwaja K, Asolati M, Harmon J, Gillingham K, et al. A prospective randomized study of cyclosporine (CSA)/cellcept (MMF) vs. tacrolimus (TAC)/sirolimus (SIR) with rapid discontinuation of prednisone (P) [abstract]. American Journal of Transplantation 2003;3(Suppl 5):198. [CENTRAL: CN‐00445992] [Google Scholar]
- Kandaswamy R, Humar A, Sturdevant M, Garcia‐Roca R, Casingal V, Tan M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: mixed results [abstract no: 329]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00766092] [Google Scholar]
- Kandaswamy R, Humar A, Sutherland DE, Gillingham K, Matas A. A prospective, randomized study of cyclosporine (CSA)/mycophenolate mofetil (MMF) versus tacrolimus (TAC)/sirolimus(SIR) with rapid discontinuation of prednisone (P) [abstract no: 084]. Transplantation 2004;78(2 Suppl):32. [CENTRAL: CN‐00509261] [Google Scholar]
- Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid‐free maintenance regimens in kidney transplant recipients‐‐an interim analysis. American Journal of Transplantation 2005;5(6):1529‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suszynski TM, Gillingham KJ, Matas AJ, Kandaswamy R. Prospective randomized trial of maintenance immunosuppression (IS) with rapid discontinuation of prednisone (RDP) in adult kidney transplantation (KTx): 10‐year results [abstract no: 1587]. American Journal of Transplantation 2012;12(Suppl S3):493. [EMBASE: 70747569] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszynski TM, Gillingham KJ, Rizzari MD, Dunn TB, Payne WD, Chinnakotla S, et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. American Journal of Transplantation 2013;13(4):961‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keitel 1999 {published data only}
- Keitel E, Michelon T, Dominguez V, Bittar AE, Santos AF, Goldani JC, et al. Long‐term evaluation of two protocols of elective cyclosporine withdrawal in renal transplant recipients. Transplantation Proceedings 1999;31(7):3013‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kovarik 2001 {published data only}
- Kaplan B, Polsky D, Weinfurt K, Fastenau J, Kim J, Ryu S, et al. Quality of life improvement and lower costs associated with simulect based induction therapy [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):733A. [CENTRAL: CN‐00401459] [Google Scholar]
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pescovitz M, Kovarik JM, Gerbeau C, Simulect US‐O1 Study Group. Pharmacokinetics of basiliximab when coadministered with MMF in kidney transplantation [abstract no: 0112]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00402225]
- Pescovitz MD, Barbeito R. Effect of "C2" cyclosporine levels and time to initiation of cyclosporine therapy on outcomes in patients receiving neoral and simulect [abstract no: A36]. Journal of the American Society of Nephrology 2000;11(Sept):703A. [CENTRAL: CN‐00433641] [Google Scholar]
- Pescovitz MD, Barbeito R, Simulect US 01 Study Group. Two‐hour post‐dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clinical Transplantation 2002;16(5):378‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Polsky D, Weinfurt KP, Kaplan B, Kim J, Fastenau J, Schulman KA. An economic and quality‐of‐life assessment of basiliximab vs antithymocyte globulin immunoprophylaxis in renal transplantation. Nephrology Dialysis Transplantation 2001;16(5):1028‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sollinger H, Kaplan B, Pescovitz M, Philosophe B, Roza A, Brayman K, et al. A multicenter randomized trial of Simulect with early neoral vs ATGAM with delayed neoral in renal transplantation [abstract no: 0113]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00402698]
- Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation 2001;72(12):1915‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sollinger H, Pescovitz M, Philosophe B, Roza A, Brayman K, Somberg K. A multicenter, randomized trial of simulect with early neoral vs ATGAM with delayed neoral in renal transplantation. A 6 month interim analysis [abstract no: 580]. Transplantation 1999;67(7):S151. [CENTRAL: CN‐00402699] [Google Scholar]
Kovarik 2003 {published data only}
- Kovarik JM, Dantal J, Civati G, Rizzo G, Rouilly M, Bettoni‐Ristic O, et al. Influence of delayed initiation of cyclosporine on everolimus pharmacokinetics in de novo renal transplant patients. American Journal of Transplantation 2003;3(12):1576‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kovarik‐2306 2004 {published data only}
- Kovarik JM, Tedesco H, Pascual J, Civati G, Bizot MN, Geissler J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced‐exposure cyclosporine in de novo kidney transplantation. Therapeutic Drug Monitoring 2004;26(5):499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Tedesco H, Pascual J, Civati G, Schmidli H, Geissler J. Everolimus therapeutic concentration range derived from prospective concentration‐controlled trial in de novo kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509289] [DOI] [PubMed] [Google Scholar]
- Magee J, Tedesco H, Pascual J, Civati G, Filho G, Garcia V, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral® in de novo kidney transplant recipients: 12 months analysis [abstract]. American Journal of Transplantation 2004;4(Suppl 8):296‐7. [CENTRAL: CN‐00509335] [Google Scholar]
- Pascual J, Cambi V, Dissegna D, Esmeraldo R, Lao M, Durlik M, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose Neoral® in de novo kidney transplant recipients: 24 months analysis [abstract no: 1010]. American Journal of Transplantation 2005;5(Suppl 11):414. [CENTRAL: CN‐00725012] [Google Scholar]
- Pascual J, Tedesco H, Civati G, Magee J, Haas T, Bernhardt P. Excellent renal function with concentration‐controlled everolimus, reduced exposure to neoral, and steroids in de novo kidney transplant recipients: results of a 6 months efficacy and safety analysis [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786. [CENTRAL: CN‐00447112] [Google Scholar]
- Pascual J, Tedesco H, Magee J, Civati G, Mazzali M, Garcia V, et al. 12 month clinical trial results of safety and efficacy of trough‐controlled certican® dosing with reduced neoral® exposure in de novo kidney transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):269. [CENTRAL: CN‐00509404] [Google Scholar]
- Tedesco‐Silva H Jr, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12‐month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transplant International 2007;20(1):27‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6‐month safety and efficacy results of two randomized studies. American Journal of Transplantation 2004;4(4):626‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2002a {published data only}
- Liu J, Guan D. Chronic allograft nephropathy: a prospective randomized trial of cyclosporin reduction or changing tacrolimus with mycophenolate mofetil [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416166]
Liu 2007b {published data only}
- Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, et al. Protective effects of sirolimus by attenuating connective tissue growth factor expression in human chronic allograft nephropathy. Transplantation Proceedings 2007;39(5):1410‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maiorano 2006 {published data only}
- Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, et al. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 2006;82(7):908‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McGrath 2001 {published data only}
- McGrath JS, Shehata M. Chronic allograft nephropathy: prospective randomised trial of cyclosporin withdrawal and mycophenolate mofetil or tacrolimus substitution. Transplantation Proceedings 2001;33(3):2193‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McGrath JS, Shehata M. Complete withdrawal of cyclosporin in chronic allograft nephropathy: a randomised prospective trial of conversion to mycophenolate mofetil or tacrolimus [abstract no: P0539]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00433636]
McMaster 1983 {published data only}
- McMaster P, Haynes IG, Michael J. Cyclosporine in cadaveric renal transplantation: a prospective randomized trial. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2523‐7. [EMBASE: 14164140] [Google Scholar]
Meier 2006 {published data only}
- Fricke L, Meier M, Mueller‐Steinhardt M, Ohltmann A, Jabs W, Steinhoff J, et al. Switching from cyclosporine to tacrolimus ‐ beneficial effects in patients with chronic allograft nephropathy over a 3 years period [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
- Meier M, Nitschke M, Weidtmann B, Jabs WJ, Wong W, Suefke S, et al. Slowing the progression of chronic allograft nephropathy by conversion from cyclosporine to tacrolimus: a randomized controlled trial. Transplantation 2006;81(7):1035‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Messa 2009 {published data only}
- Messa P, Alberti L, Montagnino G, Cafforio C, Berardinelli L, Rastaldi MP. T‐regulatory cell changes after early transition from tacrolimus to sirolimus in renal transplanted patients [abstract no: SU642]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776834]
Metcalfe 2002 {published data only}
- Brook NR, Metcalf MS, Jain S, Bicknell GR, Nicholson ML, Harper SJ. A randomised trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy [abstract no: P‐97]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550372] [DOI] [PubMed]
- Brook NR, Metcalfe MS, Waller JR, Jain S, Hosgood SA, Nicholson ML. A prospective randomised trial of mycophenolate mofetil and azathioprine after calcineurin reduction in renal allografts with established chronic allograft nephropathy [abstract]. American Journal of Transplantation 2004;4(Suppl 8):485. [CENTRAL: CN‐00509106] [Google Scholar]
- Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplantation Proceedings 2002;34(5):1812‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miserlis 2008 {published data only}
- Miserlis G, Papanikolaou V, Vergoulas G, Antoniadis N, Fouzas I, Vrochidis D, et al. Efficacy and safety of everolimus with low dose cyclosporine A compared with mycophenolate mofetil and full dose cyclosporine A in de novo renal transplant recipients [abstract no: 1641]. Transplantation 2008;86(Suppl 2):544. [CENTRAL: CN‐00740580] [Google Scholar]
Mourad 2004a {published data only}
- Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal‐transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78(4):584‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C, Lorho R, Therver E, Fares N. Simulect versus thymoglobulin with delayed introduction of neoral in renal transplantation: three month results of a French multicenter randomized trial [abstract no: 0592]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00402018]
- Mourad GJ, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. A sequential protocol using simulect vs thymoglobulin in low immunological risk renal transplant recipients: six‐month results of a French multicenter, randomized trial [abstract no: 1212]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00446849] [Google Scholar]
Mourad 2005 {published data only}
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms SG. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464. [Google Scholar]
- Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. American Journal of Transplantation 2006;6(5 Pt 1):1042‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre CH, Rostaing L, Kirchherr B, MyProms SG. Tolerability of enteric coated mycophenolate sodium (EC‐MPS) in combination with neoral and steroids in de novo kidney transplant recipients: a 12 months prospective trial [abstract no: 1209]. American Journal of Transplantation 2005;5(Suppl 11):464‐5. [Google Scholar]
- Mourad G, Karras A, Kamar N, Garrigue V, Legendre C, Lefrancois N, et al. Renal function with delayed or immediate cyclosporine microemulsion in combination with enteric‐coated mycophenolate sodium and steroids: results of follow up to 30 months post‐transplant. Clinical Transplantation 2007;21(3):295‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C. Assessment of two strategies of neoral® administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic®, steroids and anti‐il2r antibodies: 6 months interim results [abstract]. Transplantation 2004;78(2 Suppl):454. [CENTRAL: CN‐00509366] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Legendre C, Myriade FR. Assessment of two strategies of neoral administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic, steroids, and anti‐IL2R antibodies: 12‐month results of a randomized, multicentre, open, prospective controlled study. Transplantation Proceedings 2005;37(2):920‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing, Rostaing L, Legendre C. Assessment of two neoral® administration strategies on renal function and efficacy in de novo renal transplant patients receiving enteric‐coated mycophenolate sodium, steroids and anti‐IL2r antibodies: 6 months interim analysis of a randomized, multicentre, open, prospective controlled study [abstract]. American Journal of Transplantation 2004;4(Suppl 8):219. [CENTRAL: CN‐00509367] [Google Scholar]
- Pietruck F, Budde K, Salvadori M, Sollinger H, Bourbigot B, Gentil MA, et al. Efficacy and safety of enteric‐coated mycophenolate sodium in renal transplant patients with diabetes mellitus: post hoc analyses from three clinical trials. Clinical Transplantation 2007;21(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Legendre CH, Cohen D, Budde K, Zeier M, Kirchherr B, et al. Safety and tolerability of enteric coated mycophenolate sodium in combination with neoral and steroids in de novo kidney transplant recipients: 12 months analysis of a prospective trial [abstract no: T‐PO50030]. Nephrology 2005;10(Suppl 1):A215. [CENTRAL: CN‐00644301] [Google Scholar]
- Rostaing L, Mourad G, Kamar N, Garrigue V, Karras A, Lefrancois N, et al. Tolerability of enteric‐coated mycophenolate sodium to 1 year in combination with cyclosporine and corticosteroids in renal transplant recipients. Transplantation Proceedings 2006;38(9):2860‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Mourad G, Legendre C. Safety and tolerability of enteric‐coated mycophenolate sodium in combination with steroids and two regimen of neoral®, in denovo kidney transplant recipients: 6 months interim results. A randomized, multicentre, open, prospective controlled study [abstract]. American Journal of Transplantation 2004;4(Suppl 8):219. [CENTRAL: CN‐00583362] [Google Scholar]
- Rostaing L, Mourad G, Legendre C. Sustainable tolerability effects of myfortic® in combination with neoral® and steroids at 12 months, in de novo kidney transplantation: a randomized, multicentre, open, prospective controlled study [abstract no: 133]. American Journal of Transplantation 2005;5(Suppl 11):190. [Google Scholar]
Mourer 2012 {published data only}
- Mourer JS, Ewe SH, Mallat MJ, Ng AC, Rabelink TJ, Bax JJ, et al. Late calcineurin inhibitor withdrawal prevents progressive left ventricular diastolic dysfunction in renal transplant recipients.[Erratum appears in Transplantation. 2013 Apr 15;95(7):e52]. Transplantation 2012;94(7):721‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourer JS, Koning EJ, Zwet EW, Mallat MJ, Rabelink TJ, Fijter JW. Impact of late calcineurin inhibitor withdrawal on ambulatory blood pressure and carotid intima media thickness in renal transplant recipients. Transplantation 2013;96(1):49‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourer JS, Koning EJP, Berger SP, Op't Roodt J, Spaans M, Rabelink AJ, et al. Effects of CNI or MMF withdrawal on carotid intima media thickness in renal transplant recipients [abstract no: 528]. American Journal of Transplantation 2008;8(Suppl 2):319. [Google Scholar]
- Mourer JS, Hartigh J, Mallat MJ, Berger SP. Estimation of systemic exposure improves safety of withdrawal of either CNI or MMF from triple drug therapy [abstract no: F‐PO628]. Journal of the American Society of Nephrology 2007;18(Abstracts):237A‐8A. [Google Scholar]
- Mourer JS, Hartigh J, Zwet EW, Mallat MJ, Dubbeld J, Fijter JW. Randomized trial comparing late concentration‐controlled calcineurin inhibitor or mycophenolate mofetil withdrawal. Transplantation 2012;93(9):887‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noris 2007 {published data only}
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. Journal of the American Society of Nephrology 2007;18(3):1007‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84(8):956‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low‐dose rabbit anti‐thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor‐specific anti‐HLA antibody development. Journal of Immunology 2013;191(5):2818‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Novoa 2011 {published data only}
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus CSA elimination compared with CSA reduction [abstract no: P‐359]. Transplant International 2009;22(Suppl 2):183. [Google Scholar]
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation [abstract no: 1636]. American Journal of Transplantation 2010;10(Suppl 4):503. [EMBASE: 70465011] [Google Scholar]
- Novoa PA, Grinyo JM, Ramos FJ, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplantation Proceedings 2011;43(9):3331‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPTIMA‐TX Study 2008 {published data only}
- Bolin P Jr, Shihab F, Mulloy L, Henning A, Gao J, Bartucci M, et al. Optimizing tacrolimus therapy in maintenance renal allografts: 6 month results [abstract no: 390]. American Journal of Transplantation 2006;6(Suppl 2):197‐8. [CENTRAL: CN‐00671805] [DOI] [PubMed] [Google Scholar]
- Bolin P Jr, Shihab FS, Mulloy L, Henning AK, Gao J, Bartucci M, et al. Optimizing tacrolimus therapy in the maintenance of renal allografts: 12‐month results. Transplantation 2008;86(1):88‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bolin P, Shihab F, Mulloy L, Gao J, Bartucci M, Holman J, et al. Optimizing tacrolimus therapy in maintenance renal allografts: 6 month results in African Americans (AA) [abstract no: F‐PO1077]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐00602098] [Google Scholar]
Pankewycz 2011 {published data only}
- Pankewycz O, Kohli R, Wallace PK, Said M, Feng L, Patel S, et al. Low dose rabbit anti‐thymocyte globulin induction therapy results in prolonged selective CD4 T cell depletion irrespective of maintenance immunosuppression [abstract no: 957]. American Journal of Transplantation 2010;10(Suppl 4):316‐7. [EMBASE: 70464333] [Google Scholar]
- Pankewycz O, Kohli R, Wallace PK, Said M, Feng L, Patel SK, et al. Low dose rabbit anti‐thymocyte globulin induction therapy results in prolonged selective CD4 T cell depletion irrespective of maintenance immunosuppression [abstract no: 130]. Transplantation 2010;90(Suppl S2):62. [EMBASE: 71531221] [DOI] [PubMed] [Google Scholar]
- Pankewycz O, Leca N, Kohli R, Wallace PK, Said M, Feng L, et al. Low‐dose rabbit antithymocyte globulin induction therapy results in prolonged selective lymphocyte depletion irrespective of maintenance immunosuppression. Transplantation Proceedings 2011;43(2):462‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pankewycz O, Leca N, Kohli R, Weber‐Shrikant E, Said M, Alnimri M, et al. Conversion to low‐dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy‐guided study. Transplantation Proceedings 2011;43(2):519‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year and equivalent renal function at 2 years [abstract no: 948]. American Journal of Transplantation 2012;12(Suppl 3):304. [EMBASE: 70746901] [Google Scholar]
- Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year yet equivalent renal function at 2 years [abstract no: 515]. Transplantation 2012;94(Suppl 10S):967. [EMBASE: 71251715] [Google Scholar]
- Pankewycz O, Leca N, Wallace P, Said M, Feng L, Patel S, et al. Rabbit anti‐thymocyte globulin (rATG) induction therapy followed by tacrolimus conversion to sirolimus at 3 months does not increase Treg cells [abstract no: 1436]. American Journal of Transplantation 2012;12(Suppl 3):448. [EMBASE: 70747403] [Google Scholar]
- Pankewycz O, Said M, Feng L, Patel S, Alnimri M, Kohli R, et al. Conversion to low dose tacrolimus or rapamycin 3 months after kidney transplant: a prospective, protocol biopsy guided study [abstract no: 1657]. American Journal of Transplantation 2010;10(Suppl 4):509. [DOI] [PubMed] [Google Scholar]
- Pankewycz O, Said M, Feng L, Patel SK, Alnimri M, Kohli R, et al. Conversion to low dose tacrolimus or rapamycin 3 months after kidney transplant: a prospective, protocol biopsy guided study [abstract no: 106]. Transplantation 2010;90(Suppl 2S):294. [EMBASE: 71531658] [DOI] [PubMed] [Google Scholar]
Ponticelli 1988 {published data only}
- Ponticelli C, Tarantino A, et al. Prospective trial of triple therapy in renal transplants [abstract]. Nephrology Dialysis Transplantation 1988;3(1):98. [CENTRAL: CN‐00260367] [Google Scholar]
Rahamimov 2008 {published data only}
- Rahamimov R, Yusim A, Winkler J, Mashraki T, Gafter U, Mor E. Conversion from tacrolimus to sirolimus‐based protocol in renal transplant recipients: a long‐term comparative study [abstract no: 1617]. Transplantation 2008;86(Suppl 2):537. [CENTRAL: CN‐00740447] [Google Scholar]
Ritz 1998 {published data only}
- Kahn D, Botha JF, Pascoe MD, Pontin AR, Halkett J, Tandon V. Withdrawal of cyclosporine in renal transplant recipients with acute tubular necrosis improves renal function. Transplant International 2000;13(Suppl 1):S82‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ritz M, Pascoe MD, Pontin AR, Kahn D. Is cyclosporine toxic to the transplanted kidney with acute tubular necrosis?. Transplantation Proceedings 1998;30(4):1204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saunders 2003 {published data only}
- Saunders RN, Bicknell GR, Nicholson ML. The impact of cyclosporine dose reduction with or without the addition of rapamycin on functional, molecular, and histological markers of chronic allograft nephropathy. Transplantation 2003;75(6):772‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saunders RN, Carr S, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without rapamycin [abstract]. Transplantation 2000;69(8 Suppl):S226. [CENTRAL: CN‐00447600] [Google Scholar]
- Saunders RN, Carr S, Nicholson ML. Chronic allograft nephropathy: a prospective randomized trial of cyclosporin reduction with or without rapamycin [abstract]. British Journal of Surgery 2000;87 Suppl 1:44. [Google Scholar]
- Saunders RN, Carr S, Nicholson ML. Side‐effect profile of rapamycin in patients with chronic allograft nephropathy [abstract]. British Journal of Surgery 2000;87 Suppl 1:82. [CENTRAL: CN‐00339343] [Google Scholar]
SOCRATES Study 2014 {published data only}
- Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus‐based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transplant International 2014;27(3):302‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103.
Westhoff 1995 {published data only}
- Westhoff A, Heering P, Ivens K, Kutkuhn B, Grabensee B. Safe immunosuppression after kidney transplantation even without cyclosporine? A prospective, randomised study following primary triple therapy [Sichere immunsuppression nach nierentransplantation auch ohne ciclosporin? eine prospektive, randomisierte untersuchung nach primarer triple‐therapie]. Transplantationsmedizin ‐ Organ Der Deutschen Transplantationsgesellschaft 1995;7(1):27‐32. [EMBASE: 1995161557] [Google Scholar]
Wu 2007d {published data only}
- Wu MJ, Shu KH, Cheng CH, Chen CH, Yu DM. Conversion from cyclosporine to sirolimus in stable Taiwanese kidney transplant recipients ‐ one year report [abstract no: P442]. Transplant International 2007;20(Suppl 2):201. [Google Scholar]
References to ongoing studies
David‐Neto 2014 {published data only}
- David‐Neto E, Galante N, Altona M, Paula F, Triboni A, Ramos F, et al. A randomized, prospective study comparing everolimus/low tacrolimus with regular tacrolimus/MPS for the elderly renal transplant recipients [abstract]. Transplantation 2014;98(Suppl 1):549. [EMBASE: 71545379] [Google Scholar]
ERIC Study 2010 {published data only}
- Sanchez‐Fructuoso A, Ruiz JC, Hernandez D, Sanchez‐Plumed J, Fernandez A, Pastor Rodriguez A, et al. Early everolimus introduction and calcineurin inhibitor withdrawal in renal transplant patients: a multicenter, randomized, open‐label study (the ERIC study) [abstract no: 1647]. American Journal of Transplantation 2010;10(Suppl 4):506. [EMBASE: 70465022] [Google Scholar]
ISRCTN63298320 {published data only}
- Nicholson M. A prospective randomised trial of the use of cellcept to allow early tacrolimus withdrawal in live donor kidney transplantation. www.isrctn.com/ISRCTN63298320 (accessed 6 February 2017).
TRANSFORM Study 2013 {published data only}
- Chadban S, Hughes P, Campbell S, Irish A, Lim W, O'Connell P, et al. TRANSFORM trial design: a randomized, multicentre, open‐label study of everolimus with reduced calcineurin inhibitors in over 20000 de novo renal transplant recipients [abstract no:48]. Transplantation Society of Australia & New Zealand (TSANZ). 32nd Annual Scientific Meeting; 2014 Jun 11‐13; Canberra (ACT). 2014:61.
- Legendre C, Srinivas T, Pascual J, Chadban S, Citterio F, Henry M, et al. The TRANSFORM trial design: a large randomized, multicenter, open‐label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation [abstract no: P16]. Transplant International 2013;26(Suppl 3):23‐4. [EMBASE: 71356170] [Google Scholar]
- Pascual J, Chadban S, Citterio F, Henry M, Legendre C, Oppenheimer F, et al. TRANSFORM trial design: effect of everolimus on long‐term outcomes after kidney transplantation [abstract no: P308]. Transplant International 2013;26(Suppl 2):247. [EMBASE: 71359928] [Google Scholar]
- Pascual J, Srinivas TR, Chadban S, Citterio F, Henry M, Legendre C, et al. Balancing efficacy and renal function preservation after kidney transplantation with everolimus and reduced calcineurin inhibitors for better graft outcomes: design of the TRANSFORM study [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii535. [EMBASE: 71493013] [Google Scholar]
- Pascual J, Srinivas TR, Chadban S, Citterio F, Oppenheimer F, Tedesco H, et al. TRANSFORM: a novel study design to evaluate the effect of everolimus on long‐term outcomes after kidney transplantation. Open Access Journal of Clinical Trials 2013;6:45‐54. [EMBASE: 605631666] [Google Scholar]
Additional references
Ahsan 2001
- Ahsan N, Johnson C, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine oral solution (modified) plus mycophenolate mofetil after cadaveric kidney transplantation: results at 2 years. Transplantation 2001;72(2):245‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett 1996
- Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney International 1996;50(4):1089‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonwa 2002
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hariharan 2000
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. New England Journal of Medicine 2000;342(9):605‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kasiske 1996
- Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. Journal of the American Society of Nephrology 1996;7(1):158‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lim 2014
- Lim W.H, Eris J, Kannellis J, Pussell B, Wild Z, Witcombe D, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. American Journal of Transplantation 2014;14(9):2106‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mason 2014
- Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010699.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melk 2003
- Melk A, Halloran P. Immunosuppressive agents used in transplantation. In: Johnson RJ, Feehally J editor(s). Comprehensive Clinical Nephrology. 2nd Edition. Mosby, 2003:1062‐4. [Google Scholar]
Moore 2009
- Moore J, Middleton L, Cockwell P, Adu D, Ball S, Little MA, et al. Calcineurin Inhibitor sparing with mycophenolate in kidney transplantation: A systematic review and meta‐analysis. Transplantation 2009;87(4):591‐605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sharif 2011
- Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta‐analysis of calcineurin inhibitor sparing regimens in kidney transplantation. Journal of the American Society of Nephrology 2011;22(11):2107‐18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Karpe 2007
- Karpe KM, Talaulikar GS, Walters G. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006750] [DOI] [PMC free article] [PubMed] [Google Scholar]


