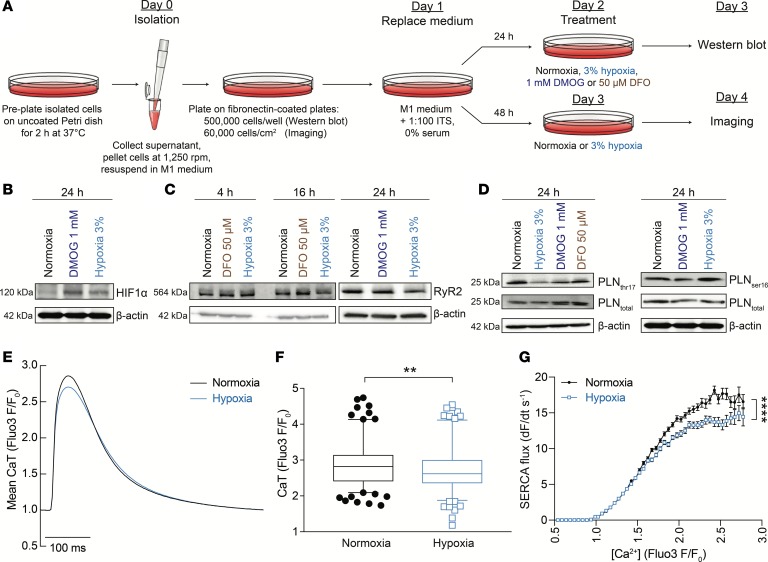
Figure 7. Ca2+ signaling in cultured neonatal ventricular myocytes.
(A) Protocol showing the method of neonatal rat ventricular myocyte (NRVM) culture including the 24-hour period under experimental conditions. (B) Immunoblot of lysates prepared from NRVMs incubated in a normal atmosphere, under 3% oxygen (hypoxia) or in the presence of 1 mM DMOG for 24 hours, showing upregulation of HIF1α, consistent with the observed HIF induction in the hearts of mice with iron-deficiency anemia. (C) RyR2 immunoreactivity in lysates of neonatal ventricular myocytes was reduced in 3% hypoxia but not in 1 mM DMOG or 50 μM DFO (iron chelator). (D) Immunoblot for PLN and its phosphorylated forms at Thr17 (left panel) or Ser16 (right panel) performed on lysates prepared from neonatal rat ventricular myocytes incubated in a normal atmosphere, under 3% oxygen (hypoxia), in the presence of 1 mM DMOG or 50 μM DFO. Phosphorylation at Thr17 (but not Ser16) was reduced in 3% hypoxia. (E) Ca2+ transients measured in electrically paced, Fluo3-loaded myocytes cultured under normoxic (19% O2) or hypoxic (3% O2) conditions for 24 hours. The low-oxygen atmosphere in hypoxia-treated cells was maintained during imaging by placing an air flow chamber over cells and superfusing cells with N2-bubbled solutions. (F) Ca2+ transient amplitude was significantly reduced in hypoxia, according to hierarchical statistical analysis. (G) Flux, measured as the recovery from systolic Ca2+, was significantly slower in hypoxia (n = 180 cells from 5 litters for control and 173 cells from 5 litters for hypoxia). See Supplemental Table 3 for details of nested (hierarchical) 1-way ANOVA analyses. Note that HIF1α in B and PLNser16 in D (blot shown on right) were blotted from the same membrane and, therefore, have the same loading control (β-actin). **P < 0.01, ****P < 0.0001.