Abstract
Background
The use of clinical signs may not be reliable in measuring the hypnotic component of anaesthesia. The use of bispectral index (BIS) to guide the dose of anaesthetic may have certain advantages over clinical signs. This is the second update of a review originally published in 2007 and updated in 2014.
Objectives
The primary objective of this review focused on whether the incorporation of BIS into the standard practice for management of anaesthesia can reduce the risk of intraoperative awareness, consumption of anaesthetic agents, recovery time and total cost of anaesthesia in surgical patients undergoing general anaesthesia.
Search methods
In this updated version, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 1), MEDLINE (1990 to 31 January 2013), Embase (1990 to 31 January 2013) and reference lists of articles. Previously, we searched to May 2009.
We reran the searches on 27 February 2017. We identified 14 potential new studies of interest which were added to a list of ‘Studies awaiting Classification' and will be incorporated into the formal review findings during the review update. In total there are 17 studies awaiting classification.
Selection criteria
We included randomized controlled trials comparing BIS with standard practice criteria for titration of anaesthetic agents.
Data collection and analysis
Two authors independently assessed trial quality, extracted data and analysed the data. We contacted study authors for further details.
Main results
We included 36 trials. In studies using clinical signs as standard practice, the results demonstrated a significant effect of the BIS‐guided anaesthesia in reducing the risk of intraoperative awareness among surgical patients at high risk for awareness (7761 participants; odds ratio (OR) 0.24, 95% confidence interval (CI) 0.12 to 0.48). This effect was not demonstrated in studies using end tidal anaesthetic gas (ETAG) monitoring as standard practice (26,530 participants; OR 1.13, 95% CI 0.56 to 2.26). BIS‐guided anaesthesia reduced the requirement for propofol by 1.32 mg/kg/hr (672 participants; 95% CI ‐1.91 to ‐0.73) and for volatile anaesthetics (desflurane, sevoflurane, isoflurane) by 0.65 standardized mean difference of minimal alveolar concentration equivalents (MAC SMD equivalences) (95% CI ‐1.01 to ‐0.28) in 985 participants. Irrespective of the anaesthetics used, BIS reduced the following recovery times: time for eye opening (2557 participants; by 1.93 min, 95% CI ‐2.70 to ‐1.16), response to verbal command (777 participants; by 2.73 min, 95% CI ‐3.92 to ‐1.54), time to extubation (1501 participants; by 2.62 min, 95% CI ‐3.46 to ‐1.78), and time to orientation (373 participants; by 3.06 min, 95% CI ‐3.63 to ‐2.50). BIS shortened the duration of postanaesthesia care unit stay by 6.75 min (1953 participants; 95% CI ‐11.20 to ‐2.31) but did not significantly reduce the time to home readiness (329 participants; ‐7.01 min, 95% CI ‐30.11 to 16.09).
Authors' conclusions
BIS‐guided anaesthesia can reduce the risk of intraoperative awareness in surgical patients at high risk for awareness in comparison to using clinical signs as a guide for anaesthetic depth. BIS‐guided anaesthesia and ETAG‐guided anaesthesia may be equivalent in protection against intraoperative awareness but the evidence for this is inconclusive. In addition, anaesthesia guided by BIS kept within the recommended range improves anaesthetic delivery and postoperative recovery from relatively deep anaesthesia.
Keywords: Humans; Anesthesia Recovery Period; Electroencephalography; Anesthesia; Anesthesia/methods; Anesthesiology; Anesthesiology/methods; Anesthesiology/organization & administration; Anesthetics; Anesthetics/administration & dosage; Intraoperative Awareness; Intraoperative Awareness/prevention & control; Monitoring, Intraoperative; Monitoring, Intraoperative/methods; Randomized Controlled Trials as Topic
Monitoring the bispectral index (BIS) to improve anaesthetic delivery and patient recovery from anaesthesia
The results from this updated review indicate that BIS can be useful in guiding the anaesthetic dose to avoid the risk of intraoperative awareness in surgical patients at high risk for awareness. Furthermore, anaesthesia guided by BIS improves anaesthetic delivery and recovery from anaesthesia.
General anaesthesia requires multiple agent administration to achieve unconsciousness (hypnotics), muscle relaxation, analgesia and haemodynamic control. Many anaesthesiologists rely on clinical signs alone to guide anaesthetic management. BIS is a scale derived from the measurement of cerebral electrical activity in anaesthetized patients so that the level of anaesthesia and drug delivery can be optimized. We systematically reviewed 36 randomized controlled studies to find out whether BIS could reduce the risk of intraoperative awareness and reduce anaesthetic use and recovery times in adult surgical patients. The risk of intraoperative awareness was determined in selected patients who were at potentially high risk for awareness. Four studies (7761 patients) that used clinical signs as a guide to anaesthetic administration in standard practice, as the control group, demonstrated a significant reduction in the risk of awareness with BIS monitoring. Four studies (26,530 patients) compared BIS monitoring with end tidal anaesthetic gas (ETAG) monitoring as a guide to management of anaesthesia and they did not demonstrate any difference in terms of intraoperative awareness. There was an overall reduction in volatile anaesthetic dose and the dose of propofol in the BIS group. Recovery from anaesthesia was quicker and postanaesthesia recovery care unit stay was shorter. The limitations of some of the clinical trials on BIS are discussed.
We reran the searches in February 2017. Fourteen potential new studies of interest were added to a list of ‘Studies awaiting Classification'. In total there are now 17 studies awaiting classification. We will deal with studies of interest when we update the review.
Summary of findings
Summary of findings for the main comparison.
Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness) for improving anaesthetic delivery and postoperative recovery
| Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness) for improving anaesthetic delivery and postoperative recovery | ||||||
| Patient or population: patients for improving anaesthetic delivery and postoperative recovery Settings: Intervention: Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness) | |||||
| Awareness in surgical patients with high risk of recall awareness ‐ using clinical signs as the guide in standard practice | Study population | OR 0.24 (0.12 to 0.48) | 7761 (4 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 8 per 1000 | 2 per 1000 (1 to 4) | |||||
| Moderate | ||||||
| 8 per 1000 | 2 per 1000 (1 to 4) | |||||
| Awareness in surgical patients with high risk of recall awareness ‐ using end tidal anaesthetic gas as the guide | Study population | OR 1.13 (0.56 to 2.26) | 26530 (4 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 1 per 1000 | 1 per 1000 (1 to 3) | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 (1 to 2) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 clinical heterogeneity 2 OR < 0.5 3 wide 95% CI
Background
Description of the condition
The practice of anaesthesia is based on the concept of components of anaesthesia resulting from separate pharmacological actions of multiple agent administration (Kissin 1997). Many anaesthesiologists rely on somatic signs (motor responses, changes in respiratory pattern) and autonomic signs (tachycardia, hypertension, lacrimation, sweating) to guide the dosages of anaesthetic agents in order to achieve the basic goals of anaesthetic management; that is unconsciousness (hypnotic effects), blockade of somatic motor responses, and suppression of autonomic responses to noxious stimulation. However, these clinical signs are not reliable measures of the conscious state of anaesthetized patients (Mahla 1997). The use of these clinical signs in judging the dosages of anaesthetic agents can lead to either overdosage or underdosage, which can result in adverse effects due to too deep or too light anaesthesia. Furthermore, there has been much concern regarding intraoperative awareness, which is an uncommon phenomenon occurring in about 0.1% to 0.2% of the general surgical population (Sebel 2004) but which can lead to a serious psychological disturbance called post‐traumatic stress disorder (PTSD), resulting in major depression and suicide. The incidence may approach 1% in surgical patients at high risk for intraoperative awareness such as patients with poor cardiac reserve, or undergoing cardiac surgery or caesarean section, where doses of anaesthetics have to be reduced to a light level of anaesthesia (Mashour 2012; Myles 2004). From a review of reported cases of intraoperative awareness, too light anaesthesia could account for 87% of the cases (Ghoneim 2009). Hence, strategies to provide optimal anaesthesia depth are required to avoid too light anaesthesia.
Description of the intervention
The bispectral index (BIS) is a dimensionless numerical scale for measuring brain electrical activity. It is derived from cerebral electrical activity (an electroencephalogram (EEG)) captured from the scalp surface at the forehead to reflect the sedative and hypnotic components of anaesthesia (Rampil 1998; Schneider 2010). Its value is a number within a range between 0 to 100, where 0 represents 'no detectable brain electrical activity' and 100 represents 'awake state'.
How the intervention might work
BIS has been recommended to guide doses of anaesthetics to achieve optimal depth of anaesthesia in individual patients. This is in order to avoid unnecessarily deep or too light anaesthesia due to overdosage or underdosage of the hypnotic medications during maintenance and recovery from anaesthesia (Schneider 2010; Sebel 2001). The recommended range of BIS is between 40 to 60 during maintenance of anaesthesia (Avidan 2011; Myles 2004) and 55 to 70 at 15 minutes prior to the end of surgery.(Gan 1997).
Why it is important to do this review
Several studies were conducted to assess the effect of BIS monitoring on the utilization of currently available anaesthetic agents, such as propofol, desflurane and sevoflurane (Gan 1997; Johansen 1998; Nelskyla 2001; Song 1997; Song 1998). There was a survey among anaesthesiologists regarding the routine use of BIS monitoring in anaesthesia (Johansen 1998). Although the majority of the respondents found that the monitor was easy to use, and it provided useful information, their comments revealed some ambivalence towards hypnotic titration using a BIS monitor. Most respondents felt that no changes occurred in their individual drug usage. Some respondents who reported a change in their practice felt that the hypnotic medication use might decrease while analgesic and haemodynamic control agent use might increase. A previous study by Song et al (Song 1997) reported an increased use of mivacurium in the BIS‐targeted group. Badrinath 1999 reported an increase in the use of intraoperative opioids in the BIS‐guided group. The increased use of either a muscle relaxant or an opioid analgesic might relate to the ability to maintain 'lighter' planes of anaesthesia with BIS, to avoid movement and increased blood pressure or heart rate during the operation. Thus, the impact of BIS monitoring on drug usage in routine clinical practice remains to be confirmed.
Since 1977, several articles and abstracts regarding the utility of BIS have been published by numerous medical researchers and academic institutions. It has been suggested that close titration of anaesthetic effect with the BIS monitor may improve some measures of patient outcomes and operating suite efficiency. However, the results are still contradictory across studies. Many studies (Anez 2001; Boztug 2006; Chiu 2007; Gan 1997; Kreuer 2003; Muralidhar 2008; Tufano 2000) have reported a significant improvement in anaesthetics delivery in terms of reduced anaesthetic consumption or requirements and improved recovery profiles but some studies (Bruhn 2005; Kreuer 2005; Luginbuhl 2003; Zohar 2006) have failed to demonstrate these effects. Moreover, the decreased anaesthetic consumption and enhanced recovery by BIS‐guided anaesthesia has to be weighed against the cost of BIS monitoring (Paventi 2001; Yli‐Hankala 1999).
Nowadays, the impact of BIS monitoring on the incidence of intraoperative awareness is a matter of interest in anaesthesia practice. The optimisation of the depth of anaesthesia may avoid too light anaesthesia which may result in intraoperative awareness. However, because of the low incidence of intraoperative awareness in an unselected surgical population undergoing surgeries with low risk of intraoperative awareness, an extremely large number of patients would be needed to determine the effects of BIS on awareness (Mashour 2012; O'Connor 2001). A previous updated systematic review (Punjasawadwong 2007) found two large randomized controlled studies (Avidan 2008;Myles 2004) reporting inconsistent results regarding the impact of BIS compared to standard practice on reduction of the risk of intraoperative awareness in surgical patients at high risk for awareness. Therefore, questions regarding the utility of BIS are valuable for the clinical practice of anaesthesia and are focused on in this systematic review.
Objectives
The primary objective of this review focused on whether the incorporation of BIS into the standard practice for management of anaesthesia can reduce the risk of intraoperative awareness, consumption of anaesthetic agents, recovery time and total cost of anaesthesia in surgical patients undergoing general anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized or quasi‐randomized controlled trials dealing with the use of the BIS compared with either clinical signs (CS) or end tidal anaesthetic gas (ETAG) as the standard practice in the titration of anaesthetic agents regardless of blinding or the language of publication of the articles.
Types of participants
We included men and women aged over 18 years undergoing any type of surgery under general anaesthesia, regardless of either a low or high risk of intraoperative awareness.
Types of interventions
We included studies with at least two arms, which used:
BIS to guide the dose of either an intravenous anaesthetic, hypnotic or volatile anaesthetic;
either CS or ETAG as the standard practice to guide the anaesthetic doses.
Types of outcome measures
Primary outcomes
The occurrence of intraoperative awareness
Secondary outcomes
Anaesthetic consumption or requirements for anaesthetics (intravenous or inhalation anaesthetics) titrated during anaesthesia
The time needed to achieve the primary recovery endpoints, namely response to command and orientation, extubation, eye opening, leaving the operating theatre and eligibility for discharge from the postanaesthesia care unit (PACU)
Amount of drugs (e.g. muscle relaxants, narcotic analgesics and other adjuvants) used during maintenance of anaesthesia
The cost (e.g. total cost during anaesthesia and PACU stay)
Search methods for identification of studies
In our second updated review we searched the literature until May 2009. In this updated version we searched the following sources for relevant trials.
Electronic searches
In this updated version we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 1), MEDLINE (1990 to 31 January 2013), Embase (1990 to 31 January 2013).
We reran the searches in February 2017). Fourteen potential new studies of interest were added to a list of Characteristics of studies awaiting classification ' . We will deal with the studies of interest when we update the review.
We identified randomized controlled trials (RCTs) using the search strategies found in Appendix 1 (MEDLINE Silver Platter); Appendix 2 (Embase Silver Platter); and Appendix 3 (CENTRAL).
Searching other resources
We searched the reference lists of retrieved trial reports and review articles for additional studies.
We did not impose any language restriction.
Data collection and analysis
We scanned the titles and abstracts of reports identified by the electronic searching to develop a list of possibly relevant reports.
Selection of studies
Two authors (YP, NB) independently assessed all selected studies to identify those to be included. We resolved disagreements by a consensus meeting between the three authors (YP, NB and AP).
Data extraction and management
We included all relevant information on the included studies in a data extraction form (Appendix 4). This included details of study method; country of investigation; number of patients; demographic characteristics; treatment groups; types of surgery; details of anaesthesia management; experience of the anaesthesiologists; BIS values during maintenance and at the end of surgery; and any relevant outcomes. We extrapolated data from figures as needed.
Assessment of risk of bias in included studies
We assessed risk of bias separately for the different domains, namely sequence generation of randomization process; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. The bias risk was classified as 'yes' for low risk of bias, 'no' for high risk of bias, and 'unclear' for unknown risk of bias due to insufficient information. For this judgement process we used the criteria and guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We used mean difference (MD) as the effect measure for continuous variables having the same units across the studies and standardized mean difference (SMD) for variables with different scales of measurement. For binary outcomes, such as the occurrence of intraoperative awareness, we used the odds ratio (OR) calculated by the Peto method as the effect measure.
In order to determine the overall effect of the BIS on the requirements for volatile anaesthetics, we converted the end tidal concentrations of volatile anaesthetics into minimal alveolar concentration (MAC) equivalents (MAC is the alveolar concentration of an anaesthetic at 1 ATM (1 ATM = 760 mm Hg) that prevents movement in response to surgical stimuli in 50% of patients). The MACs of desflurane, sevoflurane and isoflurane are 6.0, 1.8 and 1.15 for people aged 30 to 60 years; and 5.17, 1.45 and 1.0 for people older than 65 years, respectively. For studies that reported the use of volatile anaesthetics in MAC hours, for example in Luginbuhl 2003, we divided this value by the duration of anaesthesia.
We used SMD to determine the overall effect of BIS on the requirement for three volatile anaesthetics (desflurane, isoflurane, and sevoflurane) and expressed it as standardized mean difference of minimal alveolar concentration equivalents (MAC SMD equivalents). We interpreted the SMD as follows: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Higgins 2011).
To determine the overall effect of BIS on requirements of propofol, we calculated the MD of the infusion rate of propofol (mg/kg/hr). In study reports using μg/kg/hr we converted the units to mg/kg/hr.
Unit of analysis issues
We analysed the data based on two parallel groups in a randomized controlled trial, that is the use of BIS versus CS or the use of BIS versus ETAG to guide the doses of anaesthetics. For studies with more than two arms, only the arms using BIS, CS or ETAG were taken into consideration for statistical analyses.
Dealing with missing data
We performed intention‐to‐treat analysis to include all people randomized to the intervention groups. We investigated the effects of dropouts and exclusions by conducting the worst and the best scenario analyses.
We contacted the study authors to obtain missing data. In addition, for studies reporting medians and ranges or interquartile ranges (IQR) (Paventi 2001; Struys 2001; Tufano 2000) we recalculated the standard deviation (SD) by using the following formulae (Higgins 2011; Hozo 2005):
SD = IQR/1.35; SD = range/4 (for Cn < 70); or SD = range/6 (for Cn > 70).
Where, IQR is the inter‐quartile range and Cn is the number of participants.
Assessment of heterogeneity
We examined the included studies for methodological and clinical heterogeneity. We also looked for clinical heterogeneity based on sex, anaesthetics, types of operation, duration of anaesthesia, the BIS target value in the BIS group, depth of anaesthesia in the standard practice group, and the management of signs of inadequate anaesthesia and analgesia. To determine the consistency of the results between individual studies we looked at the overlap of confidence intervals. We considered the presence of statistical heterogeneity if there was poor overlap of the confidence intervals and the I2 statistic was greater than 50%.
Assessment of reporting biases
We assessed the included studies to determine whether there was a tendency for reporting bias based on the direction of the results (that is multiple or duplicate publication bias, language bias, outcome reporting bias etc). We constructed a funnel plot to determine the small studies' effect, including publication bias and other sources of bias.
Data synthesis
We used the Cochrane Collaboration's statistical package in Review Manager (RevMan 5.2) to analyse the data.
For the dichotomous variable, the occurrence of intraoperative awareness, we used the Peto's method to pool the ORs across studies. We quantified the statistical heterogeneity by using the I2 statistic. If there was statistical evidence of heterogeneity (I2 > 50%) we applied the random‐effects model. Otherwise we used the fixed‐effect model in the absence of statistical heterogeneity. For the continuous variables, the doses of anaesthetics and recovery times, we used the fixed‐effect model to pool the MDs or SMDs across studies in the absence of statistical heterogeneity as determined by the I2 statistic. We used the random‐effects model when there was statistical evidence of heterogeneity (I2 > 50%). We did not combine requirements of muscle relaxants and cost because of the limited number of studies (Paventi 2001; Song 1998).
Subgroup analysis and investigation of heterogeneity
We summarized the outcomes separately based on the type of anaesthetic agent, that is propofol and volatile anaesthetics (desflurane, isoflurane and sevoflurane).
Because of probable differences in baseline regarding depth of anaesthesia across studies depending on what they used to guide delivery of anaesthetics in their standard practice, particularly in studies focusing on the impact of BIS on the incidence of intraoperative awareness in surgical patients at high risk of awareness, we further stratified the studies into two subgroups based on the use of CS or ETAG as their standard practice guide and reported the results separately.
Sensitivity analysis
We performed sensitivity analysis to determine the effect of methodological quality on the results. We also performed sensitivity analysis to investigate the influence of missing data and assumptions regarding best or worst case scenarios on the results. We set the level of significance for all tests at a P value of 0.05.
Results
Description of studies
Results of the search
We identified 7291 possible studies from the initial search. From those studies we identified 58 potentially relevant studies and retrieved them for further assessment (see Additional Figure 1).
Figure 1.
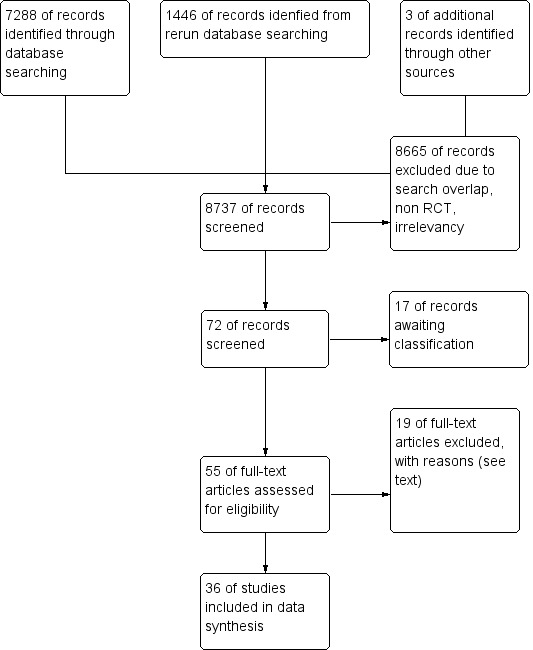
Study flow diagram.
We reran the search in CENTRAL (2017, Issue 1), MEDLINE (2013 to 27 February 2017), and Embase (2013 to 27 February 2017). We found 1446 references after removing duplicates. We found 14 potential new studies of interest and added them to a list of Studies awaiting classification. We will incorporate the 14 new studies into the formal review findings during the review update.
Included studies
We included 36 studies (Ahmad 2003; Aime 2006; Anez 2001; Assare 2002; Avidan 2008; Avidan 2011; Basar 2003; Boztug 2006; Bruhn 2005; Chiu 2007; Ellerkmann 2010; Gan 1997; Hachero 2001; Ibraheim 2008; Kamal 2009; Kreuer 2003; Kreuer 2005; Leslie 2005a; Luginbuhl 2003; Mashour 2012; Masuda 2002; Morimoto 2002; Muralidhar 2008; Myles 2004; Nelskyla 2001; Paventi 2001; Puri 2003; Recart 2003; Samarkandi 2004; Song 1997; Struys 2001; Tufano 2000; White 2004; Wong 2002; Zhang 2011; Zohar 2006) which fulfilled the inclusion criteria by comparing the use of BIS (BIS group) with either clinical signs (CS group) or end tidal anaesthetic gas (ETAG group) in guiding doses of currently used anaesthetics (propofol, desflurane, sevoflurane or isoflurane) (see the table Characteristics of included studies). Of these 36 studies, five studies were published in languages other than English: two in Japanese (Masuda 2002; Morimoto 2002); two in Spanish (Anez 2001; Hachero 2001); and one in Italian (Tufano 2000).
BIS was used to guide doses of propofol in 12 studies (Anez 2001; Chiu 2007; Ellerkmann 2010; Gan 1997; Hachero 2001; Kreuer 2003; Luginbuhl 2003; Masuda 2002; Muralidhar 2008; Struys 2001; Tufano 2000; Zhang 2011); desflurane in six studies (Bruhn 2005; Kreuer 2005; Luginbuhl 2003; Recart 2003; Song 1997; White 2004); sevoflurane in 16 studies (Ahmad 2003; Aime 2006; Assare 2002; Avidan 2008; Avidan 2011; Basar 2003; Boztug 2006; Ibraheim 2008; Kamal 2009; Mashour 2012; Morimoto 2002; Nelskyla 2001; Paventi 2001; Song 1997; Tufano 2000; Zohar 2006) and isoflurane in three studies (Muralidhar 2008; Puri 2003; Wong 2002). Six studies (Avidan 2008; Avidan 2011; Muralidhar 2008; Myles 2004; Puri 2003; Samarkandi 2004) were conducted in patients at high risk for awareness during the operation while two studies (Mashour 2012; Zhang 2011) were performed in unselected groups of patients. Eleven studies (Ahmad 2003; Anez 2001; Assare 2002; Gan 1997; Kreuer 2003; Luginbuhl 2003; Morimoto 2002; Nelskyla 2001; Paventi 2001; Song 1997; White 2004) were conducted in ambulatory surgical patients. One study (Ibraheim 2008) was conducted in obese patients and two studies (Wong 2002; Zohar 2006) were in elderly patients.
There were four studies (Luginbuhl 2003; Muralidhar 2008; Song 1997; Tufano 2000) with four treatment groups. They were divided into two substudies based on the anaesthetics titrated by either BIS or CS. There were seven studies (Aime 2006; Assare 2002; Bruhn 2005; Ellerkmann 2010; Kreuer 2003; Kreuer 2005; White 2004) with three treatment arms. Only the arms using BIS and CS were taken into consideration for statistical analyses.
The BIS target values for guiding anaesthetic doses varied across studies. The target was a BIS value of 60 in two studies (Assare 2002; Song 1997); 50 to 60 in six studies (Ahmad 2003; Kamal 2009; Nelskyla 2001; White 2004; Wong 2002; Zohar 2006); 50 in five studies (Bruhn 2005; Ellerkmann 2010; Kreuer 2003; Kreuer 2005; Struys 2001); 45 to 55 in four studies (Luginbuhl 2003; Muralidhar 2008; Puri 2003; Recart 2003); 45 to 60 in one study (Gan 1997); 40 to 50 in one study (Chiu 2007); and 40 to 60 in 16 studies (Aime 2006; Anez 2001; Avidan 2008; Avidan 2011; Basar 2003; Boztug 2006; Hachero 2001; Ibraheim 2008; Leslie 2005a; Lindholm 2008; Masuda 2002; Morimoto 2002; Myles 2004; Paventi 2001; Samarkandi 2004; Zhang 2011).
There was inconsistency across studies in the management of the signs of inadequate analgesia (hypertension and tachycardia) despite achieving target BIS values in the BIS group or target concentrations of anaesthetics in the CS group (see Additional Table 6). Most of the included studies used incremental doses of narcotics, that is fentanyl (Boztug 2006; Hachero 2001; Kamal 2009; Luginbuhl 2003; Morimoto 2002; Recart 2003; Song 1997; White 2004; Wong 2002); sufentanil (Ahmad 2003; Aime 2006; Samarkandi 2004); remifentanil (Bruhn 2005; Ellerkmann 2010; Kreuer 2003; Kreuer 2005; Paventi 2001; Struys 2001); or alfentanil (Gan 1997; Nelskyla 2001) for the management of inadequate anaesthesia or analgesia. In two studies (Basar 2003; Zohar 2006) signs of inadequate anaesthesia or analgesia were managed by increasing the concentration of sevoflurane. White et al used esmolol to treat sustained increases in heart rate (White 2004). Antihypertensive agents or labetalol were added to treat or control haemodynamic responses in three studies (Gan 1997; Kamal 2009; Wong 2002). Lidocaine was infiltrated prior to skin incision in Assare 2002 (see Additional Table 6).
Table 1.
Anaesthetic technique and strategy in management of inadequate analgesia
| Study | Anaesthetic technique | Titrating strategies |
| Ahmad 2003 | Endotracheal GA. Induction: sevoflurane Maintenance: sevoflurane‐sufentanil‐nitrous oxide‐a relaxant | Sevoflurane/sufentanil titrated for increased blood pressure/heart rate > 20%, despite a BIS value of 50‐60 or end tidal sevoflurane concentration 2% |
| Aime 2006 | Endotracheal GA, Induction: propofol‐sufentanil Intubation: atracurium Maintenance: sevoflurane and nitrous oxide in oxygen, sufentanil, atracurium |
BIS group: intermittent bolus dose of sufentanil despite BIS or Entropy values within the recommended range Control group (CS group): increased sevoflurane concentration or intermittent bolus doses of intravenous sufentanil for signs of inadequate anaesthesia, i.e. hypertension and bradycardia |
| Anez 2001 | LMA GA. Induction: propofol‐alfentanil Maintenance: propofol‐rocuronium | NA |
| Assare 2002 | LMA GA. Induction: propofol‐fentanyl Lidocaine infiltration prior to incision Maintenance: sevoflurane‐nitrous oxide (no muscle relaxant) | NA |
| Basar 2003 | Endotracheal GA. Induction: fentanyl‐thiopentone Intubation: rocuronium Maintenance: sevoflurane‐nitrous oxide | Inadequate analgesia in both groups managed by increased concentration of sevoflurane (no supplemental fentanyl) |
| Boztug 2006 | Endotracheal GA. Induction: fentanyl‐thiopentone Intubation: cis‐atracurium Maintenance: 50% O2/air mixture and 0.8%–1.5% sevoflurane, fentanyl, and cis‐atracurium | BIS group: additional fentanyl was administered in 0.1mg doses when the BIS value rose to 55. With inadequate decreases in the haemodynamic values, sevoflurane concentration was increased by 20% Control (CS) group: fentanyl was also administered in 0.1‐mg doses if MAP increased by 20% from baseline values, and in the event of inadequate decreases in the haemodynamic values, the sevoflurane concentration was increased by 20% |
| Bruhn 2005 | Endotracheal GA. Induction: remifentanil‐propofol Intubation: cis‐atracurium Maintenance: desflurane in O2/air mixture and remifentanil (no more neuromuscular blocking agents) | BIS group: desflurane during maintenance was continuously adjusted according to a target value of ‘50’. In case anaesthesia was judged inadequate despite the BIS target value, the infusion rate of remifentanil could be increased. Control (CS) group: if anaesthesia was inadequate, the desflurane concentration was increased in steps of 0.5 vol%. If this was judged insufficient, the infusion rate of remifentanil could be increased |
| Chiu 2007 | Endotracheal GA. Induction: fentanyl‐propofol Intubation:rocuronium Maintenance: before cardiopulmonary bypass ‐sevoflurane (end tidal concentration 0.5‐1.5%) with oxygen in air + infusion atracurium: during cardiopulmonary bypass ‐propofol starting TCI from 2 µG/ml in both arms | BIS group: adjustment of the propofol infusion to achieve BIS 40 to 50 Control (CS) group: titrating of TCI propofol according to perfusion pressure (70 to 90 mmHg) |
| Ellerkmann 2010 | Endotracheal GA plus regional anaesthesia for intraoperative and postoperative pain control Induction: remifentanil, propofol Intubation:cis‐atracurium Maintenance: propofol infusion, remifentanil infusion |
During maintenance of anaesthesia, all patients were assessed for signs of inadequate anaesthesia, hypotension or bradycardia. Inadequate anaesthesia was defined as hypertension, tachycardia or patient movement, eye‐opening, swallowing, grimacing, lacrimation or sweating. The definition of adverse haemodynamic responses was adapted from Garrioch et al15: responses were classified as ‘hypertension’ (SAP >40 mmHg from baseline), ‘hypotension’ (SAP <40 mmHg from baseline), ‘tachycardia’ (HR >100 beats/minute‐1) and ‘bradycardia’ (HR <45 beats/minute‐1). In the standard practice group, if anaesthesia was judged inadequate the propofol concentration was increased in steps of 1 mg/kg/hour as necessary |
| Gan 1997 | Endotracheal/LMA anaesthesia Induction: propofol alfentanil Maintenance: 50%nitrous in oxygen‐propofol‐alfentanil‐relaxants | BIS group: increasing alfentanil if BIS was within the recommended range (45‐60) SP group: increasing doses of either propofol, alfentanil or antihypertensive agents |
| Hachero 2001 | Endotracheal GA. Induction: propofol Intubation: mivacurium Maintenance: propofol‐fentanyl‐mivacurium |
Signs of inadequate anaesthesia managed in both groups by fentanyl |
| Ibraheim 2008 | Endotracheal GA. Induction: fentanyl‐propofol Intubation: succinylcholine. Maintenance: sevoflurane, nitrous oxide in oxygen, fentanyl, and atracurium | Any instances of inadequate anaesthesia were managed by increasing the concentration of sevoflurane |
| Kamal 2009 | Endotracheal GA. Induction : propofol, Intubation:atracurium Maintenance: sevoflurane, 50% nitrous oxide in oxygen, atracurium by TOF, fentanyl |
BIS group:If the patient in that group, exhibited hypertension or tachycardia the mode of treatment was dependent on the BIS index. If the BIS index was >60, anaesthesia was deepened by increasing sevoflurane concentration until BIS index was between 50 and 60. If BIS index was already in the targeted range and the patient exhibited hypertension or tachycardia, fentanyl 25‐50 μg IV was given. If BIS index was <50, sevoflurane was decreased and patient was checked for signs of lack of analgesia (i.e., lacrimation, grimacing, movement). In case of lack of analgesia, fentanyl 25‐50 μg IV was administered. But if no signs of lack of analgesia, labetalol 5‐10 mg IV was administered Standard practice group: If the patient in this group exhibited hypertension (mean arterial blood pressure >25% above baseline) (MBP) and tachycardia (heart rate (HR) >90 beats min‐1), anaesthesia was deepened either by increasing inspired sevoflurane concentration, or administering fentanyl 25‐50 μg or labetalol 5‐10 mg IV. The mode of treatment was left to anaesthesiologist’s discretion |
| Kreuer 2003 | Endotracheal GA. Induction: propofol‐remifentanil Intubation: cisatracurium. Maintenance: propofol (TCI)‐ remifentanil (constant infusion) | Remifentanil infusion was given in both groups for signs of inadequate anaesthesia despite achieving propofol target concentration or a target value of 50 for BIS |
| Kreuer 2005 | Endotracheal GA, Induction: propofol‐remifentanil Intubation: cis‐atracurium Maintenance: desflurane in O2/air mixture and remifentanil ( no more neuromuscular blocking agents) | BIS group: desflurane during maintenance was continuously adjusted according to a target value of ‘50’. In case anaesthesia was judged inadequate despite the BIS target value, the infusion rate of remifentanil could be increased. Control (CS) group: if anaesthesia was inadequate, the desflurane concentration was increased in steps of 0.5 vol%. If this was judged insufficient, the infusion rate of remifentanil could be increased |
| Leslie 2005a | Relaxant general anaesthesia. Induction: midazolam‐propofol or thiopentone Intubation: nondepolarizing muscle relaxants. Maintenance: propofol or volatiles‐nitrous oxide‐opioids. Hypnotic drugs. Combined general and regional anaesthesia | Narcotic analgesics on the discretion of the attending anaesthesiologists |
| Luginbuhl 2003 | Endotracheal GA Induction: propofol and fentanyl. Intubation: vecuronium Maintenance: propofol‐fentanyl or desflurane‐fentanyl | BIS group: propofol or desflurane to keep BIS 45‐55 and opioids according clinical criteria CS group: propofol or desflurane and opioids according to haemodynamic and vital sign criteria (within 20% of the baseline value) |
| Masuda 2002 | Endotracheal GA Induction: propofol‐fentanyl Intubation: vecuronium Maintenance: propofol‐nitrous oxide ‐ fentanyl‐vecuronium | NA |
| Morimoto 2002 | Endotracheal GA Induction:thiopentone, Intubation: vecuronium Maintenance: sevoflurane‐nitrous oxide‐ fentanyl‐vecuronium | Managed by fentanyl 50‐100 µg, despite 2% in sevoflurane in both groups |
| Myles 2004 | Relaxant general anaesthesia. Induction: midazolam‐propofol or thiopentone Intubation: nondepolarizing muscle relaxants. Maintenance: Propofol or volatiles‐nitrous oxide‐opioids. Hypnotic drugs. Combined general and regional anaesthesia | Narcotic analgesics on the discretion of the attending anaesthesiologists |
| Nelskyla 2001 | Endotracheal GA. Induction:propofol Intubation: rocuronium Maintenance: Sevoflurane (0.94%‐1.4%)‐nitrous oxide‐rocuronium | Supplemental alfentanil given for haemodynamic variables >25% of the preanaesthetic value, despite BIS of 50‐60 in BIS group or sevoflurane concentration of 1.4% in CP group |
| Paventi 2001 | Endotracheal GA. Induction: remifentanil ‐ thiopentone Intubation: vecuronium Maintenance: sevoflurane‐nitrous oxide‐remifentanil‐vecuronium |
Remifentanil infusion (0.4 µG/kg/min) for both groups |
| Puri 2003 | Endotracheal GA. Induction: midazolam‐morphine‐thiopentone Intubation:vecuronium. Maintenance: isoflurane‐nitrous oxide‐morphine | Signs of inadequate analgesia (tachycardia, hypertension, sweating, lacrimation etc) in both groups managed by morphine before vasodilators or beta‐blocker |
| Recart 2003 | Endotracheal GA Premedication: Induction: propofol‐fentanyl Intubation: rocuronium Maintenance: desflurane‐fentanyl | Intermittent intravenous fentanyl 0.5 mg/kg as needed to maintain haemodynamic variables within 15% of the baseline value Labetalol to control sympathetic responses as needed (in the presence of adequate hypnotic and analgesic states) Intermittent intravenous fentanyl 0.5 mg/kg as needed to maintain haemodynamic variables within 15% of the baseline value Labetalol to control sympathetic responses as needed (in the presence of adequate hypnotic and analgesic states) |
| Song 1997 | Endotracheal GA. Induction: fentanyl‐propofol. Intubation:succinylcholine Maintenance: desflurane or sevoflurane‐nitrous‐fentanyl‐mivacurium (at least 1‐2 TOF) | Inadequate analgesia (haemodynamic variables >20%of baseline) managed by supplemental doses of fentanyl (25‐30 µg) |
| Struys 2001 | Endotracheal GA. Induction: remifentanil, propofol .Intubation: rocuronium. Maintenance: remifentanil infusion (0.5 µg/kg/min)‐propofol infusion | Remifentanil infusion |
| Tufano 2000 | Endotracheal GA. Induction: Propofol. Intubation: Cis‐atracurium. Maintenance: propofol infusion or sevoflurane‐nitrous oxide‐cisatracurium‐fentanyl | NA |
| White 2004 | Endotracheal GA. Induction: propofol and fentanyl Intubation: succinylcholine. Maintenance: desflurane‐nitrous‐cisatracurium | Esmolol to treat sustained increased heart rate |
| Wong 2002 | Endotracheal GA. Induction: propofol‐fentanyl‐midazolam Intubation: rocuronium. Maintenance: isoflurane‐nitrous oxide‐fentanyl‐rocuronium‐fentanyl | BIS group: BIS > 60 increasing isoflurane concentration; BIS = 50‐60 giving supplemental fentanyl; BIS < 50 decreasing isoflurane concentration and supplementing fentanyl (signs of inadequate anaesthesia) or labetalol (no sign of inadequate anaesthesia) Control(CS) group: increasing isoflurane concentration or supplemental fentanyl or labetalol for management of hypertension (>25%) or tachycardia (>90 beats per minute) |
| Zohar 2006 | LMA GA. Induction: propofol‐fentanyl Maintenance: sevoflurane‐nitrous oxide (no muscle relaxant) | In both groups, the sevoflurane concentration was increased in response to signs of an inadequate “depth of anaesthesia” (e.g. movement in response to surgical stimulation) |
GA = general anaesthesia, LMA = laryngeal mask airway, TCI = target controlled infusion
NA = not available
In one study (Ellerkmann 2010) the influence of BIS was investigated in patients undergoing regional anaesthesia combined with general anaesthesia.
All but two studies (Assare 2002; Zohar 2006) used non‐depolarizing muscle relaxants either for endotracheal intubation or during maintenance of anaesthesia. Assare 2002 and Zohar 2006 were the only two studies that used laryngeal masks (LMA) without muscle relaxants for short surgical procedures, with a duration of less than 30 minutes, while the other studies were conducted for relatively longer surgical procedures with durations of at least 60 minutes.
Only three studies mentioned the length of experience of the anaesthesiologist, that is greater than one year (Basar 2003) and greater than five years (Ellerkmann 2010; Wong 2002). The others did not give any information regarding the experience of the anaesthesiologists.
Six studies (Avidan 2008; Avidan 2011; Muralidhar 2008; Myles 2004; Puri 2003; Samarkandi 2004) were conducted in surgical patients with high risk of intraoperative awareness. Two studies (Mashour 2012; Zhang 2011) were conducted in unselected groups of surgical patients with either low or high risk of intraoperative awareness. Myles 2004 and Puri 2003 used CS as a guide for anaesthetic administration in standard practice; while Avidan 2008, Avidan 2011, Mashour 2012 and Muralidhar 2008 used ETAG.
Additional Table 7 shows the BIS values during maintenance and at the end of anaesthesia in 13 studies (Basar 2003; Boztug 2006; Ellerkmann 2010; Hachero 2001; Masuda 2002; Kamal 2009; Nelskyla 2001; Paventi 2001; Recart 2003; Song 1997; White 2004; Wong 2002; Zohar 2006).
Table 2.
BIS value during anaesthesia
| Trial | Outcome | Value | BIS group | CS group | Note |
| Ahmad 2003 | Bispectral index (BIS) during operation | Mean | Not applicable | Not applicable | Data not available |
| Basar 2003 | BIS during operation | Mean | Cn = 30; mean = 44.9; SD (standard deviation) = 5.15 | Cn = 30; mean = 40.5; SD = 4.53 | |
| Boztug 2006 | BIS index during maintenance | Mean | Cn = 24; mean = 54 ; SD = 4 | Cn = 23; mean = 46; SD = 5 | |
| Bruhn 2005 | BIS index during maintenance | Mean | Data presented as a graph showing comparable BIS values between BIS and control (CS) groups at various point of anaesthesia | ||
| Chiu 2007 | BIS index during cardiopulmonary by pass | Mean | |||
| Ellerkmann 2010 | Intraoperative BIS | Mean | mean = 44.35 SD = 5.25 |
mean = 45.89 SD = 5.98 |
|
| Gan 1997 | BIS index during maintenance | Mean | Not applicable | Not applicable | Data presented as a graph showing BIS values at various points of anaesthesia in BIS group > in SP group |
| Hachero 2001 | BIS index during maintenance | Median | Cn = 20; mean = 46.4; 95% confidence interval (CI ) = 44.4 to 44.8 | Cn = 20; mean = 42.2; 95% CI = 40.1 to 44.2 | Data presented as a graph showing BIS values at various points during cardiopulmonary bypass in BIS group > in SP group |
| Ibraheim 2008 | BIS index during maintenance | Mean | Not applicable | Not applicable | Data: not available |
| Kamal 2009 | BIS index during maintenance After discontinuation of anaesthesia |
Mean | mean = 52.4 , SD = 3.4 mean = 70.1 SD = 11.2 |
Mean = 41.2 SD = 7.3 mean = 66.5 SD = 14.3 |
None of patients reported awareness |
| Kreuer 2003 | BIS index during maintenance | Mean | Not applicable | Not applicable | Data presented as a graph showing BIS values at various points of anaesthesia in BIS group >in SP group |
| Kreuer 2005 | BIS index during maintenance and at the end of surgery | Mean | Data presented as a graph showing comparable BIS values between BIS and control (CS) groups at various point during operation. At the end of surgery, BIS values were significantly higher in the BIS group | ||
| Masuda 2002 | BIS index during skin incision | Mean | Cn = 20; mean = 46; SD = 6 | Cn = 19; mean = 47; SD = 10 | |
| BIS 10 minutes before end of surgery | Mean | Cn = 20; mean = 59; SD = 6 | Cn =19; mean = 52; SD = 9 | ||
| BIS at end of surgery | Mean | Cn = 20; mean = 69; SD = 12 | Cn = 19; mean = 60; SD = 9 | ||
| BIS at end of anaesthesia | Mean | Cn = 20; mean = 92; SD = 6 | Cn = 19; mean = 88; SD = 6 | ||
| Morimoto 2002 | BIS index during maintenance | Mean | Not applicable | Not applicable | Data presented as graph showed BIS values at various points of anaesthesia in BIS group < in SP group |
| Nelskyla 2001 | BIS during surgery | Median | Cn = 32; median = 54; min‐max = 49‐61 | Cn = 30; median = 55; min‐max = 30‐65 | |
| Paventi 2001 | BIS during surgery | Median | Cn = 45; median = 46; min‐max = 36‐67 | Cn = 45; median = 42; min‐max = 39‐61 | |
| BIS after skin closure | Median | Cn = 45; median = 62; min‐max = 43‐98 | Cn = 45; median = 54; min‐max = 34‐99 | ||
| Recart 2003 | BIS index during maintenance | Mean | Cn = 30; mean = 49; SD = 13 | Cn = 30; mean = 40; SD = 11 | |
| BIS during emergence from anaesthesia | Mean | Cn = 30; mean = 88; SD =11 | Cn = 30; mean = 88; SD = 12 | At the time of eye opening before removal of endotracheal tube | |
| Song 1997 | BIS index during operation | Mean | Cn = 15; mean = 60; SD = 4 | Cn = 15; mean = 44; SD = 11 | |
| BIS during operation | Mean | Cn = 15; mean = 62; SD =3 | Cn = 15; mean = 42; SD = 8 | ||
| White 2004 | BIS index during maintenance | Mean | Cn = 20; mean = 57; SD = 12 | Cn = 20; mean = 41; SD = 10 | |
| Wong 2002 | BIS index during operation | Mean | Cn = 29; mean = 51; SD = 4.9 | Cn = 31; mean = 44.3; SD = 8.8 | |
| BIS index at discontinuation of anaesthesia | Mean | Cn = 29; mean = 68; SD =13 | Cn = 31; mean = 64; SD = 13 | ||
| Zohar 2006 | BIS index during operation | Mean | Cn = 25, mean= 57; SD = 10 | Cn = 25, mean = 59; SD =10 | |
| BIS index upon discontinuation of sevoflurane | Mean | Cn = 25, mean= 57; SD = 17 | Cn = 25, mean = 58; SD = 18 | ||
| BIS index upon removal of airway device | Mean | Cn = 25, mean = 78; SD = 13 | Cn = 25, mean = 81; SD = 14 |
Three studies (Aksun 2007; Kabukcu 2012; Qu X‐X 2011) are still awaiting assessment.
Excluded studies
We excluded 19 studies (Akcali 2008; Arnold 2007; Ballard 2012; Berti 2000; Burrow 2001; Caba 2003; Guignard 2001; Johansen 2000; Lehmann 2003; Leslie 2005b; Lindholm 2008; Mayer 2007; Pavlin 2001; Pavlin 2005; Schulz 2007; Sebel 1997; Song 1998; Vedtofte 2007; Yli‐Hankala 1999) for the reasons cited in the table Characteristics of excluded studies.
Studies awaiting classification
There are 17 studies in total awaiting classification, three from the 2013 search and 14 from the search we ran in February 2017 (Aksun 2007; Croci 2014; Fritz 2013; Golmohammadi 2014; Guo 2015; Jain 2016; Kabukcu 2012; Karaca 2014; Khoshrang 2016; Kim 2016; Martins 2013; Mozafari 2014; Nitzschke 2014; Quesada 2016; Qu X‐X 2011; Vance 2014; Villafranca 2013). For further details see the table Characteristics of studies awaiting classification.
Ongoings studies
We identified no ongoing studies
Risk of bias in included studies
Most of the included studies, with the exception of one (Anez 2001), were randomized controlled trials (RCTs). Anez 2001 was considered as a quasi‐randomized trial because it used sequential randomization.
Figure 2 and Figure 3 summarize the risks of bias, which have been described in the risk of bias table for each study.
Figure 2.
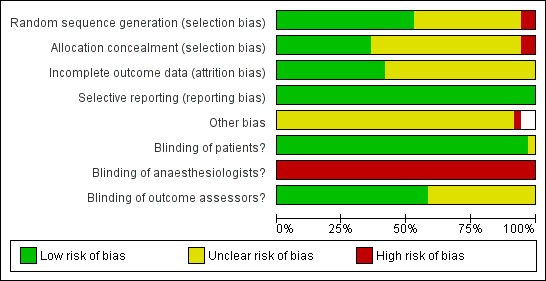
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 3.
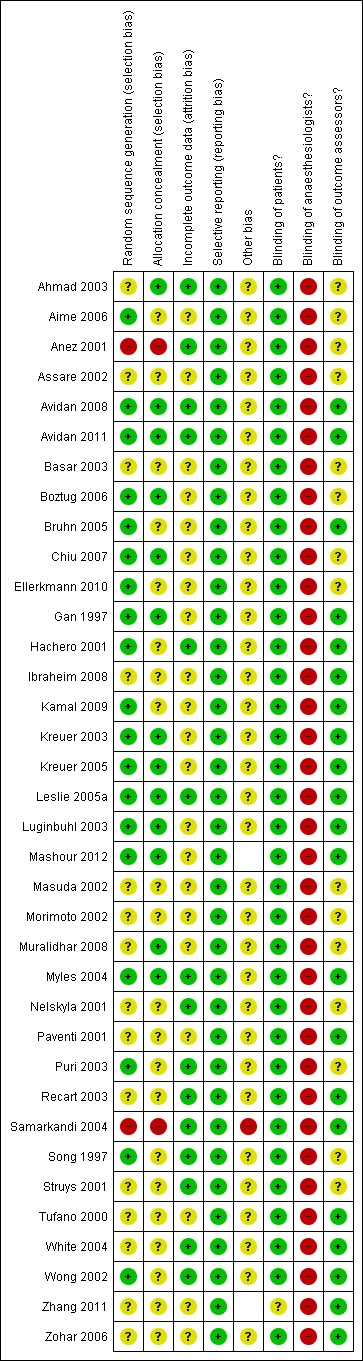
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Regarding sequence generation for the randomization process, Anez 2001 and Samarkandi 2004 were the only studies classified as 'high risk of bias', while 18 studies (Aime 2006; Avidan 2008; Avidan 2011; Boztug 2006; Bruhn 2005; Chiu 2007; Gan 1997; Hachero 2001; Kamal 2009; Kreuer 2003; Kreuer 2005; Leslie 2005a; Luginbuhl 2003; Mashour 2012; Myles 2004; Puri 2003; Song 1997; Wong 2002) were classified as 'low risk of bias' and the other 16 studies were 'unclear'.
Allocation
Allocation concealment was classified as 'low risk of bias' in 13 studies (Ahmad 2003; Avidan 2008; Avidan 2011; Boztug 2006; Chiu 2007; Gan 1997; Kreuer 2003; Kreuer 2005; Leslie 2005a; Luginbuhl 2003; Mashour 2012; Muralidhar 2008; Myles 2004). Anez 2001 was categorized as 'high risk of bias' because of its quasi‐randomization. Samarkandi 2004 was considered as 'high risk 'of bias regarding allocation concealment because of the randomization using patients' medical record numbers, that is odd numbers were assigned to group I and even numbers to group II. The other studies did not mention allocation concealment, therefore we classified them as 'unclear'.
Blinding
In all studies, the anaesthesiologists could not be blinded to the assigned groups. Twenty studies (Avidan 2008; Avidan 2011; Bruhn 2005; Gan 1997; Hachero 2001; Ibraheim 2008; Kamal 2009; Kreuer 2003; Kreuer 2005; Leslie 2005a; Luginbuhl 2003; Mashour 2012; Myles 2004; Paventi 2001; Recart 2003; Tufano 2000; White 2004; Wong 2002; Zhang 2011; Zohar 2006) blinded the outcome assessors to the assigned groups.
Incomplete outcome data
Regarding bias relating to incomplete outcome data, there were 16 studies (Ahmad 2003; Anez 2001; Avidan 2008; Avidan 2011; Boztug 2006; Hachero 2001; Leslie 2005a; Myles 2004; Nelskyla 2001; Puri 2003; Recart 2003; Samarkandi 2004; Song 1997; Struys 2001; White 2004; Wong 2002) that were classified as 'low risk of bias'. Five studies (Aime 2006; Boztug 2006; Gan 1997; Mashour 2012; Morimoto 2002) were classified as 'unclear' due to uncertainty about how missing outcome data could affect the observed effect size. The other 15 studies were classified as 'unclear' due to insufficient information about withdrawals and dropouts.
Selective reporting
All included studies were classified as at 'low risk of bias' from selective reporting because all expected outcomes were reported.
Other potential sources of bias
Anaesthesia providers participating in the trials were not blinded to the assigned group. This could introduce a 'learning contamination' bias, which involves changing clinical practice in the parallel control or unmonitored group by using the information from the BIS group (Roizen 1994).
Figure 4 and Figure 5 show the funnel plots based on the requirements for intravenous anaesthetic (propofol) and volatile anaesthetics (desflurane, isoflurane and sevoflurane). The funnel plots seem to be asymmetrical (Figure 5). This may indicate some other potential sources of bias due to both methodological and clinical heterogeneity as well as undetected publication bias.
Figure 4.
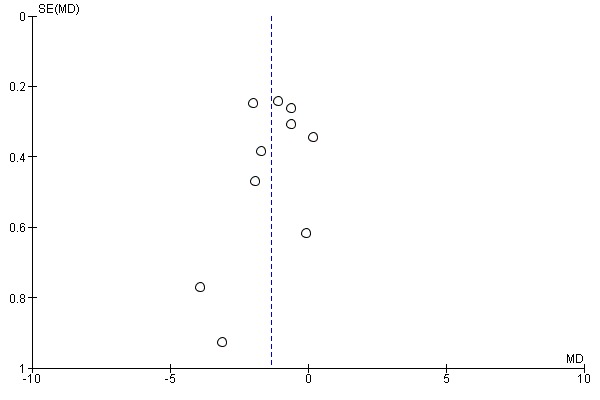
Funnel plot of comparison: bispectral index versus clinical signs on the requirement of propofol infusion rate (mg/kg/hr).
Figure 5.
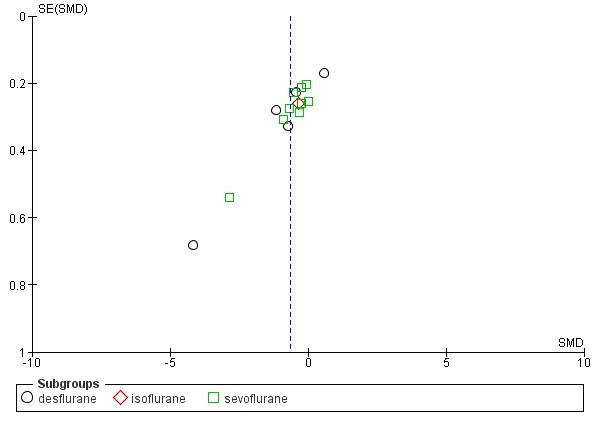
Funnel plot of comparison: bispectral index versus clinical signs on requirement of volatile anaesthetic (minimal alveolar concentration equivalents, MAC equivalents).
Effects of interventions
See: Table 1
Risk of intraoperative recall awareness
The table 'Comparison and data' (Analysis 1.1; Analysis 1.2) shows the occurrence of intraoperative awareness in eight studies (Avidan 2008; Avidan 2011; Mashour 2012; Muralidhar 2008; Myles 2004; Puri 2003; Samarkandi 2004; Zhang 2011) that were conducted in surgical patients at potentially high risk for awareness. The combined result of four studies (Myles 2004; Puri 2003; Samarkandi 2004; Zhang 2011) that used CS as the guide to anaesthetic administration in the standard practice group indicated a significant reduction in the risk of awareness with an overall OR of 0.24 (7761 participants; 95% CI 0.12 to 0.48; I2 = 0). The combined result of the other four studies (Avidan 2008; Avidan 2011; Mashour 2012; Muralidhar 2008), which used ETAG as the guide, failed to demonstrate an effect of BIS in reducing the risk of awareness. The overall effect was an OR of 1.13 (26,530 participants; 95% CI 0.56 to 2.26; I2 = 37%).
Analysis 1.1.
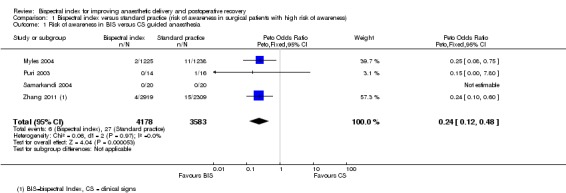
Comparison 1 Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness), Outcome 1 Risk of awareness in BIS versus CS guided anaesthesia.
Analysis 1.2.
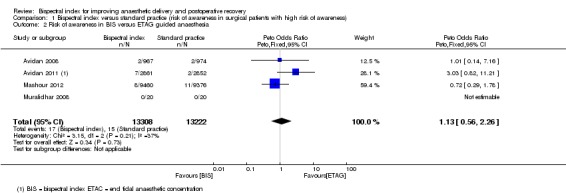
Comparison 1 Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness), Outcome 2 Risk of awareness in BIS versus ETAG guided anaesthesia.
We conducted a sensitivity analysis (Analysis 1.2) based on the best and the worst case scenario from the intention‐to‐treat analysis in Mashour 2012 where 36% of patients in the BIS group did not receive the intervention (BIS monitoring). Based on the data in this study, we assumed that the number of patients with intraoperative awareness could vary from 3 to 8 in the BIS group. If 3 out of 9460 patients in the BIS group experienced intraoperative awareness, the pooled OR of the four studies (Avidan 2008; Avidan 2011;Mashour 2012;Muralidhar 2008) that compared BIS and ETAG on the occurrence of intraoperative recall awareness would be 0.93 (26,530 participants; 95% CI 0.19 to 4.67; I2 = 68%). If 8 out of 9460 patients in the BIS group experienced intraoperative awareness the pooled OR of the four studies would be 1.13 (26,530 participants; 95% CI 0.56 to 2.26; I2 = 37%).
Recovery profiles
The early recovery times were described as time to eye opening, time to response to command, time to extubation, and time to orientation (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4). The overall effect of BIS was a reduction in early recovery times. The time to eye opening was reduced by 1.93 min (2557 participants; 95% CI ‐2.70 to ‐1.16; I2 = 82%) (Analysis 2.1), the time for response to command was reduced by 2.73 min (777 participants; 95% CI ‐3.92 to ‐1.54; I2 = 89%) (Analysis 2.2), the time to extubation was reduced by 2.62 min (1501 participants; 95% CI ‐3.46 to ‐1.78; I2 = 79%) (Analysis 2.3) and the time to orientation was reduced by 3.06 min (373 participants; 95% CI ‐3.63 to ‐2.50; I2 = 28) (Analysis 2.4).
Analysis 2.1.
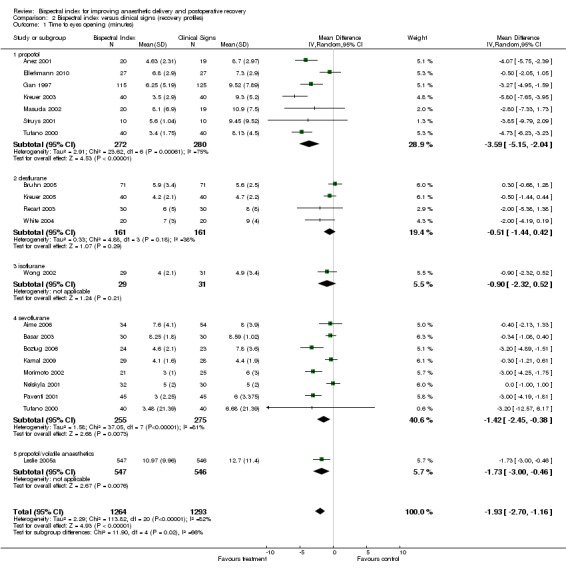
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 1 Time to eyes opening (minutes).
Analysis 2.2.
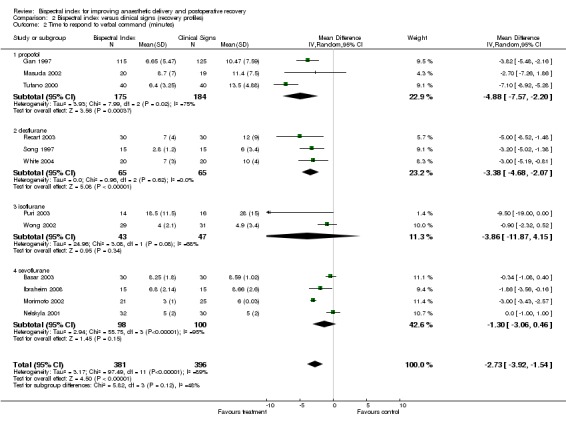
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 2 Time to respond to verbal command (minutes).
Analysis 2.3.
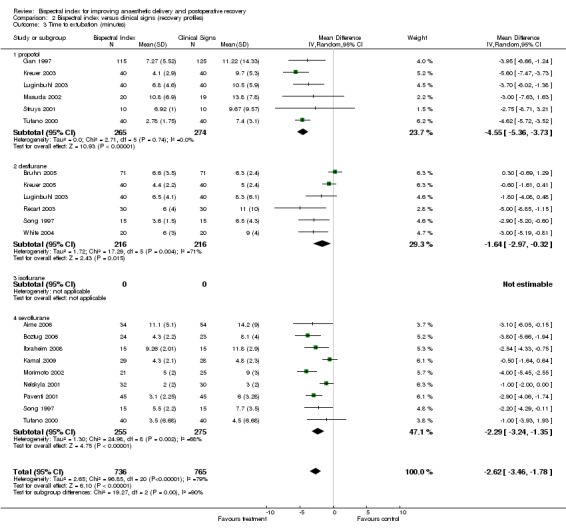
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 3 Time to extubation (minutes).
Analysis 2.4.
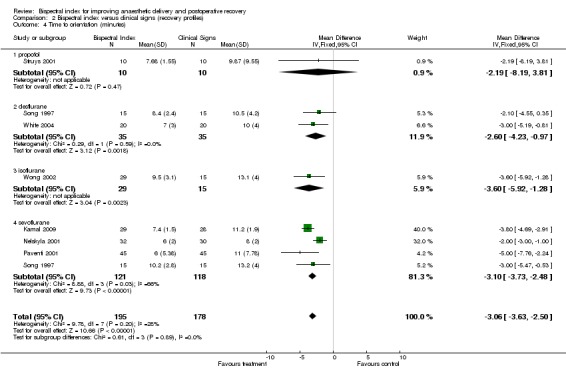
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 4 Time to orientation (minutes).
Postanaesthetic care unit (PACU) stay
The length of PACU stay is summarized in Analysis 2.5. The combined result indicated a significant effect of BIS on the length of PACU stay with an overall reduction of 6.75 min (1953 participants; 95% CI ‐11.20 to ‐2.31; I2 = 79%).
Analysis 2.5.
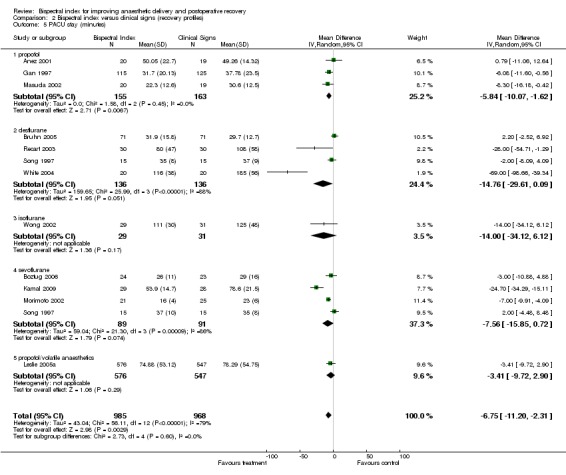
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 5 PACU stay (minutes).
Time to home readiness (discharge time)
The time to home readiness is summarized in Analysis 2.6. The combined result failed to demonstrate any effect of BIS in reducing the time to home readiness with an overall effect of ‐7.01 min (329 participants; 95% CI ‐30.11 to 16.09; I2 = 74%).
Analysis 2.6.
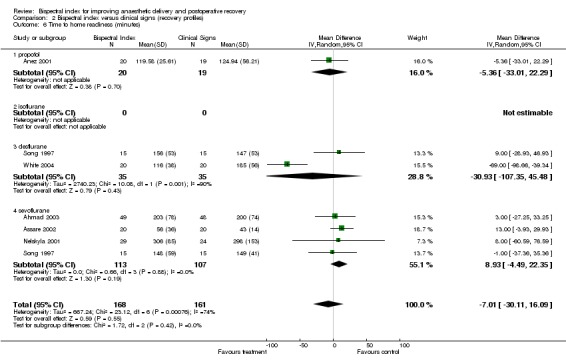
Comparison 2 Bispectral index versus clinical signs (recovery profiles), Outcome 6 Time to home readiness (minutes).
Requirement of anaesthetics
There were some variations in the results across studies regarding the consumption of anaesthetics (Analysis 3.1; Analysis 3.2).
Analysis 3.1.
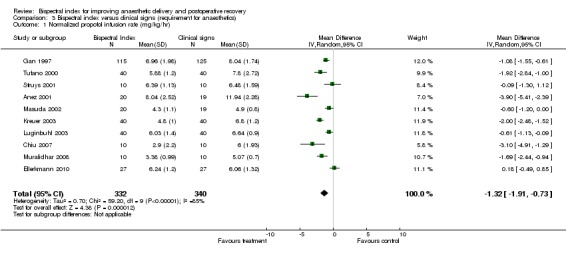
Comparison 3 Bispectral index versus clinical signs (requirement for anaesthetics), Outcome 1 Normalized propofol infusion rate (mg/kg/hr).
Analysis 3.2.
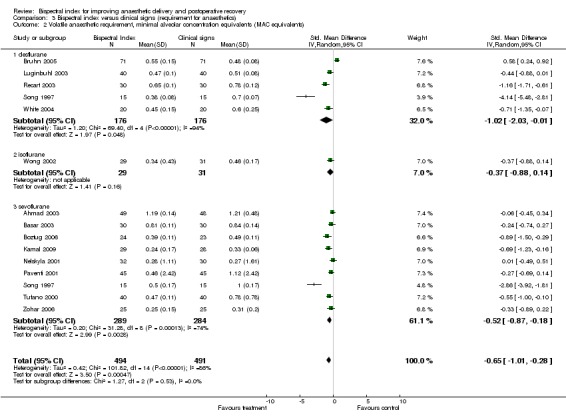
Comparison 3 Bispectral index versus clinical signs (requirement for anaesthetics), Outcome 2 Volatile anaesthetic requirement, minimal alveolar concentration equivalents (MAC equivalents).
The combined result from 10 studies involving 672 participants demonstrated the significant effect of BIS monitoring in reducing propofol consumption, with an overall decrease of 1.32 mg/kg/hr (95% CI ‐1.91 to ‐0.73; I2 = 85%) (Analysis 3.1).
The combined results for all volatile anaesthetics from 14 studies with a total of 985 participants demonstrated a significant effect of BIS monitoring in reducing the use of volatile anaesthetics, with an overall decrease of 0.65 MAC SMD equivalents (985 participants; 95% CI ‐1.01 to ‐0.28; I2 = 86%) (Analysis 3.2). The requirement for sevoflurane was decreased by 0.52 MAC SMD equivalents (573 participants; 95% CI ‐0.87 to ‐0.18; I2 = 74%). The MAC equivalent reduction for sevoflurane was ‐0.15, 95% CI (‐0.25 to ‐0.05). The requirement for desflurane was decreased by 1.02 MAC SMD equivalents (352 participants; 95% CI ‐2.03 to ‐0.10; I2 = 94%). The MAC equivalent reduction for desflurane was ‐0.11, 95% CI (‐0.25 to ‐0.03).
Requirement for intraoperative narcotic analgesics
Analysis 4.1 and Analysis 4.2 show the requirements for narcotic analgesics (fentanyl, remifentanil, sufentanil) in nine studies. Only one study (Hachero 2001) reported a significantly increased use of fentanyl in the BIS group. The combined result indicated no significant change in requirements for the narcotic analgesics in the BIS group, with an overall difference of 13.80 µg (333 participants; 95% CI ‐19.80 to 47.40; I2 = 83%) for fentanyl (Analysis 4.1) and 0.01µg/kg/min (276 participants; 95% CI ‐0.02 to 0.00; I2 = 0%) for remifentanil (Analysis 4.2). Only one study (Samarkandi 2004) reported significantly decreased use of sufentanil in the BIS group (Analysis 4.3).
Analysis 4.1.
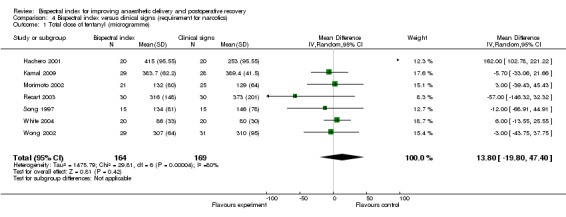
Comparison 4 Bispectral index versus clinical signs (requirement for narcotics), Outcome 1 Total dose of fentanyl (microgramme).
Analysis 4.2.
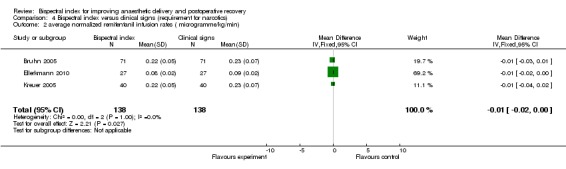
Comparison 4 Bispectral index versus clinical signs (requirement for narcotics), Outcome 2 average normalized remifentanil infusion rates ( microgramme/kg/min).
Analysis 4.3.
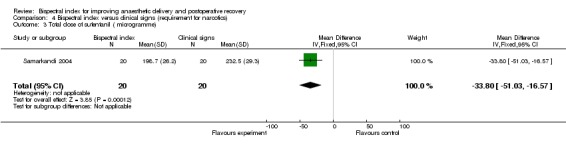
Comparison 4 Bispectral index versus clinical signs (requirement for narcotics), Outcome 3 Total dose of sufentanil ( microgramme).
Requirement for muscle relaxants
Only one study (Song 1997) reported a significant increase in the use of mivacurium in the BIS group, with an effect size of 5.70 mg (95% CI 2.77 to 8.63) in the desflurane subgroup and 4.60 mg (95% CI 0.56 to 8.64) in the sevoflurane subgroup.
Cost
Paventi 2001 reported total drug costs in the BIS and CS groups and the cost of BIS monitoring. The total drug cost was lower in the BIS group compared to the CS group (0.70 versus 0.98 EUR/min/70kg patient for sevoflurane) while the cost of BIS monitoring was 14.01 EUR/patient.
Discussion
Summary of main results
We have found that BIS‐guided anaesthesia can reduce the risk of intraoperative awareness in surgical patients at high risk for awareness compared to using CS as the guide to anaesthetic practice (Table 1). BIS‐guided and ETAG‐guided anaesthesia may be equivalent in protection against intraoperative recall awareness but the evidence for this is inconclusive (Table 1). In addition, anaesthesia guided by keeping the BIS within the recommended range can improve anaesthetic delivery and postoperative recovery from relatively deep anaesthesia. Furthermore, we have found that BIS‐guided anaesthesia can significantly reduce anaesthetic recovery times and consumption.
The relatively light anaesthesia in BIS‐guided anaesthesia has raised concerns about an increased requirement for narcotic analgesics and muscle relaxants to manage clinical signs of inadequate analgesia and relaxation. However, our current review has failed to demonstrate an effect of BIS‐guided anaesthesia on requirements for narcotic analgesics. Furthermore, only few studies (Song 1997) looked at the increased use of muscle relaxants in the BIS‐quided group. Hence, our current review has not confirmed whether or not BIS‐guided anaesthesia increases the use of narcotic analgesics and muscle relaxants.
One concern regarding the use of BIS is the cost. In this systematic review, Paventi 2001 was the only RCT that directly compared the costs for the two groups. However, only the costs of the drugs and BIS monitoring were compared. To provide sufficient evidence to support the cost‐benefit of BIS monitoring, a full economic evaluation is required. From a recent decision‐analytic model to assess the cost‐effectiveness of depth of anaesthesia monitoring (Shepherd 2013), an offset against cost savings regarding the reduced use of anaesthetics drugs could be the additional cost of depth of anaesthesia monitoring. Furthermore, the cost‐effectiveness of depth of anaesthesia monitoring appears to depend on the effectiveness of the depth monitoring in reducing intraoperative awareness and its psychological sequelae.
Overall completeness and applicability of evidence
Our review presents some evidence supporting the use of BIS to provide optimal depth of anaesthesia, avoiding unnecessarily high doses of anaesthetics. We have found a consistency across studies (80%) in a decreased propofol infusion rate. This information is very useful for anaesthesia providers who provide total intravenous anaesthesia (TIVA) with propofol.
We have found that, regardless of the anaesthetics used, BIS‐guided anaesthesia reduces all components of early recovery times, that is time to eye opening, response to verbal command, extubation and orientation. This information will help anaesthesia providers to tail off doses of anaesthetics at the end of surgery to optimal light levels of anaesthesia, by using the BIS, and to facilitate recovery from anaesthesia. Despite a decreased PACU stay, our review did not demonstrate the impact of BIS‐guided anaesthesia on time to home readiness following ambulatory surgery. Factors that might have affected the discharge following the ambulatory surgery were not only anaesthetic or surgical factors, such as drowsiness, nausea and vomiting, and pain, but also a system factor such as lack of immediate availability of an escort (Pavlin 1998).
From our systematic review, we have provided sufficient evidence supporting the use of BIS to guide doses of anaesthetics in the prevention of intraoperative awareness in either selected (Myles 2004) or unselected (Zhang 2011) risk groups for intraoperative awareness. However, we have failed to demonstrate either the superiority or the inferiority of BIS monitoring over ETAG monitoring in guiding the delivery of volatile anaesthetics on the incidence of intraoperative awareness. From the combined result it seems that the effects of both the BIS and ETAG techniques were equivalent on the incidence of intraoperative awareness, however a good quality equivalence trial is required to provide stronger evidence regarding this matter.
In our current review we have not evaluated the impact of BIS‐guided anaesthesia on the incidence of other outcomes such as postoperative nausea and vomiting, postoperative cognitive dysfunction (POCD) and mortality.
Quality of the evidence
We found clinical heterogeneity across the studies in this review in anaesthetic administration, the protocol for management of insufficient anaesthesia or analgesia, and clinical endpoints (see Additional Table 6). This could explain the statistical heterogeneity (I2 > 50%) of the trial results in our review. Therefore, we decided to combine the results using the random‐effects model and found that BIS‐guided anaesthesia could significantly reduce anaesthetic recovery times. Furthermore, we tried to explore the high statistical heterogeneity regarding the measured reduced anaesthetic consumption (Analysis 3.2). We found extremely different results from two studies (Bruhn 2005; Song 1997), which went in opposite directions. Song 1997 favoured BIS (Analysis 3.2.1; Analysis 3.2.2) and Bruhn 2005 favoured CS (Analysis 3.2.1). When removing these studies from the analyses we found the I2 was reduced from 94% to 50% and 77% to 29% in Analysis 3.2.1 and Analysis 3.2.3, respectively. However, the removal of these studies from the analyses did not change the conclusion regarding the decreased requirement of anaesthetics in the BIS group. Therefore, we concluded that BIS could improve the drug delivery in terms of decreased requirements of anaesthetics.
Potential biases in the review process
In this updated review we included 36 RCTs. Of these 36 studies, 14 studies were considered to have high methodological quality with regard to the allocation concealment. Although some studies did not mention blinding with regard to the outcome assessors, both patients and the outcome assessors were blinded to the allocation assignment in most studies (Figure 3), particularly in those concerned about intraoperative awareness (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004; Zhang 2011). From the sensitivity analyses, the exclusion of studies with an unclear blinding procedure did not affect the conclusion for the main outcome (intraoperative awareness).
Anaesthesia providers participating in the trials were not blinded to the assigned group. This could introduce a 'learning contamination' bias, which involves changing clinical practice in the parallel control or unmonitored group by using the information from the BIS group (Roizen 1994). This could have affected the results in some studies, which failed to demonstrate a reduction of the requirement for anaesthetics and recovery times with BIS monitoring (Bruhn 2005).
Agreements and disagreements with other studies or reviews
The results from our analysis were similar to the results from a previous meta‐analysis (Liu 2004), which was conducted in ambulatory surgical patients. The greater use of anaesthetics in the standard practice group of many studies indicated that the anaesthesia providers tended to use high doses of hypnotics (in a hypnotic‐based anaesthesia regimen) to manage signs of inadequate anaesthesia or analgesia, which resulted in too deep anaesthesia as indicated by the BIS values in some studies (see Additional Table 7). Hence, BIS‐guided anaesthesia could be helpful in optimising the dose of hypnotics.
There have been debates for years (Avidan 2008; Myles 2004) regarding the incorporation of BIS into routine practice for prevention of intraoperative awareness, particularly for surgical patients at high risk of awareness during general anaesthesia. Evidence supporting each side depends on what monitors (CS versus ETAG) to guide doses of anaesthetics in the standard practice group. In our current review we have found that BIS can significantly reduce the risk of recall awareness in studies using CS‐guided anaesthesia as standard practice (Myles 2004; Zhang 2011). For those studies in which ETAG‐guided anaesthesia was used as standard practice, maintaining a concentration of end tidal anaesthetics at a target of 0.7 MAC or above might be enough to decrease the likelihood of intraoperative awareness (Avidan 2008; Gonsowski 1995). This may explain why we have failed to demonstrate the role of BIS in prevention of intraoperative awareness in studies using ETAG‐guided anaesthesia as the comparison group. However, whether BIS is equivalent to ETAG in preventing intraoperative awareness requires a large, good quality equivalence trial for confirmation.
The result of our updated review published in 2014 seems contradictory to the result in a recent Cochrane review published in 2016 by Messina et.al.(Messina 2016), regarding the effect of BIS‐guided anaesthesia on the risk of intraoperative recall awareness. This could be explained by the differences between the two reviews. Our review focused only on studies which were conducted in surgical patients at a high risk of intraoperative recall awareness. Whereas Messina 2016, included studies with mixed groups of surgical patients (with or without risk of intraoperative recall awareness). Furthermore, our review performed sub‐group analyses based on studies using clinical signs or ETAG as their anaesthetic guide in the standard practice group. While Messina 2016, included all studies regardless as to whether they used clinical signs or ETAG as an anaesthetics guide in the standard practice group. The result favouring BIS monitoring for definite awareness could only be demonstrated y in our sub‐group analysis, where clinical signs were used as an anaesthetic guide in the standard practice group.
Authors' conclusions
BIS‐guided anaesthesia can reduce the risk of intraoperative awareness in surgical patients at high risk for awareness compared to using clinical signs as the guide for anaesthetic depth. BIS‐guided and ETAG‐guided anaesthesia may be equivalent in protection against intraoperative awareness but the evidence for this is inconclusive. In addition, anaesthesia guided by keeping the BIS within the recommended range improves anaesthetic delivery and postoperative recovery from relatively deep anaesthesia.
1. The information on the decreased risk of intraoperative recall awareness, anaesthetic use, and recovery times may be useful for further full economic evaluation in terms of the cost savings of BIS monitoring in various clinical aspects and settings in the real world.
2. A further large, good quality equivalence trial is needed to elucidate the effect of BIS‐guided anaesthesia compared to ETGA‐guided anaesthesia on the incidence of intraoperative recall awareness.
3. Further systematic reviews should be conducted to evaluate the impact of BIS‐guided anaesthesia on the incidence of other interesting outcomes such as postoperative nausea and vomiting, postoperative cognitive dysfunction, and mortality.
Acknowledgements
We would like to thank:
Dr Jan Jakobsson of the Karolinska Institute, Stockholm, for providing us with more details of his study; Dr Tong J Gan for providing additional information on his study; Denna N Braaksma for searching the literature; Ms Chompunuch Boonyawan of the Internet Resources and Retrieval Unit, Chiang Mai University Library, for searching MEDLINE; Anupa Shah for searching EMBASE in the 2007 version of this review; Karen Hovhannisyan for establishing the search terms and searching MEDLINE, EMBASE and CENTRAL in this updated version; Valeria Salerno (a medical student) for extracting data from an Italian article; Dr Andrea Casati for commenting on a study; Dr Sandro Salzano for commenting on an Italian article; Prof Martha Delgado, Dr Cesar Carcamo, Dr Idoris Cordero and Ivan Sola for translating and extracting data from Spanish articles; Dr Paul Manberg for details about BIS in an unpublished abstract; Dr Tomoki Hashimoto for quality assessment and data extraction of Japanese articles; Dr Simonia Vecchi for extracting data from an Italian article; Dr Kate Leslie for providing means and standard deviations from her study; Dr Mathew Zacharias (content editor), Prof Nathan Pace (statistical editor), Dr Michel Struys and Dr Chris Pomfrett (peer reviewers) for kindly commenting on the 2007 version of this review; Dr Janet Wale (CARG consumer editor) for kindly helping to rewrite our plain language summary; and Jane Cracknell for co‐ordinating the updated review.
We would like to thank Dr Stephan C Kettner and Dr Mathew Zacharias (content editors), Dr Cathal Walsh (statistical editor), Dr Melissa Giraldo‐Duque, Dr Maurizio Solca and Dr Paul Myles (peer reviewers) for their help and editorial advice during the preparation of the current updated systematic review.
Appendices
Appendix 1. MEDLINE SilverPlatter
#1 explode "Electroencephalography‐" / all SUBHEADINGS in MIME,MJME,PT #2 "Monitoring‐Physiologic" / all SUBHEADINGS in MIME,MJME,PT #3 (intra?operative* near monitoring) or (intra?operative* and monitoring) #4 intra?operative* near patient #5 BIS or bispectral* #6 (bispectral near index*) or (bispectral and index*) #7 electro?encephalograph* #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 ("Anesthesia‐and‐Analgesia" / all SUBHEADINGS in MIME,MJME,PT) or ("Anesthesia‐" / all SUBHEADINGS in MIME,MJME,PT) #10 (explode "Anesthetics‐General" / all SUBHEADINGS in MIME,MJME,PT) or(explode "Anesthesia‐General" / all SUBHEADINGS in MIME,MJME,PT) #11 an?esth* in TI, AB #12 explode "Postoperative‐Period" / WITHOUT SUBHEADINGS in MIME,MJME,PT #13 #9 or #10 or #11 or #12 #14 #8 and #13 #15 CLINICAL‐TRIAL in PT #16 randomised in AB #17 placebo in AB #18 (clinical trials) in MESH #19 randomly in AB #20 trial in TI #21 #15 or #16 or #17 or #18 or #19 or #20 #22 TG=animals #23 TG=humans #24 #22 not (#22 and #23) #25 #21 not #24 #26 #14 and #25 #27 #26 and (PY>1990)
Appendix 2. EMBASE SilverPlatter
#1 explode ELECTROENCEPHALOGRAPHY/ all subheadings #2 "patient‐monitoring" / all SUBHEADINGS in DEM,DER,DRM,DRR #3 (intra?operative* near monitoring) or (intra?operative* and monitoring) #4 electro?encephalograph* #5 explode "bispectral‐index" / all SUBHEADINGS in DEM,DER,DRM,DRR #6 (bispectral near index*) or (bispectral index* ) #7 #1 or #2 or #3 or #4 or #5 #8 explode "general‐anesthesia" / all subheadings #9 explode "anesthetic‐agent" / all subheadings #10 an?esthe* #11 #8 or #9 or #10 #12 #7 and #11 #13 "RANDOMIZED‐CONTROLLED‐TRIAL"/ all subheadings #14 "RANDOMIZATION"/ all subheadings #15 "CONTROLLED‐STUDY"/ all subheadings #16 "MULTICENTER‐STUDY"/ all subheadings #17 "PHASE‐3‐CLINICAL‐TRIAL"/ all subheadings #18 "PHASE‐4‐CLINICAL‐TRIAL"/ all subheadings #19 "DOUBLE‐BLIND‐PROCEDURE"/ all subheadings #20 "SINGLE‐BLIND‐PROCEDURE"/ all subheadings #21 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 #22 (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI,AB #23 (SINGL* or DOUBL* or TREBL* or TRIPL*) near ((BLIND* or MASK*) in TI,AB) #24 #21 or #22 or #23 #25 HUMAN in DER #26 (ANIMAL or NONHUMAN) in DER #27 #25 and #26 #28 #26 not #27 #29 #24 not #28 #30 #12 and #29 #31 #30 and (PY > 1990)
Appendix 3. CENTRAL
#1 MeSH descriptor Electroencephalography explode all trees #2 MeSH descriptor Monitoring, Physiologic, this term only #3 intraoperative monitoring #4 intraoperative near (patient* or monitoring) #5 BIS or bispectral* #6 bispectral near index* #7 bispectral index* #8 electroencephalograph* #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Anesthesia and Analgesia explode all trees #11 (anaesth* or anesth*):ti,ab #12 MeSH descriptor Postoperative Period, this term only #13 (#10 OR #11 OR #12) #14 (#9 AND #13)
Appendix 4. Data extraction form
Checklists for selection of study
| Study ID |
|
| Reviewer |
|
| Study Title |
|
| Source of data base |
MEDLINE EMBASE CENTRAL Handsearch |
| The study is published Not published Is the topic relevant? Is the study randomized /quasi‐ randomized? Are the participant adults (> 18 years)? |
Yes/No Yes/NO Yes/No/Unclear Yes/No/Unclear Yes/No/Unclear |
| Is the surgery under general anaesthesia? |
Yes/No/Unclear |
| Did the study group use BIS monitoring guiding the dose of anaesthetics? | Yes/No/Unclear |
| Did the control group use clinical signs guiding the dose of anaesthetics? | Yes/No/Unclear |
| Does the study fulfil the inclusion criteria? If no, state why? |
Yes/No/Unclear |
DATA EXTRACTION FORM
| Study ID | |
| Authors | |
| MEDLINE Journal ID | |
| Year of Publication | |
| Language | |
| Type of study | RCT Quasi‐RCT Non‐ RCT |
| Comments on study design | |
| Does the study compare the use BIS (BIS group) and the use of clinical signs (SP group) in guiding doses of anaesthetics? | |
| Was the assignment of subjects to treatment groups randomized? | |
| Was there blinding? If so, who was blinded | Subject ‐Blinded? Yes/No/Unclear Anaesthesiologist Blinded? Yes/No/Unclear Outcome accessor blinded? Yes/No/Unclear |
| Were the BIS and SP groups similar at the start of the trial? | |
| Apart from the treatment under investigation, were the groups treated equally? | |
| Are all relevant outcomes measured in a standard, valid and reliable way? | |
| What percentage of the individuals or clusters recruited into the study are included in the analysis? | |
| Were all the subjects analysed in the groups to which they were randomly allocated? |
QUALITY OF CONCEALMENT OF ALLOCATION
| Was an adequate concealment method used? | A = (adequate) if the allocation concealment was described as central randomization; serially numbered; opaque; or sealed envelopes B = (uncertain) if there was no mention about the allocation concealment C = (inadequate) if the allocation concealment was not used D = the randomization was not used |
|
PARTICIPANTS How many patients participated in the study? Overall number, and in each arm of the study. |
Total number: Number in each arm of study: Withdrawals: Yes/No/Unclear Number of withdrawals in each arm: |
| What are the characteristics of the study population? E.g. age range, sex, and disease characteristics of the population, disease prevalence. | BIS group SP group Age Sex ASA Operation |
| What are the characteristics of the study setting? E.g. rural, urban, hospital inpatient or outpatient, general practice, community. | |
| How many groups/sites are there in the study? If the study is carried out on more than one group of patients, or at more than one site, indicate how many are involved. | |
| Are there any specific issues raised by this study? Make any general comments on the study results and their implications | |
|
INTERVENTION: What interventions are evaluated in this study? |
OUTCOMES:
| Outcomes | Interventions | ||
| BIS group |
Non‐BIS (SP) group | Difference, P |
|
| Dose of anaesthetic agents |
Mean SD |
Mean SD |
|
| Time to recovery (please specify end point) Time to eye opening: Time to response to command: Time to extubation: Time to: Time to: Time to: |
Mean SD |
Mean SD |
|
| Relaxants | |
|
|
| Narcotics | |
||
| Awareness (Cn/N, %) |
|
|
|
CONTACT WITH AUTHOR:
REMARKS:
REVIEWER
Data and analyses
Comparison 1.
Bispectral index versus standard practice (risk of awareness in surgical patients with high risk of awareness)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of awareness in BIS versus CS guided anaesthesia | 4 | 7761 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.12, 0.48] |
| 2 Risk of awareness in BIS versus ETAG guided anaesthesia | 4 | 26530 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.56, 2.26] |
Comparison 2.
Bispectral index versus clinical signs (recovery profiles)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to eyes opening (minutes) | 20 | 2557 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐2.70, ‐1.16] |
| 1.1 propofol | 7 | 552 | Mean Difference (IV, Random, 95% CI) | ‐3.59 [‐5.15, ‐2.04] |
| 1.2 desflurane | 4 | 322 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.44, 0.42] |
| 1.3 isoflurane | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.32, 0.52] |
| 1.4 sevoflurane | 8 | 530 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.45, ‐0.38] |
| 1.5 propofol/volatile anaesthetics | 1 | 1093 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐1.00, ‐0.46] |
| 2 Time to respond to verbal command (minutes) | 12 | 777 | Mean Difference (IV, Random, 95% CI) | ‐2.73 [‐3.92, ‐1.54] |
| 2.1 propofol | 3 | 359 | Mean Difference (IV, Random, 95% CI) | ‐4.88 [‐7.57, ‐2.20] |
| 2.2 desflurane | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐3.38 [‐4.68, ‐2.07] |
| 2.3 isoflurane | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐11.87, 4.15] |
| 2.4 sevoflurane | 4 | 198 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐3.06, 0.46] |
| 3 Time to extubation (minutes) | 18 | 1501 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐3.46, ‐1.78] |
| 3.1 propofol | 6 | 539 | Mean Difference (IV, Random, 95% CI) | ‐4.55 [‐5.36, ‐3.73] |
| 3.2 desflurane | 6 | 432 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐2.97, ‐0.32] |
| 3.3 isoflurane | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 sevoflurane | 9 | 530 | Mean Difference (IV, Random, 95% CI) | ‐2.29 [‐3.24, ‐1.35] |
| 4 Time to orientation (minutes) | 7 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐3.06 [‐3.63, ‐2.50] |
| 4.1 propofol | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐8.19, 3.81] |
| 4.2 desflurane | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐4.23, ‐0.97] |
| 4.3 isoflurane | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐3.6 [‐5.92, ‐1.28] |
| 4.4 sevoflurane | 4 | 239 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐3.73, ‐2.48] |
| 5 PACU stay (minutes) | 12 | 1953 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐11.20, ‐2.31] |
| 5.1 propofol | 3 | 318 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐10.07, ‐1.62] |
| 5.2 desflurane | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐14.76 [‐29.61, 0.09] |
| 5.3 isoflurane | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐14.00 [‐34.12, 6.12] |
| 5.4 sevoflurane | 4 | 180 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐15.85, 0.72] |
| 5.5 propofol/volatile anaesthetics | 1 | 1123 | Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐9.72, 2.90] |
| 6 Time to home readiness (minutes) | 6 | 329 | Mean Difference (IV, Random, 95% CI) | ‐7.01 [‐30.11, 16.09] |
| 6.1 propofol | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.36 [‐33.01, 22.29] |
| 6.2 isoflurane | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 desflurane | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐30.93 [‐107.35, 45.48] |
| 6.4 sevoflurane | 4 | 220 | Mean Difference (IV, Random, 95% CI) | 8.93 [‐4.49, 22.35] |
Comparison 3.
Bispectral index versus clinical signs (requirement for anaesthetics)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Normalized propofol infusion rate (mg/kg/hr) | 10 | 672 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐1.91, ‐0.73] |
| 2 Volatile anaesthetic requirement, minimal alveolar concentration equivalents (MAC equivalents) | 14 | 985 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.28] |
| 2.1 desflurane | 5 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.03, ‐0.01] |
| 2.2 isoflurane | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.88, 0.14] |
| 2.3 sevoflurane | 9 | 573 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.87, ‐0.18] |
Comparison 4.
Bispectral index versus clinical signs (requirement for narcotics)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total dose of fentanyl (microgramme) | 7 | 333 | Mean Difference (IV, Random, 95% CI) | 13.80 [‐19.80, 47.40] |
| 2 average normalized remifentanil infusion rates ( microgramme/kg/min) | 3 | 276 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 3 Total dose of sufentanil ( microgramme) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐33.80 [‐51.03, ‐16.57] |
What's new
Last assessed as up‐to‐date: 27 January 2013.
| Date | Event | Description |
|---|---|---|
| 30 June 2018 | Amended | Typo in 'what's new corrected' (the following line: 'the result of our updated review published in 2014 seems contradictory to the result in a recent review published in 2016 by Messina et al' was repeated. |
| 11 September 2017 | Amended | We made the following corrections to the published review:
|
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 10 June 2014 | New search has been performed |
|
| 10 June 2014 | New citation required and conclusions have changed |
|
| 3 May 2009 | New search has been performed |
|
Differences between protocol and review
In the published protocol, the standard practice group was clinical signs‐guided anaesthesia. In this review, we categorized the standard practice into two subgroups: clinical signs‐guided anaesthesia (CS‐guided group) and end tidal anaesthetic gas‐guided anaesthesia (ETAG‐guided group).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Country: USA N = 99 ASA: I/II Gender: female Age: 31.5±8.7, 35.4±8.9 Exclusion: not mentioned Operation: gynaecologic laparoscopy Duration of anaesthesia: 67±36; 6937 min | |
| Interventions |
|
|
| Outcomes | Successful fast track rate (using modified Aldrete Score, main outcome) mean concentration of sevoflurane (%, sevoflurane requirement) mean dose of sufentanil mean dose of rocuronium mean duration of phase II recovery room stay (time to discharge) pain in phase II recovery area (Cn, %) nausea/vomiting in phase II recovery area (Cn, %) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | "...99 patients...were enrolled and randomised, using a closed envelope technique with random numbers,..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "...2 patients required inpatient hospitalisation postoperatively for surgical complications and were withdrawn from the final analysis." Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All expected outcomes have been reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In the BIS‐monitored group, sevoflurane was titrated to maintain the BIS value in the 50‐60 range...." This indicates no blinding of the anaesthesia provider |
| Blinding of outcome assessors? | Unclear risk | The study has not mentioned outcome assessor blinding |
| Methods | RCT | |
| Participants | Country: USA N = 125 ASA: I/II/III 13/16/5, 14/19/4, 26/24/4 Gender: M/F 14/20, 23/14, 23/33 Age: 57±19, 58±18, 54±15 years Exclusion: a history of any disabling central nervous or cerebrovascular disease, hypersensitivity to opioids or substance abuse, treatment with opioids or any psychoactive medication, or a body weight 70% or more than 130% of ideal body weight Operation: elective abdominal, gynaecologic, urologic, or orthopedic surgery expected to last at least 1 hour Duration of anaesthesia: 182.8±85.3, 190.8±84.9, 170.8±90.6 min |
|
| Interventions |
|
|
| Outcomes | Sevoflurane consumption (gm/kg/hr) (primary outcome) Recovery times (min) ‐ time to spontaneous eye opening ‐ time to tracheal extubation Sufentanil consumption (µg/kg/hr) Intraoperative recall by using a standardized interview (Cn, %) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...140 adult patients were randomly allocated to one of three groups, the standard practice group, the BIS‐guided group, or the spectral entropy‐guided group, using a randomization list performed with computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No mention about allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Six patients were excluded from the standard practice group (1 was not extubated at the end of surgery because of hypothermia, 3 required intraoperative propofol administration, and there were missing data in 2 cases), six patients were excluded from the BIS‐guided group (3 were not extubated at the end of surgery because of hypothermia, 2 required intraoperative propofol administration, and monitor data were lost in 1 case) and three from the spectral entropy‐guided group (all were not extubated at the end of surgery due to hypothermia, 2 required intraoperative propofol administration) (ns)." The study has not clearly stated how to deal with these excluded patients |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In both EEG‐groups, anaesthesiologists were instructed to adjust the sevoflurane concentration to keep BIS, SE, and RE values, in the respective group, in the range of 40‐60. ." It was unlikely to blind the anaesthesia providers |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | Quasi‐randomization | |
| Participants | Country: Spain N = 40 ASA: I/II Gender: ? Age: 40 (average) Exclusion: using psychotropic medication Operation: vascular (venous) or orthopaedic outpatient surgery | |
| Interventions |
|
|
| Outcomes | Propofol consumption Immediate and total recovery times Presence of intraoperative alertness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study used sequential randomization (quasi‐randomization). The rational for this 'sequence' was to avoid any contamination or influence of the 'BIS guided anaesthesia' on the 'standard anaesthesia' administered subsequently |
| Allocation concealment (selection bias) | High risk | The allocation concealment was not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One in the control group was excluded from the analysis. Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Insufficient information |
| Blinding of patients? | Low risk | The patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "Anesthesia administered guided by BIS monitor." (Ivan Sola, translator) |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | RCT | |
| Participants | Country: Sweden N = 60 (20,20,20) ASA: I/II Gender: not stated Age: 45±12, 45±12, 44±11 yr (mean±SD) Exclusion: not stated Operation: elective arthroscopy (ambulatory surgery) Duration of anaesthesia: 15±5, 15±5.5, 17±4.8 (min) | |
| Interventions |
|
|
| Outcomes | Sevoflurane consumption (g/min) Emergence times: ‐time to removal of laryngeal mask (min) ‐time to state of birth and name (min) ‐time to ready for discharge (min) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information regarding the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | No detailed information regarding allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information regarding withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes have been reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "...sevoflurane was titrated to maintain a target BIS of 60 during surgery." This indicates no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Unclear risk | No detailed information regarding blinding of outcomes assessors |
| Methods | RCT, multicentre | |
| Participants | Country: USA N = 1961 ASA: I/II/III/IV 21/ 265/ 454/ 222, 15/ 252/ 503/ 202 Gender: Male, Cn(%): 516 (53.4%), 5323(53.7%) Age: 59.5±14.8, 59.2±14.6 yr Inclusion: patients with at least one major criterion (preoperative long‐term use of anticonvulsant agents, opiates, benzodiazepines, or cocaine; a cardiac ejection fraction less than 40%; a history of anaesthesia awareness; a history of difficult intubation or anticipated difficult intubation, ASA physical status class 4 or class 5; aortic stenosis; end‐stage lung disease; marginal exercise tolerance not resulting from musculoskeletal dysfunction; pulmonary hypertension; planned open‐heart surgery; and daily alcohol consumption) or two minor criteria (preoperative use of beta‐blockers, chronic obstructive pulmonary disease, moderate exercise tolerance not resulting from musculoskeletal dysfunction, smoking two or more packs of cigarettes per day, and obesity, defined as a body‐mass index (the weight in kilograms divided by the square of the of more than 30) Exclusion: the surgical procedure or positioning of the patient prevented BIS monitoring or if the surgery required a wake‐up test Duration of anaesthesia: NA | |
| Interventions |
|
|
| Outcomes | Definite intraoperative awareness (Cn, %) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ".... in which 2000 patients underwent prerandomization electronically in blocks of 100, with 50 patients assigned to a BIS‐guided protocol and 50 to an ETAG‐guided protocol." This indicates adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | The design was a single‐centre, prospective study, in which 2000 patients underwent prerandomization electronically in blocks of 100, with 50 patients assigned to a BIS‐guided protocol and 50 to an ETAG‐guided protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 7 of the study shows 33 in the BIS group and 20 in the ETAG group were excluded. Intention‐to‐treat analysis was planned |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Blinding of anaesthesiologists? | High risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Blinding of outcome assessors? | Low risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Methods | RCT, multicentre | |
| Participants | Country: USA N = 5413 ASA: I/II/III/IV 23/ 468/ 1416/ 954, 19/ 407/ 1407/ 1019 Gender: Male, Cn(%): 1621 (56.7%), 1679(58.8%) Age: 60±14.12, 61±14.4 yr Inclusion: patients with at least one major criterion (preoperative long‐term use of anticonvulsant agents, opiates, benzodiazepines, or cocaine; a cardiac ejection fraction less than 40%; a history of anaesthesia awareness; a history of difficult intubation or anticipated difficult intubation, ASA physical status class 4 or class 5; aortic stenosis; end‐stage lung disease; marginal exercise tolerance not resulting from musculoskeletal dysfunction; pulmonary hypertension; planned open‐heart surgery; and daily alcohol consumption) or two minor criteria (preoperative use of beta‐blockers, chronic obstructive pulmonary disease, moderate exercise tolerance not resulting from musculoskeletal dysfunction, smoking two or more packs of cigarettes per day, and obesity, defined as a body‐mass index (the weight in kilograms divided by the square of the of more than 30) Exclusion: the surgical procedure or positioning of the patient prevented BIS monitoring or if the surgery required a wake‐up test Duration of anaesthesia: NA | |
| Interventions |
|
|
| Outcomes | Definite intraoperative awareness (Cn, %) using Michigan Awareness Classification Instrument | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...6100 prerandomization designations were generated electronically in blocks of 100, divided equally between the groups." |
| Allocation concealment (selection bias) | Low risk | " Labels indicating BIS group to EATC group were sealed in opaque, number envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 in the BIS group and 50 in the EATC group were lost to follow up. A modified intention‐to‐treat analysis were performed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Blinding of anaesthesiologists? | High risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Blinding of outcome assessors? | Low risk | "The anaesthesia practitioners were aware of the assignments of the patients, but the patients, the postoperative interviewers, the expert reviewers, and the statistician were not." |
| Methods | RCT | |
| Participants | Country: Turkey N = 60 ASA: I/II Gender: male/female, 17/13,18/12 Age: 42.1±3.3, 39±4.5 yrs Exclusion‐ renal, hepatic or neurological dysfunction, use of benzodiazepines, anticonvulsants, alcohol, opioids or other psychotropic drugs Operation: open abdominal surgery Duration of anaesthesia: 85±10.5, 90.4±8.7 min | |
| Interventions |
|
|
| Outcomes | Mean sevoflurane exposure (aged adjusted minimal alveolar concentration, main outcome) Amount of sevoflurane used (ml, main outcome) Immediate recovery times (time to open eyes on verbal command, time to motor respond to verbal command) Aldrete score at 10 min | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information regarding withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "..the anaesthesiologist had access to the monitor and adjusted the concentration of sevoflurane to achieve a target BIS in the range of 40‐60." This indicates no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Unclear risk | The author did not mention about blinding of the outcome assessors |
| Methods | RCT | |
| Participants | Turkey N = 50 ASA: I/II Gender: male/female 13/11, 11/12 Age: 45±11, 50±10 yrs Exclusion: any medication interaction with the central nervous system (antidepressant drugs, anti seizure drugs) or cardiopulmonary system (antihypertensive drugs, beta blockers), or a need for postoperative ventilation or other psychotropic drugs) Operation: Supratentorial craniotomy Duration of anaesthesia: 239±30, 222±32 min | |
| Interventions |
|
|
| Outcomes | Average end tidal concentrations (mean±SD) of sevoflurane Recovery times (min): ‐from end of surgery to first spontaneous breathing ‐from end of surgery to eye opening ‐from end of surgery to extubation PACU stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated sequence of number was used |
| Allocation concealment (selection bias) | Low risk | A sealed envelope technique was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Three patients were excluded from the study due to disconnection of BIS probe (2) or artefact contamination (1)." The study has not been mentioned how to deal with the missing outcome data in the analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "...sevoflurane was adjusted in an effort to achieve a target BIS of 40‐60..." This indicates no blinding of the anaesthesia provider |
| Blinding of outcome assessors? | Unclear risk | The study has not mentioned clearly about the blinding of outcomes assessors |
| Methods | RCT, multicentre | |
| Participants | Country: Germany N = 200 ASA: I/II/III 32/38/1, 23/34/1, 22/45/4 Gender: male/female Age: 46.3±13.0, 47.8±14.1, 48.6±14.5 years Exclusion‐ a history of any disabling central nervous or cerebrovascular diseases, hypersensitivity to opioids or substance abuse, or a treatment with opioids or any psychoactive medication Operation: Minor surgery expected to last at least 1 hour Duration of anaesthesia: 122.2±62.2, 117.1±48.5, 120.4±55.4 min | |
| Interventions |
|
|
| Outcomes | Desflurane consumption (end tidal concentrations) Recovery times: ‐Time to open eyes (min, primary outcome) ‐Time to be extubated (min) ‐Time to stating name ‐Time to arrive in PACU (min) ‐Time to discharge from ICU | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrolment the patients were randomized by drawing lots from a closed box |
| Allocation concealment (selection bias) | Unclear risk | No mention about method of allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detailed information |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | Patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | ".. desflurane was sequentially adjusted according to the predetermined target values of BIS or AAI, or clinical parameters." The blinding of anaesthesia care providers is unlikely |
| Blinding of outcome assessors? | Low risk | "Recovery times were recorded by a blinded investigator." This indicates blinding of the outcome assessors |
| Methods | RCT | |
| Participants | Country: Malaysia N = 20 ASA: I/II/III Gender: male/female 7:3, 8:2 Age: 52±12, 51±16 yrs Exclusion: previous cardiac surgery, preoperative neurologic disease, ejection fraction of less than 30%, known allergy to one of the drugs used, and severe renal and hepatic impairment Operation: cardiac surgery requiring cardiopulmonary bypass Duration of anaesthesia during cardiopulmonary bypass: 138 (120,181), 128 (120,175) min | |
| Interventions |
|
|
| Outcomes | ‐Propofol requirement during cardiopulmonary bypass ‐Haemodynamic stability during cardiopulmonary bypass | |
| Notes | ‐Both arms were conducted during cardiopulmonary bypass | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated by computer generated random numbers in closed envelopes." |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated by computer‐generated random numbers in closed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially introduce 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In group B, BIS‐controlled adjustment of the propofol infusion was used to achieve a BIS value of 40 to 50." This indicates no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | RCT | |
| Participants | Country: Germany N = 90 (20,20,20) ASA: I/II/III 10/16/1, 4/15/6, 10/10/7 Gender: male/female 9/18, 10/15, 12/15 Age: 50.6±15.7, 58±14.2, 53.6±18.4yr (mean±SD) Exclusion: history of any disabling central nervous or cerebrovascular diseases, hypersensitivity to opioids or substance abuse, or a treatment with opioids or any psychoactive medication Operation: minor surgery expected to last at least one hour (orthopaedic patients receiving regional anaesthesia for intra‐ and postoperative pain control for surgery to the upper or lower extremity in combination with general anaesthesia) Duration of anaesthesia: 100±30.7, 123.7±44.6, 119.5±50.6 (min) |
|
| Interventions | 1. Propofol guided by BIS (A‐2000 BIS® monitor (version XP, software version 4.0), Target BIS=50 2.Propofol guided by Entropy (an Entropy Module®), Target entropy=50 3. Propofol guided by clinical parameters (blood pressure, heart rate, sweating, tear production, movement) |
|
| Outcomes | ‐drug consumption ‐recovery times ‐intraoperative recall awareness |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized by drawing lots from a closed box |
| Allocation concealment (selection bias) | Unclear risk | No information regarding concealed randomization |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Due to insufficient regional anaesthesia or EEG data loss, five patients in the entropy group and three patients in each of the BIS and standard practice groups had to be excluded from further investigation |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially introduce 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "Propofol was sequentially adjusted according to the predetermined target values of BIS or Entropy (SE) or clinical parameters." |
| Blinding of outcome assessors? | Unclear risk | No information |
| Methods | RCT, multicentre | |
| Participants | Country: USA N = 268 ASA: I/II/III 45/65/5, 45/72/8 Gender: Male/Female 37/78, 45/84 Age: 41 (39‐43), 40 (37‐43) yr Exclusion: known neurologic disorders, uncontrolled hypertension,baseline systolic BP <106 HR<55, other serious medical conditions Operation: General surgical procedures >1 hour Duration of anaesthesia: 108 (95% CI 99 to 119); 125 (95% CI 114 to 135) min | |
| Interventions |
|
|
| Outcomes | ‐Normalized propofol infusion rate (µg/kg/hr) ‐Mean propofol used (mg) ‐Normalized alfentanil infusion rate (µg/kg/min) ‐Time to open eyes (min) ‐Time to respond to command (min) ‐Time to be extubated ‐Time to be eligible to discharge/readiness to home ‐Number of unwanted somatic and haemodynamic responses ‐Intraoperative global assessment score % of patients arrived fully oriented to the postanaesthesia care unit (PACU) Overall global nursing impression score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The sequence of treatments was determined in blocks of 10 using a random number generator." |
| Allocation concealment (selection bias) | Low risk | "Assignment to the study condition was determined using sequential coded envelopes." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twenty‐eight patients were excluded from efficacy analysis due to protocol violations for various reasons." As a result, there were 125 CS and 115 BIS group patients. There is uncertainty how much these missing outcome data could affect the observed effect size |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "The anaesthesiologists viewed the monitor in the BIS treatment group." This indicate no blinding of the anaesthesia providers |
| Blinding of outcome assessors? | Low risk | "Patients were assessed continuously by a recovery room nurse who blinded to the intraoperative treatment group assignment." This indicates blinding of the assessor for the main outcome |
| Methods | RCT | |
| Participants | Country: Spain N = 40 ASA: I/II Gender: female Age: 18‐65 years Exclusion: extreme obesity, cardiovascular and metabolic illnesses, hepatic or renal diseases and history of abuse of alcohol or drugs Operation: gynaecologic procedures including myomectomy, hysterectomy, oophorectomy and infra‐umbilical laparotomy Duration of anaesthesia: 73 (64‐82), 64 (56‐74) | |
| Interventions |
|
|
| Outcomes | ‐Total dose of fentanyl during maintenance (main outcome) ‐Propofol used during maintenance (mg) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No mention about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in the analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes have been reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | Patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | According to Ivan Sola (translator) "The propofol perfusion was controlled on depending of the BIS values to maintain patients' values between 40 and 60". This indicates no blinding of the anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | According to Ivan Sola (translator) ".. Nurse on the PACU assessed blinded the patients' self reported pain level' |
| Methods | RCT | |
| Participants | Participants country: Saudi Arabia N = 30 ASA: I/II 8/7, 10/5 Morbidity obese: body‐mass index of greater than 35 Gender: male/female 9/6, 11/4 Age: 39± 4.50, 41.21± 5.07 years Exclusion: renal, hepatic or neurological dysfunction or use of benzodiazepines, anticonvulsants, alcohol, opioids or other psychotropic drugs Operation: gastric banding procedures Duration of anaesthesia: 136.6±113.7, 138.9±13.8 minutes |
|
| Interventions | 1) Sevoflurane administration guided by BIS (BIS A‐2000 software 2.21, Aspect Medical Systems, Newton, and Mass), BIS value of 40‐60 during maintenance (BIS group), Cn = 15 2) Sevoflurane administration guided by signs of inadequate anaesthesia (increased blood pressure of greater than 20%, increased heart rate of greater than 90 beats per minutes and other somatic responses) (CS group), Cn = 15 |
|
| Outcomes | Sevoflurane used during maintenance (ml/hr) Recovery times (min) ‐time to awakening (opening eyes on verbal command) ‐time to extubation ‐time to Aldrete score of 9 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficiet information regarding withdrawal/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcome reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "Group BIS: the anaesthesiologist had access the monitor.." This indicates no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Low risk | "Blinded study personnel recorded the time ...." This is blinding of outcomes assessors |
| Methods | RCT | |
| Participants | Country: Egypt N = 60 ASA: I/II/III Gender: male/female 18/11, 20/8 Age: 51.6±7.4 , 52.1±5.2 years Exclusion: a history of any disabling central nervous or cerebrovascular disease, hypersensitivity to opioids, substance abuse, treatment with opioids or any psychoactive medication and a body mass index >40 Operation: elective moderate abdominal surgical procedures Anaesthesia: propofol induction, atracurium, sevoflurane, nitrous in oxygen, fentanyl Duration of anaesthesia: 111.7±14.6, 108.7±10.5 minutes |
|
| Interventions | 1) Sevoflurane administration guided by BIS (Aspect Medical Systems, model A‐2000,Newton, MA, USA), Maintenance BIS :50‐60, end of surgery BIS 55‐70 2) Sevoflurane or fentanyl administration guided by clinical signs (mean arterial blood pressure > 25 above baseline >25% above baseline or heart rate > 90 beats per minutes) or labetalol based on anaesthesiologist's discretion |
|
| Outcomes | ‐recovery times ‐anaesthetic drug consumption ‐amount of sevoflurane (ml) ‐end tidal sevoflurane concentration ‐incidence of awareness |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly selected and assigned into two groups of 30 patients each |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three patients were discarded, two from BIS‐b group and one from BIS‐g group |
| Selective reporting (reporting bias) | Low risk | All expected outcome reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "exhibited hypertension or tachycardia the mode of treatment was dependent on the BIS index" |
| Blinding of outcome assessors? | Low risk | "...Aldrete score assessment is expressed in Table 6 and performed at 15 min interval by a research assistant blinded to group assignment..." |
| Methods | RCT | |
| Participants | Country: Germany N = 120 ASA: I/II/III 12/25/3, 12/24/4,13/24/3 Gender: male/female 20/20,20/20,20/20 Age: 43.8±4.2, 46.1±14.5, 44.8±15.9 years Exclusion: disabling, central nervous or cerebrovascular diseases, hypersensitivity to opioid or substance abuse, or treatment with opioids or any psychoactive medication Operation: minor orthopaedic surgery lasted at least 1 hr Duration of anaesthesia: 121.2±40.9; 108.2±44.2 min | |
| Interventions |
|
|
| Outcomes | ‐Normalized propofol infusion rate (µg/kg/hr) ‐Normalized remifentanil infusion rate (µg/kg/min) ‐Time to open eyes (min, primary outcome) ‐Time to be extubated (min) ‐Time to arrive in PACU (min) ‐Awareness (Cn, %) ‐Number of patients receiving intervention to treat intraoperative hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ".. patients were randomized by drawing lots from a closed box." |
| Allocation concealment (selection bias) | Low risk | ".. patients were randomized by drawing lots from a closed box." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study has not mentioned about the withdrawal/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "..Propofol TCI during maintenance of anaesthesia was continuously adjusted according to a target value of.......50 for BIS.". This indicates no blinding of anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | "Recovery times and propofol consumption were recorded by a blinded investigator." |
| Methods | RCT | |
| Participants | Country: Germany N = 120 ASA: I/II/III 7/30/3, 13/23/4, 11/27/2 Gender: male/female 20/20, 20/20, 20/20 Age: 46.5±14.1, 44.7±15.6, 43.6±16.0 years. Exclusion: history of any disabling central nervous or cerebrovascular disease, hypersensitivity to opioids or substance abuse, or a treatment with opioids or any psychoactive medication Operation: minor orthopaedic surgery expected to last at least 1 hour Duration of anaesthesia: 113±57, 122±50, 125±51 min | |
| Interventions |
|
|
| Outcomes | Outcomes ‐ desflurane consumption (mg/min) Recovery times: ‐Time to open eyes (min, primary outcome) ‐Time to be extubated (min) ‐Time to arrive in PACU (min) | |
| Notes | . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ".. patients were randomized by drawing lots from a closed box." |
| Allocation concealment (selection bias) | Low risk | ".. patients were randomized by drawing lots from a closed box." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study has not mentioned about the withdrawal/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "..desflurane during maintenance of anaesthesia was continuously adjusted according to a target value of.......50 for BIS ". This indicates no blinding of anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | "Recovery times were recorded by a blinded investigator." |
| Methods | RCT, multicentre | |
| Participants | Country: Australia N = 2463 ASA: I/II/III/IV 111/179/542/388/5, 127/227/520/354/10 Gender: Male/Female 752/473, 784/454 Age: 58.1 (16.5), 57.5 (16.9) years Inclusion : at least one of risk factors for awareness, i.e. caesarean section, high risk cardiac surgery, acute trauma with hypovolaemia, rigid bronchoscopy, significant impairment of cardiovascular status, severe end stage lung disease, past history of awareness, unplanned awake intubation, known or suspected heavy alcohol intake, chronic benzodiazepine or opioid use , or current protease inhibitor therapy Operation: minor/intermediate/major 104/216/905, 104/231/903 Duration of anaesthesia: 3.2 (1.5‐4.4), 3.1 ( 1.3‐4.5) hours |
|
| Interventions |
|
|
| Outcomes | ‐Confirmed awareness (Cn, %) ‐Recovery times* |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random group allocation |
| Allocation concealment (selection bias) | Low risk | A central allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | " ..40 patients were withdrawn because of cancellation of surgery ( BIS group13, routine group13), withdrawal of consent ( six, twoO, surgery done without general anaesthesia ( four, none), or the patients was under‐age (none, two)" and " All patients.. were included in the intention‐to‐treat population for all analyses." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | Unlikely to blind the anaesthesia providers to the allocated groups |
| Blinding of outcome assessors? | Low risk | "Follow‐up was undertaken by a blind observer." |
| Methods | RCT | |
| Participants | Country: Switzerland N = 160 Sex: female Exclusion:central nervous system disease (i.e. history of cerebrovascular disease or epilepsy) or taking EEG‐affecting drug ans ASA > 3 Operation: gynaecological surgery lasted >15 min Desflurane subgroups ‐ASA: I/II/III 22/15/3, 15/22/3 ‐Gender: female ‐Age: 45.2±17.5, 47.1±17.8 years ‐Duration of anaesthesia: 100.5±58.2; 90.9±53.6 min Propofol subgroup (N = 80) ‐ASA: III/III 21/18/1, 22/16/2 ‐Gender: female ‐Age: 46.3±15.4, 48.7±15.7 years ‐Duration of anaesthesia: 100.5±58.2; 90.9±53.6 min | |
| Interventions |
|
|
| Outcomes | Mean propofol infusion rate (mg/kg/hr) Desflurane usage (age‐adjusted MAC‐hours) ‐Recovery profiles ‐Aldrete score ‐Global clinical impression score ‐Extubation time ‐Duration of PACU stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the patients were randomized into four groups by drawing lots from sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | "...the patients were randomized into four groups by drawing lots from sealed envelopes." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information regarding withdrawal or dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcome reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | "The patients, the PACU nurses and the nurses on the ward were blinded to the allocation of the patients" |
| Blinding of anaesthesiologists? | High risk | "In the BIS group, the hypnotic drug concentration... was adjusted to keep the BIS between 45 and 55 during surgery" This indicates no blinding of the anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | "The patients, the PACU nurses and the nurses on the ward were blinded to the allocation of the patients." |
| Methods | RCT | |
| Participants | Country: USA N = 18836 9460, 9376 Inclusion criteria ‐Age more than 18 yr, ‐Anaesthesia using inhalational or intravenous technique ‐Surgery any surgical case that did not involve the forehead ‐Availability for follow‐up interviews Exclusion criteria ‐intracranial procedures ‐adhesive allergy ‐psychosis, or history of traumatic brain injury |
|
| Interventions | 1. BIS group: electronic alerts in the event of median BIS values more than 60 2. ETAG group: electronic alerts for median age‐adjusted MAC level of less than 0.5 |
|
| Outcomes | The incidence of definite intraoperative awareness (using modified intention‐to‐treat analysis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a random‐number, computer‐generated block scheme based on even or odd operating room number" |
| Allocation concealment (selection bias) | Low risk | "...practitioners were not made aware of the randomization scheme or dates for randomization change during the study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of the 9,460 patients randomized to the BIS intervention and successfully interviewed, 3,384 or 36% did not have BIS data recorded because of technical issues described in Materials and Methods.This population was used for secondary analysis only as a post hoc control group because it had neither intervention;" |
| Selective reporting (reporting bias) | Low risk | Selective reporting (reporting bias) |
| Blinding of patients? | Low risk | "Patients, postoperative interviewers, and all case reviewers were blinded to group assignment" |
| Blinding of anaesthesiologists? | High risk | "Practitioners receiving pages regarding BIS or MAC values were not blinded to group assignment." |
| Blinding of outcome assessors? | Low risk | "Patients, postoperative interviewers, and all case reviewers were blinded to group assignment" |
| Methods | RCT | |
| Participants | Country: Japan N = 46 ASA: I/II Gender: Female/male 15/5, 15/4 Age: 33±9, 37±14 years. Exclusion ‐ not mentioned Operation: laparotomy (6;4), laparoscopy (7;3), surgery on extremities (5;5), arthroscopy (1;2), surface (1;1), head and neck (0;3) Duration of anaesthesia: 190±45, 191±57 | |
| Interventions |
|
|
| Outcomes | ‐Propofol infusion rate ‐Total amount of propofol used ‐Recovery profiles ‐Patients with undesirable responses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Insufficient information |
| Blinding of patients? | Low risk | Patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | It was unlikely to blind the anaesthesia provider from the assigned groups |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | RCT | |
| Participants | Country: Japan N = 60 (enrolled) ASA: I/II Gender: Male/Female 21/25 Age: 18‐70 yr Operation: not specified Duration of anaesthesia: 284±85; 256±172 | |
| Interventions |
|
|
| Outcomes | ‐Anaesthetic ‐ sevoflurane consumption (ml‐1) ‐Fentanyl required ‐Vecuronium required ‐Time to open eyes on verbal command ‐Time to extubate ‐Time to discharge from the recovery room | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 subjects were excluded: 11 subjects excluded because surgery was either longer than 6 hrs or shorter than 2 hours, and 3 patients excluded because of mechanical dysfunction of BIS.How these missing data affect on the result is unclear |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | Patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | It was unlikely to blind the anaesthesiologists from the assignment groups because they had to adjust the anaesthetic according to the target BIS values in the BIS group |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | RCT | |
| Participants | Country: India N = 40 (enrolled) (20 isoflurane, 20 propofol) Operation: elective off‐pump coronary artery bypass grafting (CABG) Exclusion: Patients with poor ventricular function of lesser than 40%; left ventricular aneurysms; and renal/hepatic dysfunction, requiring extra corporeal circulation; preoperative or intraoperative intraaortic balloon pump, presence of unstable angina, carotid stenosis, cerebrovascular accident; excessive alcohol intake and drug abuse Isoflurane Gender: male/female 9/1, 8/2 Age: 50±6, 50±4 years Weight: 71±5, 71±6 kg Propofol Gender: male/female 8/2, 10/0 Age: 52±7, 47±5 years Weight: 71±6, 71±4 kg |
|
| Interventions |
|
|
| Outcomes | ‐Amount of isoflurane (ml) or propofol (ml) ‐Time to extubation ‐Intraoperative recall awareness |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information regarding the sequence generation process |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly divided into four groups by a sealed envelope technique.." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information regarding withdrawal/dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to ' learning contamination bias" during administration of the anaesthetics |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | It is unlikely to blind the anaesthesia providers who delivery the anaesthetics |
| Blinding of outcome assessors? | Unclear risk | insufficient information. The study has not stated clearly whether the intensive care unit research fellow, who was an interviewer, blinded to the group assignment or not |
| Methods | RCT, multicentre | |
| Participants | Country: Australia N = 2463 ASA: I/II/III/IV 111/179/542/388/5, 127/227/520/354/10 Gender: Male/Female 752/473, 784/454 Age: 58.1 (16.5), 57.5 (16.9) Inclusion: at least one of risk factors for awareness, i.e. caesarean section, high risk cardiac surgery, acute trauma with hypovolaemia, rigid bronchoscopy, significant impairment of cardiovascular status, severe end‐stage lung disease, past history of awareness, unplanned awake intubation, known or suspected heavy alcohol intake, chronic benzodiazepine or opioid use , or current protease inhibitor therapy Operation: minor/intermediate/major 104/216/905, 104/231/903 Duration of anaesthesia: 3.2 (1.5‐4.4), 3.1 (1.3‐4.5) hrs | |
| Interventions |
|
|
| Outcomes | Primary outcome: incidence of confirmed awareness Secondary outcomes: ‐Possible awareness ‐Hypnotic drug administration ‐Marked hypotension (Cn, %) ‐Patient satisfaction ‐Recovery times | |
| Notes | Relaxant general anaesthesia Induction: midazolam (62%, 62%) + propofol (63%, 63%) or thiopentone (15%, 15%) Intubation: non‐depolarizing muscle relaxants (93%, 95%) Maintenance: propofol infusion (43%, 42%) nitrous oxide (35%, 37%) ‐opioids ‐volatiles ‐hypnotic drugs (7%,6%) and combined general and regional anaesthesia (18%, 15%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random group allocation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | " ..40 patients were withdrawn because of cancellation of surgery ( BIS group13, routine group13), withdrawal of consent ( six, twoO, surgery done without general anaesthesia ( four, none), or the patients was under‐age (none, two)" and " All patients.. were included in the intention‐to‐treat population for all .analyses." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | |
| Blinding of anaesthesiologists? | High risk | Unlikely to blind the anaesthesia providers to the allocated groups |
| Blinding of outcome assessors? | Low risk | "Follow‐up was undertaken by a blind observer." |
| Methods | RCT | |
| Participants | Country: Finland N = 62 ASA :I/II Gender: Female Age: 32±6 Operation: gynaecologic laparoscopy (tubal ligation excluded) Duration of anaesthesia: 59±39; 55±50 min | |
| Interventions |
|
|
| Outcomes | ‐Nausea and vomiting (N/V) in PACU (main outcome) (Cn, %) ‐Anaesthetic exposure (sevoflurane exposure; sevoflurane end tidal concentration, %.h) ‐Number of patients required alfentanil ‐Time to open eyes spontaneously (min) ‐Time to follow command (squeezing hand) (min) ‐Time to be extubated (min) ‐Time to be eligible to discharge/home readiness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information regarding adequate sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | No detailed information regarding allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Table 6 |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In the BIS group, sevoflurane was titrated to maintain a BIS value of 50‐60...." This indicates no blinding of the anaesthesia provider |
| Blinding of outcome assessors? | Unclear risk | The authors did not mention about the outcome assessors blinding |
| Methods | RCT | |
| Participants | Country: Italy N = 90 ASA: no information Gender: no information Age: mean 42‐48 years Exclusion: history of neurologic disease, medication affecting central nervous system (CNS) and alcohol and drug abuse Operation: general abdominal surgery >30 min Duration of anaesthesia 74‐102 min | |
| Interventions | 1) Sevoflurane and remifentanil administration guided by BIS (Version 3.22) of 40‐60 during maintenance, Cn = 45 2) Anaesthetic administration without BIS information, Cn = 45 | |
| Outcomes | ‐Direct cost of anaesthesia management (total drug cost/min versus cost of BIS electrodes and monitor) (main outcome) ‐% sevoflurane required (median and range) ‐Remifentanil required, µg/kg/hr) (median and range) ‐Recovery times 1. Time to breath spontaneously (min) 2. Time to be extubated (min) 3. Time to eye opening (min) 4. Time to orientation (min) ‐Cost 1. total drug cost/min 2. Cost of BIS electrodes (EUR/patient) ‐Sevoflurane requirement (median, range) | |
| Notes | Withdrawals ‐ not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information regarding withdrawal or dropouts of the participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could probably lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In group 1 the anaesthetics were given according to the BIS value rate between 40 to 60." This indicates no blinding of the anaesthesia providers |
| Blinding of outcome assessors? | Low risk | "All recovery parameters were assessed by the same research coordinator not involved in treatment of the patient." This indicates blinding of the outcome assessors |
| Methods | RCT | |
| Participants | Country: India N = 30, ASA: III or greater Gender: no information Age: 38.25±14.02, 32.08±13.84 Inclusion: undergoing either coronary artery grafting (CAGB) or valve replacement under cardiopulmonary bypass (CP) Exclusion: neurological disorders, poor ventricular function, New York Heart Association grade IV, diabetes mellitus, and impaired renal or hepatic function Operation: coronary artery grafting (CAGB) or valve replacement under cardiopulmonary bypass (CP) Duration of surgery: 295±45, 285±40 minutes | |
| Interventions |
|
|
| Outcomes | Number of haemodynamic disturbances: hypertension, tachycardia, hypotension, bradycardia Recovery endpoint ‐ time from switching off anaesthetic vaporizer to opening eyes or response to verbal commands Time to tracheal extubation Awareness* | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "......were randomized into ......using computer‐generated numbers." This indicate adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From table 1 of the study, it is likely that all patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In the study group, the anaesthesiologist was allowed to see and use the monitor.." This indicates no blinding of anaesthesia care providers |
| Blinding of outcome assessors? | Unclear risk | Insufficient information regarding blinding of outcome assessors |
| Methods | RCT | |
| Participants | Country: USA N = 90 ASA: NA Gender: Male/Female 21/9, 20/10, 24/6 Age: 47±17,46±15,42±14 Exclusion: history of CNS disease, chronic use of psychoactive medication, and clinical significant cardiovascular, renal, hepatic or endocrinology disorders Operation: laparoscopic general surgery procedures (cholecystectomy, gastric bypass/banding, hernia repair) Duration of anaesthesia: 125±52; 127±38 min | |
| Interventions |
|
|
| Outcomes | ‐End tidal concentrations of desflurane (%) (main outcome) ‐Total fentanyl used ‐Total rocuronium used (mg) ‐ Requirement of labetalol (Cn,%) ‐Time to open eyes ‐Time to obey simple verbal commands ‐Time to orientation ‐Time to be extubated ‐Time to achieve White fast‐track score ≥12 ‐Time to achieve Aldrete discharge score of 10 ‐Length of stay in the postanaesthesia care unit (PACU) ‐Patients with recall of intraoperative awareness (Cn, %) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to ' learning information bias' |
| Blinding of patients? | Low risk | Patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | ".... , the real time AAI and BIS values were only made available during the procedure to those anaesthesiologists caring for patients in the AEP or BIS‐guided groups,..." This indicates no blinding of anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | " Emergence times were determined .......by a blinded observer." This indicates blinding of outcome assessors |
| Methods | RCT | |
| Participants | Country: Saudi Arabia N =40; 20, 20 ASA: not specified Age: mean (SD) 55.3 (10.4), 60.8 (10.2) yr sex: not specified Operation: cardiac revascularization procedure by the off‐pump technique Anaesthesia: intravenous (midazolam, and sufentanil), relaxant (rocuronium), and supplemented sevoflurane Duration: of anaesthesia, mean (SD) min 239.8 (20); 230 (24.5) |
|
| Interventions | 1. BIS‐guided anaesthetics for maintaining BIS values of 40‐60 2. No BIS monitoring |
|
| Outcomes | 1. Anaesthetic requirements 2. The need for circulatory support (dosage of phenylephrine) 3. Extubation time 4. Intraoperative recall awareness (Cn, %) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was performed using patient's medical record number, being odds related to group I and evens related to group II" |
| Allocation concealment (selection bias) | High risk | "Randomization was performed using patient's medical record number, being odds related to group I and evens related to group II" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | |
| Blinding of patients? | Low risk | All patients were anaesthetised |
| Blinding of anaesthesiologists? | High risk | It was not possible to blind the anaesthesiologists |
| Blinding of outcome assessors? | Low risk | "Postoperatively, patients were visited on the second postoperative day by one of the medical staff about the grouping |
| Methods | RCT | |
| Participants | Country: USA N = 60 (30 sevoflurane, 30 desflurane) Sex: female Exclusion: neurologic disease, CVS or metabolic diseases, impaired renal or hepatic function, BW > 100% above the ideal or history of alcohol or drug abuse Operation: laparoscopic tubal ligation Desflurane subgroup (treatment, control) ‐ASA: I/II; 10/5, 11/4 ‐Age: 28±4, 27±6 ‐Duration of anaesthesia:76±20;78±22 min Sevoflurane subgroup (treatment, control) ‐ASA: I/II; 11/4, 10/5 ‐Age: 26±6, 26±7 ‐Duration of anaesthesia: 74±21, 75±21 min | |
| Interventions |
|
|
| Outcomes | ‐End tidal concentration (%) ‐Exposure to desflurane (MAC. hrs) ‐Consumption of desflurane (ml) ‐Consumption of mivacurium (mg) ‐Consumption of fentanyl (µg) ‐Time to verbal response (min) ‐Time to extubation (min) ‐Time to orientation (min) ‐Time to PACU stay (min) ‐Time to oral intake (min) ‐Time to home readiness (min) ‐Patients with recall awareness ‐Patients with increased airway pressure ‐Patient with coughing and bucking | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " Patients were randomly assigned to one of four study groups according to a computer‐generated random numbers table." |
| Allocation concealment (selection bias) | Unclear risk | The study has not mentioned about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In the BIS‐titrated groups, the volatile anaesthetics were titrated to maintain a BIS index of 60." This indicates no blinding of anaesthesia care providers |
| Blinding of outcome assessors? | Unclear risk | The study has not mentioned about outcome assessor blinding |
| Methods | RCT | |
| Participants | Country: Belgium N = 20 Sex: female Exclusion: neurologic disorders, psychoactive medication including alcohol, body weight above 130% or below 70% of the ideal body weight Operation: gynaecologic laparotomy ‐ASA: I/II ‐Age: 42±8, 46±4 ‐Duration of anaesthesia: 6798±2085; 6896±2018 second | |
| Interventions |
|
|
| Outcomes | ‐Time to spontaneous breathing ‐Time to eye opening ‐Time to extubation ‐Time to orientation ‐Propofol use (mg/kg/hr) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patients were excluded from analysis." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes have been reported |
| Other bias | Unclear risk | Insufficient information about the blinding of the anaesthesiologists |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | It was unlikely to blind the anaesthesia providers to the assigned groups |
| Blinding of outcome assessors? | Unclear risk | Insufficient information |
| Methods | RCT | |
| Participants | Country: Italy N = 160 (80 propofol, 80 sevoflurane) ASA? Gender? Age 18‐70 yr Operation: abdominal surgery | |
| Interventions |
|
|
| Outcomes | ‐Propofol or sevoflurane consumption ‐Fentanyl consumption ‐Time to spontaneous breathing ‐Time to extubation ‐Time to follow simple commands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Insufficient information |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | It was unlikely to blind the anaesthesia providers to the assigned groups |
| Blinding of outcome assessors? | Low risk | Accordng to Valeria Salerno translation and comments |
| Methods | RCT | |
| Participants | Country: USA N = 60 ASA I/II/II 9/10/1 9/11/0, 7/12/1 Gender: female Exclusion: known neurologic or psychiatric disorders, currently using anticonvulsants or other centrally actives medications, clinically significant cardiovascular, respiratory, hepatic, renal or metabolic diseases, long term drug or alcohol abuse; or a body weight greater than 50% above the ideal body weight Operation: gynaecologic laparoscopic surgery Duration of anaesthesia: 58±22; 66±16 min | |
| Interventions |
|
|
| Outcomes | ‐End tidal concentration ‐Desflurane consumption (ml) ‐Time to open eyes (main outcome) ‐Time to follow simple commands (e.g. squeeze the investigator's hand) ‐Time to orientation ‐White fast‐track score on arrival in PACU ‐Modified Aldrete score on arrival in PACU ‐Time to fit for discharge (sitting up, standing, ambulating and tolerating oral fluids) ‐Actual discharge time ‐Quality recovery score before discharge ‐Intraoperative recall | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The unblinded anaesthesiologists could potentially lead to ' learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "....the BIS or AEP monitor, respectively, was positioned to enable the anaesthesiologist to use the displayed index value to titrate the concentration of desflurane..." This indictees no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Low risk | ".......the times at which patients were able to open their eyes,....by a third investigator who was unaware of the monitoring group.. " This indicates blinding of outcome assessors |
| Methods | RCT | |
| Participants | Country: Canada N = 68 ASA: I/II/II 2/24/3, 3/27/1 Gender: Male/Female 10/10, 21/10 Age: 71±15, 70±6 yr Exclusion: significant cardiopulmonary diseases or other end‐organ disease, depression or psychiatric disorders, dementia previous CVA, head trauma, inadequate command of English and drugs and all alcohol abuse, preoperative baseline of Mini Mental state examination (MMSE) <24 Operation: elective orthopaedic surgery or hip replacement Duration of anaesthesia: 120±17, 121±17 min | |
| Interventions |
|
|
| Outcomes | ‐Time to orientation to person, place and time (main outcome) ‐End tidal concentration (%) ‐Consumption of isoflurane (ml) ‐Time to awakening (eye opening to verbal commands) ‐Time to extubation ‐Time to readiness for transfer to PACU ‐Time to readiness for discharge from PACU (Aldrete score >9) ‐Symptoms of postoperative cognitive dysfunction ‐Recall awareness of intraoperative events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization with concealed varying block sizes was performed with computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | The process of allocation concealment is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "....., eight patients (three from the SP group, and five from the BIS group) were excluded from the analysis for protocol violations." The missing outcome data seem to balance across intervention group. The plausible effect size (difference in mean) among missing outcome probably not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to "learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "In the BIS group, the anaesthesiologist adjusted the administration of isoflurane and fentanyl to maintain a BIS index of 50‐60." This indicates no blinding of the anaesthesia care providers |
| Blinding of outcome assessors? | Low risk | "The Aldrete score was assessed at 15 min intervals by a research nurse blinded to the group assignment ......." |
| Methods | RCT, multicentre | |
| Participants | Country: China N = 5309 ASA: I/II/III‐V 1386/1128/138, 1323/834/65 Gender: Male/Female 1237/1656, 971/1309 Age: 46.95 (14.86), 46.06 (14.59) Inclusion : patients scheduled for total intravenous anaesthesia (TIVA) Operation: neurosurgery 25, 19 craniofacial and cervical surgery 774, 780 heart surgery 24, 22 gynaecologic and obstetric surgery 401, 296 chest and abdominal surgery 1217, 840 urinary surgery 213, 198 spine and limb surgery 149, 185 others 38, 37 Duration of anaesthesia: not specified |
|
| Interventions | 1. Propofol guided by BIS (A‐2000, Aspect Medical System, USA) to maintain BIS values between 40‐60 2.Control group: no BIS‐guided TIVA |
|
| Outcomes | Confirmed awareness (Cn, %) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Despite using computer‐generated random numbers, we are uncertain regarding this type of bias because the information of group allocation was not available in 54 cases. Furthermore, there was a significant difference of ASA of greater than or equal to 3 between the two groups, 138 (5.2%) versus 65 (2.9%) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Fifty‐four cases were withdrawn because the information of group allocation was unavailable and another 21 patients were excluded due to age younger than 18 years old ( 11/10) and a further six patients were excluded because of failure to be interviewed (2/2)." |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Blinding of patients? | Unclear risk | "Interviewers and patients were blinded to the group allocation" |
| Blinding of anaesthesiologists? | High risk | "...the doses administered were left to the discretion of the anaesthetist taking charge of the TIVA..." |
| Blinding of outcome assessors? | Low risk | "Interviewers and patients were blinded to the group allocation" |
| Methods | RCT | |
| Participants | Country: Canada N = 50 ASA: I/II/II 2/19/4, 2/20/3 Gender: Male/Female 21/4, 22/3 Age: 73 ± 8, 76 ± 7 yr Exclusion: a history of unstable cardiovascular, pulmonary, hepatic, renal, neurologic, psychiatric or metabolic diseases Operation: short elective transurethral surgical procedures Duration of anaesthesia: 31 ± 22, 28 ± 16 min | |
| Interventions |
In both groups, the sevoflurane concentration was increased in response to signs of an inadequate “depth of anaesthesia” (e.g. movement in response to surgical stimulation) |
|
| Outcomes | Anaesthetic requirement: ‐sevoflurane minimal alveolar concentration (MAC) during maintenance (MAC/hr) Recovery times (min): ‐time to spontaneous eye opening ‐time to remove laryngeal mask airway (LMA) device ‐time to responding to simple verbal commands ‐time to correctly state name, age, and personal identification number ‐time to achieve fast‐track ability (main outcome) ‐time from awakening from anaesthesia to achieve post anaesthesia care unit (PACU) discharge eligibility The occurrence of any side effects The occurrence of need for therapeutic interventions The occurrence of intraoperative recall awareness Patients' satisfaction scores |
|
| Notes | Muscle relaxants were not used (spontaneous breathing) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information about withdrawals/dropouts of the participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
| Blinding of patients? | Low risk | All patients were anaesthetized |
| Blinding of anaesthesiologists? | High risk | "The anaesthesiologists was instructed to maintain the BIS value in the 50 to 60 range by varying the inspired concentration of sevoflurane." This indicates no blinding of the anaesthesia care provider |
| Blinding of outcome assessors? | Low risk | "Early recovery endpoints were recorded....by a blinded observer,....." This indicates blinding of the assessor |
RCT = randomized controlled trial BIS = bispectral index TCI = target controlled infusion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akcali 2008 | The study was not a RCT (historical control) |
| Arnold 2007 | The study was not a RCT |
| Ballard 2012 | The study was a RCT comparing active anaesthetic monitoring (bispectral index and cerebral oxygen saturation) with a control condition on the incidence of postoperative cognitive decline in older adults undergoing surgery. The outcome (postoperative cognitive decline ) was not in the scope of this review) |
| Berti 2000 | The study was a RCT comparing three groups (i.e. subarachnoid anaesthesia versus general anaesthesia with bispectral index versus general anaesthesia without bispectral index) but did not provide data on the relevant outcomes |
| Burrow 2001 | The study was not a RCT |
| Caba 2003 | Outcome was not relevant (the need for postoperative analgesia) |
| Guignard 2001 | The study was not a RCT (historical control) |
| Johansen 2000 | The study was not a RCT. It was an open, observational trial with retrospective analysis |
| Lehmann 2003 | This study was a RCT but compared 2 levels of BIS‐guided anaesthesia. Its publication has been withdrawn by a journal |
| Leslie 2005b | The study was a substudy of the B‐Aware randomized controlled trial (Myles 2004) and focused on dreaming during anaesthesia (PMID: 15710008) |
| Lindholm 2008 | The study investigated how increasing experience from BIS in clinical practice affect the hypnotic level, drug consumption, as well as subjective opinions on this monitoring. Therefore, it did not fulfil the objective of our review |
| Mayer 2007 | Its publication has been withdrawn by a journal |
| Pavlin 2001 | The study was a RCT but the randomization was different from the other studies. It allocated healthcare providers to use or not use BIS for guiding doses of anaesthetics. Therefore, the study design did not fulfil the inclusion criteria of the study selection in terms of randomization process |
| Pavlin 2005 | The study was a RCT but the randomization was different from the other studies. It allocated healthcare providers to use or not use BIS for guiding doses of anaesthetics. Therefore, the study design did not fulfil the inclusion criteria of the study selection in terms of randomization process |
| Schulz 2007 | The study was not a RCT |
| Sebel 1997 | It was a multicentre RCT to evaluate the real‐time utility of BIS in predicting movement response incision. Hence, it did not fulfil the objective of this review |
| Song 1998 | This study was a RCT but did not use BIS guiding doses of anaesthetics but used it as a tool to measure the effect of two anaesthetics |
| Vedtofte 2007 | The study was a RCT but the randomization was different from the other studies. It allocated healthcare providers to use or not use BIS for guiding doses of anaesthetics. Therefore, the study design did not fulfil the inclusion criteria of the study selection in terms of randomization process |
| Yli‐Hankala 1999 | This study was an RCT but was excluded as it randomly allocated participant into two groups based on the anaesthetic use (propofol versus sevoflurane). The comparison group was an historical control group |
RCT = randomized controlled trial BIS = bispectral index
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | N=40 Operation: cholecystectomy |
| Interventions | 1. BIS‐guided sevoflurane (BIS 40‐60) 2. standard practice sevoflurane 3. BIS‐guided desflurane (BIS 40‐60) 4. standard practice desflurane |
| Outcomes | ‐ drug consumption ‐ recovery times |
| Notes | ‐ A non‐English article waiting for translation |
| Methods | RCT |
| Participants | N=480 operation: gynaecological laparoscopy surgery |
| Interventions | 1.Bispectral index‐guide anaesthesia (BIGA) 2.Non‐bispectral index‐guide anaesthesia |
| Outcomes | ‐Postoperative nausea and vomiting (PONV) ‐Desflurane consumption ‐Cost |
| Notes |
| Methods | RCT |
| Participants | N=2949 Patients at high risk of intraoperative awareness |
| Interventions | 1. BIS‐guided general anaesthesia 2. End‐tidal anaesthetic concentration‐guided general anaesthesia |
| Outcomes | ‐recovery time ‐postoperative complications such as postoperative nausea and vomiting and severe postoperative pain |
| Notes | a substudy of the B‐Unaware and BAG‐RECALL trials |
| Methods | RCT |
| Participants | N = 50 morbidly obese adult patients undergoing elective laparoscopic cholecystectomy |
| Interventions | 1. BIS guided isoflurane anaesthesia (BIS value 40‐60 during maintenance and 60‐70 at 15 minutes before the end of surgery 2. Standard clinical practice |
| Outcomes | 1. isoflurane utilization 2. early recovery profiles |
| Notes |
| Methods | RCT |
| Participants | N=80 severe burn undergoing elective escharectomy |
| Interventions | 1.BIS guided intravenous target‐controlled infusion (TCI) of remifentanil and propofol. 2. Control: non‐BIS guided anaesthesia |
| Outcomes | ‐target concentrations of remifentanil and propofol ‐time from drug withdrawal to eye opening |
| Notes |
| Methods | RCT |
| Participants | N=60 Halothane based anaesthesia |
| Interventions | 1.BIS‐guided anaesthesia 2.ETAG‐guided anaesthesia |
| Outcomes | ‐time to tracheal extubation |
| Notes |
| Methods | RCT |
| Participants | Open heart surgery |
| Interventions | 1.BIS‐guided anaesthesia 2. No BIS |
| Outcomes | Consumption of anaesthetics Intraoperative recall awareness |
| Notes | Full text: not available |
| Methods | RCT |
| Participants | N=82 Adults (20‐60 years) Supratentorial neurosurgery |
| Interventions | 1. BIS guided anaesthesia (BIS values 40‐60) 2. Standard control group; Clinical signs (haemodynamics) guided anaesthesia |
| Outcomes | ‐Drugs including anaesthetics used during anaesthesia ‐Haemodynamic changes ‐Recovery time |
| Notes |
| Methods | RCT |
| Participants | N=96 Open renal surgery |
| Interventions | 1. BIS group 2.Control group (clinical assessment) |
| Outcomes | ‐Depth of anaesthesia ‐Recovery time |
| Notes |
| Methods | RCT |
| Participants | N=42 Desflurane anaesthesia balanced with remifentanil |
| Interventions | 1. BIS guided anaesthesia (BIS at 50 during maintenance) 2. Fixed gas concentration method (1 MAC desflurane) |
| Outcomes | ‐Dose and adjustment frequency of anaesthetics ‐Recovery time ‐Cost |
| Notes |
| Methods | RCT |
| Participants | N= not mentioned Coronary artery bypass surgery without cardiopulmonary bypass (CPB) |
| Interventions | 1. BIS visible 2. BIS not visible ((BIS is hidden and monitoring of anaesthetic depth is based on clinical signs associated with the monitoring of expiratory fraction of halogenated anaesthetic agent) |
| Outcomes | ‐Anaesthetic depth ‐Associate costs |
| Notes | a research protocol |
| Methods | RCT |
| Participants | N=333 Elective abdominal surgery |
| Interventions | 1. BIS monitoring 2. Routine monitoring |
| Outcomes | ‐ Awareness ‐Changes in haemodynamic parameter |
| Notes |
| Methods | A prospective, controlled, sequential two‐arm clinical study |
| Participants | N=60 elective on‐pump cardiac surgery |
| Interventions | 1. BIS guided sevoflurane anaesthesia (BIS target between 40 and 60) 2. a sustained inspired concentration of sevoflurane 1.8% |
| Outcomes | ‐sevoflurane plasma concentration (SPC) ‐intraoperative vasopressor doses during on‐pump ‐intraoperative awareness, postoperative blood lactate concentration, duration of mechanical ventilation, intensive care unit length of stay and kidney injury |
| Notes |
| Methods | RCT |
| Participants | Country: China N=300 Anaesthesia: total intravenous anaesthesia |
| Interventions | 1. BIS‐guided anaesthesia 2. No BIS |
| Outcomes | Intraoperative recall awareness |
| Notes | Full text: not available |
| Methods | RCT |
| Participants | N=90 Endobronchial ultrasound (EBUS) under sedation1 |
| Interventions | 1.BIS guided sedation 2 .modified observer's assessment of alertness/sedation scale clinical evaluation |
| Outcomes | ‐Drug doses ‐Waking time ‐Adverse events, and tolerance of the procedure |
| Notes |
| Methods | RCT |
| Participants | N = 294 Cardiac surgery |
| Interventions | 1.BIS guided anaesthesia 2.MAC guided anaesthesia |
| Outcomes | ‐Time to extubation ‐Length of stay in the ICU and total postoperative hospital length of stay. |
| Notes |
| Methods | RCT |
| Participants | N=723 Patients undergoing cardiac surgery. |
| Interventions | 1. BIS guided anaesthesia (target BIS 40‐60) 2. End‐tidal anaesthetic concentration (ETAC) guided anaesthesia |
| Outcomes | ‐Time to tracheal extubation. |
| Notes | A single institution who were enrolled in the larger, multicentre BIS or Anaesthesia Gas to Reduce Explicit Recall (BAG‐RECALL) clinical trial. |
Acronyms and abbreviations used in these tables
BAG‐RECALL: a multi‐centre, randomized, controlled clinical trial comparing bispectral index (BIS) guided versus end‐tidal anaesthetic concentration (ETAC) guided anaesthesia on explicit recall in patients at high risk of intraoperative recall awareness; BIGA: Bispectral index‐guide anaesthesia; BIS: Bispectral index; EBUS: Endobronchial ultrasound; :ETAC: End‐tidal anaesthetic concentration; ICU: Intensive care unit..
Contributions of authors
Conceiving the review: Yodying Punjasawadwong (YP) Co‐ordinating the review: YP Undertaking manual searches: YP, Aram Phongchiewboon (AP) and Nutchanart Bunchuungmonkol (NB) Screening search results: YP, NB Organizing retrieval of papers: YP Screening retrieved papers against inclusion criteria: YP and NB Appraising quality of papers: YP, AP and NB Abstracting data from papers: YP and NB Writing to authors of papers for additional information: YP Providing additional data about papers: YP and NB Obtaining and screening data on unpublished studies: YP Data management for the review: YP Entering data into Review Manager (RevMan 5.2): YP and NB RevMan statistical data: YP Other statistical analysis not using RevMan: YP Double entry of data: data entered by person one YP; data entered by person two NB Interpretation of data: YP Statistical analysis: YP Writing the review: YP Securing funding for the review: YP Performing previous work that was the foundation of the present review: YP Guarantor for the review (one author): YP Responsible for reading and checking review before submission: YP
Sources of support
Internal sources
The faculty of medicine, Chiang Mai University, Thailand.
External sources
No sources of support supplied
Declarations of interest
Yodying Punjasawadwong: none known
Aram Phongchiewboon: none known
Nutchanart Bunchungmongkol: none known
Edited (no change to conclusions)
References
References to studies included in this review
- Ahmad S, Yilmaz M, Marcus RJ, Glisson S, Kinsella A. Impact of bispectral index monitoring on fast tracking of gynecologic patients undergoing laparoscopic surgery. Anesthesiology 2003;98(4):849‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Aime I, Verroust N, Masson‐Lefoll C, Taylor G, Laloe P, Liu N, et al. Does monitoring bispectral index or spectral entropy reduce sevoflurane use?. Anesthesia and Analgesia 2006;103(6):1469‐77. [PUBMED: 17959961] [DOI] [PubMed] [Google Scholar]
- Anez C, Papaceit J, Sala JM, Fuentes A, Rull M. The effect of encephalogram bispectral index monitoring during total intravenous anesthesia with propofol in outpatient surgery. Revista Espanola de Anestesiologia Reanimacion 2001;48(6):264‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Assare H, Anderson RE, Jakobsson J. Sevoflurane requirements during ambulatory surgery: a clinical study of bispectral index and auditory evoked potential guided anaesthesia. Ambulatory Surgery 2002;9:207‐11. [PII:S0966‐6532(02)00004‐5] [Google Scholar]
- Avidan MS, Zhang L, Burnside BA, Fink KJ, Searleman AC, Selvidge JA, et al. Anesthesia awareness and the bispectral index. New England Journal of Medicine 2008;358(11):1097‐108. [PUBMED: 18337600] [DOI] [PubMed] [Google Scholar]
- Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, et al. Prevention of intraoperative awareness in a high‐risk surgical population. New England Journal of Medicine 2011;365(7):591‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Basar H, Ozcan S, Buyukkocak U, Akpinar S, Apan A. Effect of bispectral index monitoring on sevoflurane consumption. European Journal of Anaesthesiology 2003;20(5):396‐400. [MEDLINE: ] [PubMed] [Google Scholar]
- Boztug N, Bigat Z, Akyuz M, Demir S, Ertok E. Does using the bispectral index (BIS) during craniotomy affect the quality of recovery?. Journal of Neurosurgical Anesthesiology 2006;18(1):1‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bruhn J, Kreuer S, Bischoff P, Kessler P, Schmidt GN, Grzesiak A, et al. Bispectral index and A‐line AAI index as guidance for desflurane‐remifentanil anaesthesia compared with a standard practice group: a multicentre study. British Journal of Anaesthesia 2005;94(1):63‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chiu CL, Ong G, Majid AA. Impact of bispectral index monitoring on propofol administration in patients undergoing cardiopulmonary bypass. Anaesthesia and Intensive Care 2007;35(3):342‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ellerkmann RK, Soehle M, Kreuer S. The Entropy Module® and Bispectral Index® as guidance for propofol‐remifentanil anaesthesia in combination with regional anaesthesia compared with a standard clinical practice group. Anaesthesia and Intensive Care 2010;38(1):159‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, et al. Bispectral Index monitoring allows faster emergence and improved recovery from propofol, alfentanil and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology 1997;87(4):808‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hachero A, Alamo F, Caba F, Echevarria M, Merino S, Gomez P, et al. Influence of bispectral index monitoring on fentanyl requirements during total intravenous anesthesia for major gynecological surgery. Revista Espanola de Anestesiologia Reanimacion 2001;48(8):364‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ibraheim O, Alshaer A, Mazen K, El‐Dawlaty A, Turkistani A, Alkathery K, et al. Effect of bispectral index (BIS) monitoring on postoperative recovery and sevoflurane consumption among morbidly obese patients undergoing laparoscopic gastric banding. Middle East Journal of Anesthesiology 2008;19(4):819‐30. [MEDLINE: ] [PubMed] [Google Scholar]
- Kamal NM, Omar SH, Radwan KG, Youssef A. Bispectral index monitoring tailors clinical anesthetic delivery and reduces anesthetic drug consumption. Journal of Medical Sciences 2009;9:10‐6. [DOI: 10.3923/jms.2009.10.16] [DOI] [Google Scholar]
- Kreuer S, Biedler A, Larsen R, Altmann S, Wilhelm W. Narcotrend monitoring allows faster emergence and a reduction of drug consumption in propofol‐remifentanil anesthesia. Anesthesiology 2003;99(1):34‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kreuer S, Bruhn J, Stracke C, Aniset L, Silomon M, Larsen R, et al. Narcotrend or bispectral index monitoring during desflurane‐remifentanil anesthesia: a comparison with a standard practice protocol. Anesthesia and Analgesia 2005;101(2):427‐34. [PUBMED: 16037157] [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles PS, Forbes A, Chan MT, Short TG, Swallow SK. Recovery from bispectral index‐guided anaesthesia in a large randomized controlled trial of patients at high risk of awareness. Anaesthesia and Intensive Care 2005;33(4):443‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Luginbuhl M, Wuthrich S, Petersen‐Felix S, Zbinden AM, Schnider TW. Different benefit of bispectral index (BIS) in desflurane and propofol anesthesia. Acta Anaesthesiologica Scandinavica 2003;47:165‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner CR, Ramachandran SK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology 2012;117(4):717‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Yamada H, Takada K, Sagata Y, Yamaguchi M, Tomiyama Y, et al. Bispectral index monitoring is useful to reduce total amount of propofol and to obtain immediate recovery after propofol anesthesia. Masui 2002;51(4):394‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Morimoto Y, Oka S, Mii M, Shinjo Y, Yamashita A, Gohara T, et al. Efficacy of bispectral index monitoring in improving anesthetic management, economics, and use of the operating theater. Masui 2002;51(8):862‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Muralidhar K, Banakal S, Murthy K, Garg R, Rani GR, Dinesh R. Bispectral index‐guided anaesthesia for off‐pump coronary artery bypass grafting. Annals of Cardiac Anaesthesia 2008;11(2):105‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B‐Aware randomised controlled trial. Lancet 2004;363(9423):1757‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nelskyla KA, Yli‐Hankala AM, Puro PH, Korttila KT. Sevoflurane titration using bispectral index decreases postoperative vomiting in phase II recovery after ambulatory surgery. Anesthesia and Analgesia 2001;93(5):1165‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paventi S, Santevecchi A, Metta E, Annetta MG, Perilli V, Sollazzi L. Bispectral index monitoring in sevoflurane and remifentanil anesthesia. Analysis of drugs management and immediate recovery. Minerva Anestesiologica 2001;67(6):435‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Puri GD, Murthy SS. Bispectral index monitoring in patients undergoing cardiac surgery under cardiopulmonary bypass. European Journal of Anaesthesiology 2003;20(6):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Recart A, Gasanova I, White PF, Thomas T, Ogunnaike B, Hamza M, et al. The effect of cerebral monitoring on recovery after general anesthesia: a comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesthesia and Analgesia 2003;97(6):1667‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Samarkandi AH, Abdel‐Meguid ME, Abdullah KM, Riad W. Bispectral index monitoring and titration of anaesthetics during off‐pump coronary artery bypass surgery. Egyptian Journal of Anaesthesia 2004;20(4):357‐61. [Google Scholar]
- Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology 1997;87:842‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Struys MM, Smet T, Versichelen LF, Velde S, Broecke R, Mortier EP. Comparison of closed‐loop controlled administration of propofol using Bispectral Index as the controlled variable versus "standard practice" controlled administration. Anesthesiology 2001;1:6‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tufano R, Palomba R, Lambiase G, Giurleo LG. The utility of bispectral index monitoring in general anesthesia. Minerva Anestesiologica 2000;66(6):389‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- White PF, Ma H, Tang J, Wender RH, Sloninsky A, Kariger R. Does the use of electroencephalographic bispectral index or auditory evoked potential index monitoring facilitate recovery after desflurane anesthesia in the ambulatory setting?. Anesthesiology 2004;100(4):811‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wong J, Song D, Blanshard H, Grady D, Chung F. Titration of isoflurane using BIS index improves early recovery of elderly patients undergoing orthopedic surgeries. Canadian Journal of Anaesthesia 2002;49(1):13‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu L, Ma YQ, Sun YX, Li YH, Zhang L, et al. Bispectral index monitoring prevent awareness during total intravenous anesthesia: a prospective, randomized, double‐blinded, multi‐center controlled trial. Chinese Medical Journal 2011;124(22):3664‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Zohar E, Luban I, White PF, Ramati E, Shabat S, Fredman B. Bispectral index monitoring does not improve early recovery of geriatric outpatients undergoing brief surgical procedures. Canadian Journal of Anesthesia 2006;53(1):20‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Akçali DT, Ozköse Z, Yardim S. Do we need bispectral index monitoring during total intravenous anesthesia for lumbar discectomies? [Lomber Diskektomilerde Totalntravenöz Anestezide Bispektralndeks Monitörleme Gerekli midir?]. Turkish Neurosurgery 2008;18(2):125‐33. [MEDLINE: ] [PubMed] [Google Scholar]
- Arnold G, Kluger M, Voss L, Sleigh J. BIS and Entropy in the elderly. Anaesthesia 2007;62(9):907. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One 2012;7(6):e37410. doi: 10.1371/journal.pone.0037410. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti M, Danelli G, Albertin A, Casati A, Cucchi C, Torri G. Bispectral index: clinical effectiveness and role in reducing anesthetic drug consumption. Minerva Anestesiologica 2000;66(5):394‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Burrow B, McKenzie B, Case C. Do anaesthetized patients recover better after bispectral index monitoring?. Anaesthesia and Intensive Care 2001;29(3):239‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Caba F, Merino S, Martinez Navas A, Nunez Garcia A, Diaz Fernandez F, Marcos E, et al. Influence of BIS monitoring on the need of postoperative analgesia [Influencia de la monitorizacion on BIS en los requerimientos de analgesia postoperatoria]. Revista de la Sociedad Espanola del Dolor 2003;10(6):341‐8. [Google Scholar]
- Guignard B, Coste C, Menigaux C, Chauvin M. Reduced isoflurane consumption with bispectral index monitoring. Acta Anaesthesiologica Scandinavica 2001;45(3):308‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johansen JW, Sebel PS, Sigl JC. Clinical impact of hypnotic‐titration guidelines based on EEG bispectral index (BIS) monitoring during routine anesthetic care. Clinical Anesthesia 2000;12(6):433‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lehmann A, Karzau J, Boldt J, Thaler E, Lang J, Isgro F. Bespectral index‐guided anesthesia in patients undergoing aortocoronary bypass grafting. Anesthesia and Analgesia 2003;96(2):336‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles PS, Forbes A, Chan MT, Swallow SK, Short TG. Dreaming during anaesthesia in patients at high risk of awareness. Anaesthesia 2005;60:239‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindhom ML, Brudin L, Sandin RH. Bispectral index monitoring: appreciated but does not affect drug dosing and hypnotic levels. Acta Anaesthesiologica Scandinavica 2008;52:88‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mayer J, Boldt J, Schellhaaß A, Hiller B, Suttner SW. Bispectral index‐guided general anesthesia in combination with thoracic epidural analgesia reduces recovery time in fast‐track colon surgery. Anesthesia and Analgesia 2007;104(5):1145‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pavlin DJ, Hong JY, Freund PR, Koerschgen ME, Bower JO, Bowdle TA. The effect of bispectral index monitoring on end‐tidal gas concentration and recovery duration after outpatient anesthesia. Anesthesia and Analgesia 2001;93(3):613‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pavlin JD, Souter KJ, Hong JY, Freund PR, Bowdle TA, Bower JO. Effects of bispectral index monitoring on recovery from surgical anesthesia in 1,580 inpatients from an academic medical center. Anesthesiology 2005;102(3):566‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulz U, Keh D, Barner C, Kaisers U, Boemke W. Bispectral index monitoring does not improve anesthesia performance in patients with movement disorders undergoing deep brain stimulating electrode implantation. Neurosurgical Anesthesia 2007;104(6):1481‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sebel PS, Lang E, Rampil IJ, White PF, Cork R, Jopling M, et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesthesia and Analgesia 1997;84(4):891‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Song D, Vlymen J, White PF. Is the bispectral index useful in predicting fast‐track eligibility after ambulatory anesthesia with propofol and desflurane?. Anesthesia and Analgesia 1998;87(6):1245‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vedtofte JI, Rasmussen LS. The use of bispectral index monitoring in education ‐ a tool to improve nurse‐anaesthetists practice. Journal of Advanced Nursing 2007;59(6):577‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yli‐Hankala A, Vankkuri A, Annila P, Korttila K. EEG bispectral index monitoring in sevoflurane or propofol anesthesia: analysis of direct costs and immediate recovery. Acta Anaesthesiologica Scandinavica 1999;43:545‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Aksun M, Aydin O, Aran G, Atasoy N, Savaci S. The effects of bispectral index (BIS) monitorization on recovery from sevoflurane and desflurane anesthesia. Anestezi‐Derg Anestezi‐Dergisi 2007;15(1):14. [EMBASE: 2007166072] [Google Scholar]
- Croci M, Hudecova S, Fracassi S, Greco S. Control of the postoperative nausea and vomiting using a bispectral index‐guide anesthesia and its economic impact: 1AP1‐8. European Journal of Anaesthesiology 2014;31(supplement 52):5. [Google Scholar]
- Fritz BA, Rao P, Mashour GA, Abdallah AB, Burnside BA, Jacobsohn E, et al. Postoperative recovery with bispectral index versus anesthetic concentration‐guided protocols. Anesthesiology 2013;118(5):1113‐22. [DOI] [PubMed] [Google Scholar]
- Golmohammadi M, Abasgholizadeh M. Bispectral index monitoring in isoflurane anesthesia in laparoscopic cholecystectomy of morbid obese patients. Tehran University Medical Journal 2014;72(7):471‐9. [Google Scholar]
- Guo ZG, Jia XP, Wang XY, Li P, Su XJ, Hao JH. Bispectral index for monitoring anesthetic depth in patients with severe burns receiving target‐controlled infusion of remifentanil and propofol. Genetic Molecular Research 2015;14(3):7597‐604. [DOI] [PubMed] [Google Scholar]
- Jain N, Mathur PR, Khan S, Khare A, Mathur V, Sethi S. Effect of bispectral index versus end‐tidal anesthetic gas concentration‐guided protocol on time to tracheal extubation for halothane‐based general anesthesia. Anesthesia Essays and Researches 2016;10(3):591‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabukcu HK, Sahin N, Ozkaloglu K, Golbasi Y, Titiz TA. Bispectral index monitoring in open heart surgery. British Journal of Anaesthesia. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca İ, Akçıl FE, Dilmen ÖK, Köksal GM, Tunalı Y. The effect of BIS usage on anaesthetic agent consumption, haemodynamics and recovery time in supratentorial mass aurgery. Turkish Journal of Anaesthesiology and Reanimation 2014;42(3):117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshrang H, Nemati M, Mokhtari G, Pourreza F, Roshan ZA, Najmabadi AD, et al. Comparative efficacy of bispectral index monitoring and clinical assessment in the recovery of patients undergoing open renal surgery: A randomized, double‐blind study. Annual Anesthesiology Critical Care 2016;(in press). [Google Scholar]
- Kim B, Park S, Jung Y, Lee H, Jung C. Anesthesia maintenance with desflurane and target‐controlled remifentanil: Comparison of bispectral index‐guided and fixed‐gas concentration techniques. Anesthesia and Analgesia. 2016; Vol. 122, issue supplement 5. [Google Scholar]
- Martins A, Pereira A, Seabra H, Gomes D, Fonte Boa A, Lima F. Influence of processed EEG monitoring in the anesthetic management and its cost in off‐pump coronary surgery: a research protocol. Revista Portuguesa De Cirurgia Cardio‐Toracica E Vascular 2013;20(2):67‐71. [PMID: 24730013 ] [PubMed] [Google Scholar]
- Mozafari H, Fakhr AA, Salehi I, Moghimbigi A. The ability of bispectral‐guided management compared to routine monitoring for reflecting awareness rate in patients undergoing abdominal surgery. Iranian Red Crescent Medical Journal 2014;16(9):e13584. [PMCID: PMC4270681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzschke R, Wilgusch J, Kersten JF, Trepte CJ, Haas SA, Reuter DA, et al. Bispectral index guided titration of sevoflurane in on‐pump cardiac surgery reduces plasma sevoflurane concentration and vasopressor requirements: a prospective, controlled, sequential two‐arm clinical study. European Journal of Anaesthesiology 2014;31(9):482‐90. [DOI] [PubMed] [Google Scholar]
- Quesada N, Júdez D, Martínez Ubieto J, Pascual A, Chacón E, Pablo F, et al. Bispectral index monitoring reduces the dosage of propofol and adverse events in sedation for endobronchial ultrasound. Respiration 2016;92(3):166‐75. [DOI] [PubMed] [Google Scholar]
- Qu X‐X, Ma H‐C, Yuan Y, Feng C‐S. Effect of bispectral index on intraoperative awareness under total intravenous anesthesia. Journal of Jilin University Medicine Edition 2011;37:308‐11. [Google Scholar]
- Vance JL, Shanks AM, Woodrum DT. Intraoperative bispectral index monitoring and time to extubation after cardiac surgery: secondary analysis of a randomized controlled trial. BMC Anesthesiology 2014;18(14):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafranca A, Thomson IA, Grocott HP, Avidan MS, Kahn S, Jacobsohn E. The impact of bispectral index versus end‐tidal anesthetic concentration‐guided anesthesia on time to tracheal extubation in fast‐track cardiac surgery. Anesthesia and Analgesia 2013;116(3):541‐8. [DOI] [PubMed] [Google Scholar]
Additional references
- Badrinath S, Avramov MN, Papaioannou BS, Ivankovich AD. The impact of EEG‐bispectral index monitoring on drug usage and recovery during ambulatory anesthesia. Anesthesia and Analgesia 1999;88 Suppl:51. [Google Scholar]
- Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesthesia and Analgesia 2009;108(2):527‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gonsowski CT, Chortkoff BS, Eger EI 2nd, Bennett HL, Weiskopf RB. Subanesthetic concentrations of desflurane and isoflurane suppress explicit and implicit learning. Anesthesia and Analgesia 1995;80(3):568‐72. [PUBMED: PMID: 7864427 ] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. West Sussex: John Wiley & Sons Ltd, 2011. [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5(1):13. [PUBMED: PMID: 15840177 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JW. Provider survey of bispectral index utility. Anesthesia and Analgesia 1998;86 Suppl:212. [Google Scholar]
- Kissin I. A concept for assessing interactions of general anesthetics. Anesthesia and Analgesia 1997;85(1):204‐10. [PUBMED: PMID: 9212148 ] [DOI] [PubMed] [Google Scholar]
- Liu SS. Effects of bispectral index monitoring on ambulatory anesthesia: a meta‐analysis of randomized controlled trials and a cost analysis. Anesthesiology 2004;101(2):311‐5. [PUBMED: PMID: 15277912] [DOI] [PubMed] [Google Scholar]
- Mahla ME. Electroencephalogram in the OR. Seminars in Anesthesia 1997;16(1):3‐13. [Google Scholar]
- Messina AG, Wang M, Ward MJ, Wilker CC, Smith BB, Vezina DP, et al. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD007272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Daves SM, Tung A, Cook RI, Thisted R, Apfelbaum J. BIS monitoring to prevent awareness during general anesthesia. Anesthesiology 2001;94(3):520‐2. [PUBMED: PMID: 11374615 ] [DOI] [PubMed] [Google Scholar]
- Pavlin DJ, Rapp SE, Polissar NL, Malmgren JA, Koerschgen M, Keyes H. Factors affecting discharge time in adult outpatients. Anesthesia and Analgesia 1998;87(4):816‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rampil IJ. A Primer for EEG signal processing in anesthesia. Anesthesiology 1998;89(4):815‐7. [PUBMED: PMID: 9778016 ] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Roizen MF, Toledano A. Technology assessment and the "learning contamination" bias. Anesthesia and Analgesia 1994;79(3):410‐2. [PUBMED: PMID: 8067542 ] [DOI] [PubMed] [Google Scholar]
- Schneiderder G. Monitoring anesthetic depth. In: Mashour GA editor(s). Consciousness, Awareness, and Anesthesia. 1st Edition. New York: Cambridge University Press, 2010:122‐30. [Google Scholar]
- Sebel PS. Can we monitor depth of anesthesia. International Anesthesia Research Society Review Course Lectures. 2001:95‐7. [Google Scholar]
- Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, Domino KB. The incidence of awareness during anesthesia: a multicenter United States study. Anesthesia and Analgesia 2004;99(3):833‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Jones J, Frampton G, Bryant J, Baxter L, Cooper K. Clinical effectiveness and cost‐effectiveness of depth of anaesthesia monitoring (E‐Entropy, Bispectral Index and Narcotrend): a systematic review and economic evaluation. Health Technology Assessment 2013;17(34):1‐264. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003843.pub2] [DOI] [PubMed] [Google Scholar]


