Abstract
Background
Cardiovascular disease, which includes coronary artery disease, stroke and peripheral vascular disease, is a leading cause of death worldwide. Homocysteine is an amino acid with biological functions in methionine metabolism. A postulated risk factor for cardiovascular disease is an elevated circulating total homocysteine level. The impact of homocysteine‐lowering interventions, given to patients in the form of vitamins B6, B9 or B12 supplements, on cardiovascular events has been investigated. This is an update of a review previously published in 2009, 2013, and 2015.
Objectives
To determine whether homocysteine‐lowering interventions, provided to patients with and without pre‐existing cardiovascular disease are effective in preventing cardiovascular events, as well as reducing all‐cause mortality, and to evaluate their safety.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 5), MEDLINE (1946 to 1 June 2017), Embase (1980 to 2017 week 22) and LILACS (1986 to 1 June 2017). We also searched Web of Science (1970 to 1 June 2017). We handsearched the reference lists of included papers. We also contacted researchers in the field. There was no language restriction in the search.
Selection criteria
We included randomised controlled trials assessing the effects of homocysteine‐lowering interventions for preventing cardiovascular events with a follow‐up period of one year or longer. We considered myocardial infarction and stroke as the primary outcomes. We excluded studies in patients with end‐stage renal disease.
Data collection and analysis
We performed study selection, 'Risk of bias' assessment and data extraction in duplicate. We estimated risk ratios (RR) for dichotomous outcomes. We calculated the number needed to treat for an additional beneficial outcome (NNTB). We measured statistical heterogeneity using the I2 statistic. We used a random‐effects model. We conducted trial sequential analyses, Bayes factor, and fragility indices where appropriate.
Main results
In this third update, we identified three new randomised controlled trials, for a total of 15 randomised controlled trials involving 71,422 participants. Nine trials (60%) had low risk of bias, length of follow‐up ranged from one to 7.3 years. Compared with placebo, there were no differences in effects of homocysteine‐lowering interventions on myocardial infarction (homocysteine‐lowering = 7.1% versus placebo = 6.0%; RR 1.02, 95% confidence interval (CI) 0.95 to 1.10, I2 = 0%, 12 trials; N = 46,699; Bayes factor 1.04, high‐quality evidence), death from any cause (homocysteine‐lowering = 11.7% versus placebo = 12.3%, RR 1.01, 95% CI 0.96 to 1.06, I2 = 0%, 11 trials, N = 44,817; Bayes factor = 1.05, high‐quality evidence), or serious adverse events (homocysteine‐lowering = 8.3% versus comparator = 8.5%, RR 1.07, 95% CI 1.00 to 1.14, I2 = 0%, eight trials, N = 35,788; high‐quality evidence). Compared with placebo, homocysteine‐lowering interventions were associated with reduced stroke outcome (homocysteine‐lowering = 4.3% versus comparator = 5.1%, RR 0.90, 95% CI 0.82 to 0.99, I2 = 8%, 10 trials, N = 44,224; high‐quality evidence). Compared with low doses, there were uncertain effects of high doses of homocysteine‐lowering interventions on stroke (high = 10.8% versus low = 11.2%, RR 0.90, 95% CI 0.66 to 1.22, I2 = 72%, two trials, N = 3929; very low‐quality evidence).
We found no evidence of publication bias.
Authors' conclusions
In this third update of the Cochrane review, there were no differences in effects of homocysteine‐lowering interventions in the form of supplements of vitamins B6, B9 or B12 given alone or in combination comparing with placebo on myocardial infarction, death from any cause or adverse events. In terms of stroke, this review found a small difference in effect favouring to homocysteine‐lowering interventions in the form of supplements of vitamins B6, B9 or B12 given alone or in combination comparing with placebo.
There were uncertain effects of enalapril plus folic acid compared with enalapril on stroke; approximately 143 (95% CI 85 to 428) people would need to be treated for 5.4 years to prevent 1 stroke, this evidence emerged from one mega‐trial.
Trial sequential analyses showed that additional trials are unlikely to increase the certainty about the findings of this issue regarding homocysteine‐lowering interventions versus placebo. There is a need for additional trials comparing homocysteine‐lowering interventions combined with antihypertensive medication versus antihypertensive medication, and homocysteine‐lowering interventions at high doses versus homocysteine‐lowering interventions at low doses. Potential trials should be large and co‐operative.
Plain language summary
Homocysteine‐lowering interventions (B‐complex vitamin therapy) for preventing cardiovascular events
Review question We reviewed whether particular vitamins, which lower homocysteine, prevent cardiovascular events such as heart attack and stroke.
Background Cardiovascular disease, which includes heart attacks and strokes, is the number one cause of death worldwide. Many people with cardiovascular disease may not have symptoms, but be at high risk. Diabetes mellitus, high blood pressure, smoking and a high cholesterol, as well as a family history of cardiovascular disease are well known risk factors. Elevated total homocysteine levels have recently been identified as a risk factor for cardiovascular disease. Homocysteine is an amino acid, its levels in the blood are influenced by blood levels of B vitamins: cyanocobalamin (B12), folic acid (B9) and pyridoxine (B6). This report is an update from a previous review published in 2015.
Study characteristics The evidence is current to June 2017. We included 15 studies involving 71,422 participants living in countries with or without mandatory supplementation of foods with vitamins. These studies compared different regimens of B vitamins (cyanocobalamin (B12), folic acid (B9) and pyridoxine (B6)) with a control or any other comparison group. The studies were published between 2002 and 2015.
Key results We found no evidence that homocysteine‐lowering interventions, in the form of supplements of vitamins B6, B9 or B12 given alone or in combination, at any dosage compared with placebo, or standard care, prevented heart attack or reduced death rates in participants at risk of, or living with cardiovascular disease. Homocysteine‐lowering interventions combined with antihypertensive medication had uncertain effects on stroke, approximately 143 people would need to be treated for 5.4 years to prevent 1 stroke. Homocysteine‐lowering interventions compared with placebo or any other comparison did not affect serious adverse events (cancer).
Quality of evidence The quality of evidence from these studies was generally high.
Summary of findings
Background
Description of the condition
The burden of cardiovascular disease
Cardiovascular disease is the number one cause of death worldwide (Barquera 2015; Smith 2012). The term cardiovascular disease covers a wide array of disorders, including diseases of the cardiac muscle and of the vascular system supplying the heart, brain and other vital organs. The most common causes of cardiovascular disease‐related morbidity and mortality are ischaemic heart disease and stroke (Li 2016; Maredza 2015; Oliveira 2015; Prabhakaran 2016).
The burden of cardiovascular disease is significant and ischaemic heart disease is the single largest cause of death worldwide (Bansilal 2015; Kwan 2016). Global deaths from cardiovascular disease increased by 41% between 1990 and 2013 (Roth 2015a). It has been pointed out that cardiovascular diseases cause more than 4 million deaths/year in the 53 countries of the World Health Organization European Region and over 1.9 million deaths in the European Union (Bansilal 2015). It has been estimated that there will be 7.8 million premature cardiovascular deaths in 2025 (Roth 2015b).
Cardiovascular diseases account for about one‐half of non communicable diseases deaths (Benziger 2016). The majority of cardiovascular disease deaths occur in low‐ and middle‐income countries (Barquera 2015; Benziger 2016; Oliveira 2015; Prabhakaran 2016). The major risk factors for cardiovascular diseases include tobacco use, high blood pressure, high blood glucose, lipid abnormalities, high levels of body mass index and physical inactivity (Barquera 2015; Lackland 2015; Li 2016; Roth 2015b; Singh 2015; Tzoulaki 2016; Yeates 2015).
Homocysteine as a risk factor for cardiovascular disease
In 1962, it was hypothesised that increased levels of total homocysteine may cause vascular disease: the homocysteine theory of arteriosclerosis (McCully 2015a). The pathways through which total homocysteine levels may cause damage to endothelial cells and lead to atherosclerosis have been widely described (Ganguly 2015; McCully 2015bPushpakumar 2014). It has been pointed out that homocysteine reduces the bioavailability of the nitric oxide, a potent vasodilator (Lai 2015b). Another mechanism would be through an integration of the roles of homocysteine and folic acid in cardiovascular pathobiology, known as methoxistasis (Joseph 2013). The molecular and cellular effect of homocysteine metabolism imbalance yields oxidative stress which is cytotoxic (Skovierova 2016). The cellular status of homocysteine is not correlated with the homocysteine levels in plasma, which may explain the considerable differences that there are between epidemiological, intervention and basic research reports (Hannibal 2016).
Homocysteine is a non‐proteinogenic amino acid derived in methionine metabolism (Skovierova 2016). Several observational studies had shown that a raised blood homocysteine level was a risk factor for cardiovascular events (Casas 2005; Danesh 1998; Eikelboom 1999; Ford 2002; Guthikonda 2006; HSC 2002; Jacobsen 2005; Kardesoglu 2011; Refsum 1998; Splaver 2004; Stampfer 1992; Wald 2002; Wang 2005; Williams 2010; Wu 2013). The public significance of raised circulating blood homocysteine levels has been considered (Shelhub 2008). Currently, there is no evidence to support cardiovascular risk reduction by homocysteine‐lowering interventions (Cybulska 2015; Li 2015; Martí‐Carvajal 2009; Martí‐Carvajal 2013; Martí‐Carvajal 2015 (three previous versions of this review)). The American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Goff 2014) and The European Guidelines on cardiovascular disease prevention in clinical practice (Perk 2012) ratify that homocysteine is not a causal risk factor for cardiovascular disease.
Circulating total homocysteine levels are composed of protein (albumin)‐homocysteine mixed disulfide, sulfhydryl form and low molecular weight disulfides (Mudd 2000). The normal levels of total homocysteine are close to 10 µmol/L (Mudd 2000). Hyperhomocysteinaemia is defined as the presence of an abnormally elevated concentration of plasma or serum total homocysteine (Mudd 2000). However, there is some controversy about the definition of the degree of hyperhomocysteinaemia. Fasting total homocysteine level concentrations between 12 µmol/L and 30 µmol/L are termed mild or moderate, while intermediate hyperhomocysteinaemia includes levels between 31 µmol/L to 100 µmol/L, and severe hyperhomocysteinaemia reflects values above 100 µmol/L (Maron 2006; Maron 2009). In the general population, the prevalence of hyperhomocysteinaemia is between 5% and 10% (Refsum 1998). However, rates may be as high as 30% to 40% in the elderly population (Selhub 1993).
Description of the intervention
B‐complex vitamins, cyanocobalamin (B12) (Fedosov 2012; Herrmann 2012; Kräutler 2012), folic acid (B9) (Crider 2011; Molloy 2012; Ohrvik 2011; Yetley 2011), and pyridoxine (B6) (di Salvo 2011; di Salvo 2012; Friso 2012; Mukherjee 2011), given as a supplement.
How the intervention might work
The B‐complex vitamins are essential for homocysteine metabolism; they are involved in both the transformation and excretion pathways of homocysteine (McCully 2015a; McCully 2015b). Supplementation with B‐complex vitamins reduces total homocysteine levels (Clarke 2007; HLTC 2005). There is some ambiguity regarding the function of pyridoxine (vitamin B6). Vitamin B6 supplementation has been shown to lower total homocysteine levels after a methionine load, which occurs in experimental situations. However, at least two studies have shown the contrary (Gori 2007; Sofi 2008). It is, as a result, believed to be a weak determinant of circulating total homocysteine levels.
Why it is important to do this review
This is the third update of this Cochrane review and has been performed to identify and review the latest evidence.
Objectives
To determine whether homocysteine‐lowering interventions, provided to patients with and without pre‐existing cardiovascular disease:
are effective in preventing cardiovascular events and/or all‐cause mortality;
are safe;
differ in efficacy or safety.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a follow‐up period of one year or longer.
Types of participants
Adults (over 18 years) at risk of, or with established cardiovascular disease. We excluded studies in patients with end‐stage renal disease.
Types of interventions
The interventions considered were vitamins B6 (pyridoxine; pyridoxal), B9 (folic acid) or B12 (cyanocobalamin) given alone or in combination, at any dosage, and via any administration route.
We made comparisons with placebo, or with differing regimens of vitamins B6, B9 or B12. When the included population was at risk of cardiovascular disease, we considered combinations of homocysteine‐lowering interventions with standard treatment (such as antihypertensives and statins) versus standard treatment alone.
Types of outcome measures
Primary outcomes
Non‐fatal or fatal myocardial infarction.
Non‐fatal or fatal stroke (ischaemic or haemorrhagic stroke).
Secondary outcomes
First unstable angina pectoris episode requiring hospitalisation.
Hospitalisation for heart failure.
Death from any cause.
Serious or non‐serious adverse events.
We defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997), as any event that leads to death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation and/or results in persistent or significant disability. We considered all other adverse events non‐serious.
Search methods for identification of studies
Electronic searches
We reran the searches previously run in 2008 (Appendix 1), 2012 (Appendix 2), and 2014 (Appendix 3). Search strategies for 2017 are shown in Appendix 4.
We updated the searches of the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 5), MEDLINE OVID (1946 to 1 June 2017), Embase OVID (1980 to 2017 week 22) and Web of Science (Thomson Reuters, 1970 to 1 June 2017). The search of LILACS was last run on 1 June 2017. In a previous version (Martí‐Carvajal 2009), we searched Allied and Complementary Medicine ‐ AMED (accessed through Ovid) and the Cochrane Stroke Group Specialised Register.
We used the Cochrane sensitive‐maximising RCT filters to search MEDLINE and Embase (Lefebvre 2011).
We imposed no language restrictions.
Searching other resources
We also checked the reference lists of all trials identified.
We also searched the World Health Organization International Clinical Trials Platform search portal (http://apps.who.int./trialsearch) and ClinicalTrials.gov (https://clinicaltrials.gov/).
We also searched websites of U.S. Food and Drug Administration (www.fda.gov) and European Medicines Agency (www.ema.europa.eu) for unpublished information on homocysteine‐lowering interventions.
We contacted authors and researchers to obtain further details for published studies.
Data collection and analysis
We conducted data collection and analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors (AMC and IS) independently screened the results of the search strategy for potentially relevant trials and independently assessed them for inclusion based on the inclusion criteria.
Data extraction and management
Two review authors (AMC and IS) carried out data extraction using a pre‐designed data extraction form that included publication details, patient population, randomisation, allocation concealment, details of blinding measures, description of interventions and results. We resolved discrepancies through discussion. We involved a third review author (DL) to check the data entered into the Review Manager software. Two review authors (AMC and IS) assessed the included studies and entered the information into tables; see Characteristics of included studies.
Assessment of risk of bias in included studies
All review authors independently assessed the risk of bias of the trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following domains.
Generation of the allocation sequence
Allocation concealment
Blinding (or masking)
Incomplete outcome data
Selective outcome reporting
Other bias
See Appendix 5 for details of domains.
Measures of treatment effect
We pooled the risk ratios (RR) with 95% confidence interval (CI) for the following binary outcomes: non‐fatal or fatal myocardial infarction, non‐fatal or fatal stroke (ischaemic or haemorrhagic), first unstable angina pectoris episode requiring hospitalisation, hospitalisation for heart failure, death from any cause and serious or non‐serious adverse events as recommended by Higgins 2011. We calculated the number needed to treat for an additional beneficial outcome (NNTB) if the RR was significant (P value = < 0.05). NNTB is a measure of assessment of clinical useful of the consequences of treatment (Laupacis 1988). We estimated NNTB with GraphPad software.
Dealing with missing data
For all included trials, we noted the levels of attrition. We contacted the first author of the paper if data were missing. We extracted data on the number of participants by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up. If we were not able to do so, we recorded for each study whether the results pertained to an intention‐to‐treat analysis or to available‐case analysis.
Assessment of heterogeneity
We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). We considered statistical heterogeneity to be present if the I2 value was greater than 50% (Higgins 2011). When significant heterogeneity was detected (I2 > 50%), we attempted to identify the possible causes.
Assessment of reporting biases
We assessed asymmetry in funnel plots for myocardial infarction, stroke and death from any cause, and devoted to detect potential publication bias and other causes of asymmetry (Sterne 2001). We used the contour‐enhanced funnel plot for differentiating asymmetry due to publication bias from that due to other factors (Peters 2008). We assessed likelihood of publication bias with Harbord and Peters tests (Sterne 2011a; Sterne 2011b). We used STATA statistical software V.14.0 (StataCorp LP) to perform conventional and contour funnel plots.
Data synthesis
We pooled the results from the trials using the Review Manager software (RevMan 2014). We summarised the findings using a random‐effects model.
Trial Sequential Analysis
Meta‐analysis of cumulative data may run the risk of random errors ('play of chance') due to sparse data and repetitive analyses of the same data (Brok 2008; Brok 2009; Thorlund 2010; Thorlund 2011; Wetterslev 2008; Wetterslev 2009; Wetterslev 2017). In order to assess the risks of random errors in our cumulative meta‐analyses, we conducted diversity‐adjusted trial sequential analyses based upon the proportion with the outcome in the control group, an a priori set relative risk reduction of 20%, an alpha of 5%, a beta of 20% and the diversity in the meta‐analysis (CTU 2011; Thorlund 2009; Thorlund 2011). We conducted sensitivity analysis of the trial sequential analysis to estimate the potential need for further trials.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis according to the type of intervention, and by trials including participants without cardiovascular disease versus trials including participants with cardiovascular disease.
Sensitivity analysis
We conducted a sensitivity analysis comparing the results using all studies and using only those with a low risk of bias.
'Summary of findings' tables
We used The Grading of Recommendations Assessment, Development and Evaluation (GRADE) proposals to assess the quality of the body of evidence associated with the following outcomes: myocardial infarction, stroke, death from any cause and cancer (Guyatt 2011). One review author constructed Table 1; Table 2; Table 3 using the GRADEpro software (GRADEpro 2008). We involved a second review author to check the data.
Summary of findings 1. Homocysteine‐lowering interventions (Vitamin B6 (pyridoxine; pyridoxal); B9 (folic acid) or B12 (cyanocobalamin) compared with placebo or standard care for preventing cardiovascular events.
| Homocysteine‐lowering interventions (vitamins B6 (pyridoxine; pyridoxal); B9 (folic acid) or B12 (cyanocobalamin) compared with placebo or standard care for preventing cardiovascular events | ||||||
| Patient or population: adults at risk of or with established cardiovascular disease Settings: outpatients Intervention: homocysteine‐lowering interventions (vitamins B6 (pyridoxine; pyridoxal), B9 (folic acid) or B12 (cyanocobalamin). Comparison: placebo or standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Homocysteine‐lowering interventions (vitamins B6 (pyridoxine; pyridoxal); B9 (folic acid) or B12 (cyanocobalamin) | |||||
| Myocardial infarction Follow‐up: 1 to 7.3 years | 60 per 1000 | 61 per 1000 (57 to 66) | RR 1.02 (0.95 to 1.10) | 46,699 (12 trials) | ⊕⊕⊕⊕ high | |
| Stroke Follow‐up: 1 to 7.3 years | 51 per 1000 | 46 per 1000 (42 to 50) | RR 0.90 (0.82 to 0.99) | 44,224 (10 trials) | ⊕⊕⊕⊕ high | |
| Death by any cause Follow‐up: 1 to 7.3 years | 123 per 1000 | 124 per 1000 (118 to 130) | RR 1.01 (0.96 to 1.06) | 44,817 (11 trials) | ⊕⊕⊕⊕ high | |
| Adverse events Follow‐up: 3.4 to 7.3 years | 85 per 1000 | 91 per 1000 (85 to 97) | RR 1.07 (1.00 to 1.14) | 35,788 (8 trials) | ⊕⊕⊕⊕ high | Cancer is the only reported adverse event. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Homocysteine‐lowering interventions (high dose) compared with homocysteine‐lowering interventions (low dose) for preventing cardiovascular events.
| Homocysteine‐lowering interventions (high dose) compared with homocysteine lowering interventions (low dose) for preventing cardiovascular events | ||||||
| Patient or population: adults at risk of or with established cardiovascular disease Settings: outpatients Intervention: homocysteine‐lowering interventions (high dose) either (folic acid; vitamin B12 (cyanocobalamin) and vitamin B6 (pyridoxine; pyridoxal) or folic acid Comparison: homocysteine‐lowering interventions (low dose) either (folic acid; vitamin B12; vitamin B6 per day) or folic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Homocysteine‐lowering interventions (low‐dose) | Homocysteine‐lowering interventions (high‐dose) | |||||
| Myocardial infarction Follow‐up: 2 years | 44 per 1000 | 40 per 1000 (29 to 54) | RR 0.90 (0.66 to 1.23) | 3649 (1 trial) | ⊕⊕⊕⊝ moderate1 |
VISP 2004:
|
| Stroke Follow‐up: 2 to 5 years | 112 per 1000 | 101 per 1000 (74 to 137) | RR 0.90 (0.66 to 1.22) | 3929 (2 trials) | ⊕⊝⊝⊝ very low1, 2, 3 | 1. Li 2015a was conducted including only Chinese elderly females.Trial used only folic acid as homocysteine‐lowering intervention.
2. VISP 2004:
|
| Death by any cause Follow‐up: 2 years | 64 per 1000 | 55 per 1000 (42 to 71) | RR 0.86 (0.66 to 1.11) | 3649 (1 trial) | ⊕⊕⊕⊝ moderate1 |
VISP 2004:
|
| Cancer | Not estimable | ‐ | Li 2015a and VISP 2004 reported no information on this outcome. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level for imprecision due to low number of events 2 Dowgraded one level for risk of bias as one trial (Li 2015a) was rated as having unclear risk of selection, conduction and detection biases 3 Downgraded one level for heterogeneity (I‐squared: 72%).
Summary of findings 3. Enalapril plus folic acid compared with enalapril for adults with hypertension.
| Enalapril plus folic acid compared with folic acid for adults with hypertension | ||||||
| Patient or population: adults with hypertension Settings: Chinese outpatients Intervention: enalapril (10 mg) plus folic acid (0.8 mg) Comparison: enalapril (10 mg) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Folic acid | Enalapril plus folic acid | |||||
| Myocardial infarction Follow‐up: median 4.5 years | 2 per 1000 | 2 per 1000 (1 to 4) | RR 1.04 (0.60 to 1.82) | 20,702 (1 trial) | ⊕⊕⊕⊝ moderate1 | |
| Stroke Follow‐up: median 4.5 years | 34 per 1000 | 27 per 1000 (23 to 32) | RR 0.79 (0.68 to 0.93) | 20,702 (1 trial) | ⊕⊕⊕⊕ high | |
| First unstable angina pectoris episode requiring hospitalisation | Not estimable | ‐ | CSPPT 2015 did not assess this outcome. | |||
| Death from any cause Follow‐up: median 4.5 years | 31 per 1000 | 29 per 1000 (25 to 34) | RR 0.94 (0.81 to 1.10) | 20,702 (1 trial) | ⊕⊕⊕⊕ high | |
| Serious adverse event (cancer) Follow‐up: median 4.5 years | 8 per 1000 | 8 per 1000 (6 to 11) | RR 0.96 (0.71 to 1.31) | 20,243 (1 trial) | ⊕⊕⊕⊝ moderate1 | CSPPT 2015 included either neoplasms benign, malignant or unspecified |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for imprecision due to low number of events.
GRADE classifies the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the outcome being assessed (Guyatt 2008; Guyatt 2013).
Fragility Index
We calculated the fragility index (FI) if the RR was significant (P value = < 0.05). FI is a measure to identify the number of events required to change statistically significant results to non‐significant results (Walsh 2014). The FI was only applied to RCTs where the allocation 1:1 and to binary data. We estimated the FI with the Fragility Index Calculator.
Bayes Factors
We estimated the threshold for clinical relevance using a Bayes factor (Jakobsen 2014). This is a likelihood ratio indicate the relative strength of evidence for two theories (Dienes 2014; Goodman 1999; Goodman 2005). A Bayes factor is a comparison of how well two hypotheses (the null hypothesis ‐H0‐ and the alternative hypothesis ‐H1‐) predict the data (Goodman 1999). A Bayes factor provides a continuous measure of evidence for H1 over H0. When a Bayes factor is 1, the evidence does not favour either model over the other. As a Bayes factor increase above 1 (towards infinity) the evidence favours H1 over H0. As a Bayes factor decreases below 1 (towards 0) the evidence favours H0 over H1 (Dienes 2008; Dienes 2014; Dienes 2017). We used Dienes' Calculator for estimating Bayes factors.
Results
Description of studies
The search in June 2017 identified 1464 records, which resulted in 1073 unique references after duplicates were removed. After examining the titles and abstracts we excluded 1036 references. We obtained full reprints of the remaining 37 references for more detailed examination, of which 22 reports were excluded. The remaining 15 references identified were for three new randomised clinical trials (B‐PROOF 2015; CSPPT 2015; Li 2015a), 11 of which related to one of the new trials (CSPPT 2015).
In total, this updated review includes 15 randomised clinical trials, published between 2002 and 2015, involving 71,422 participants (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). See Figure 1 for details.
1.
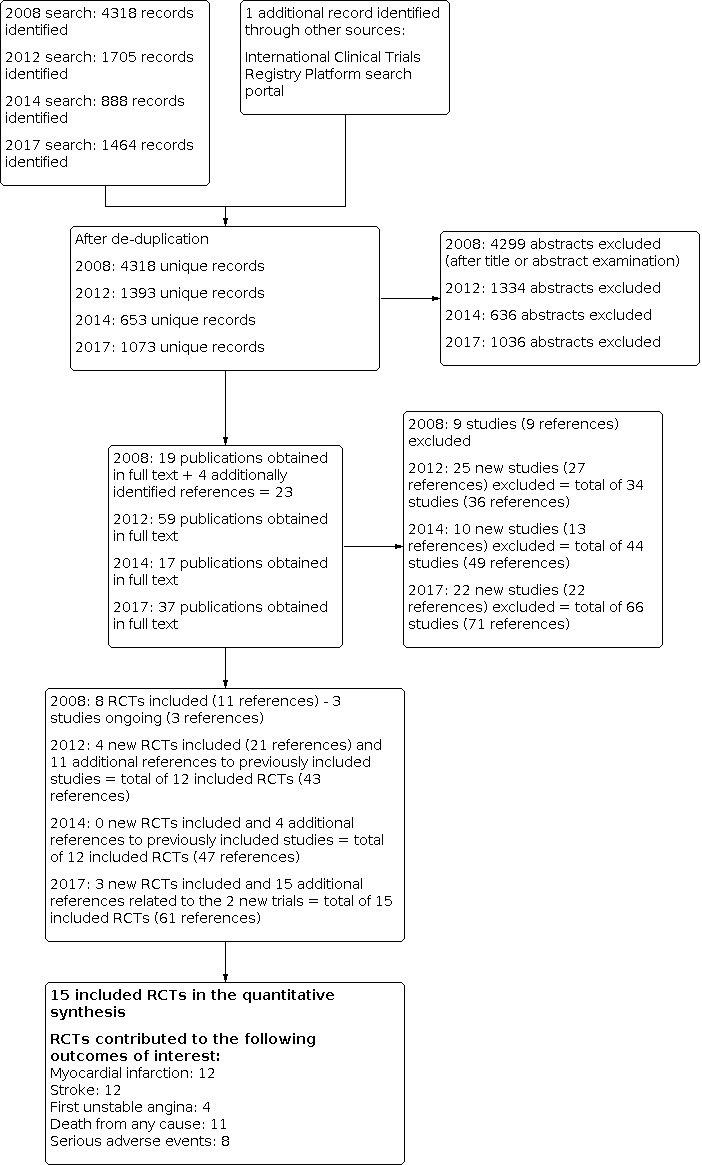
Study flow diagram.
These trials are described in the section Characteristics of included studies. The length of follow‐up ranged from one to 7.3 years. The trials varied in size, characteristics of participant populations, duration, drug dosage and experimental design.
Included studies
Thirteen trials were conducted in participants with known cardiovascular disease, such as coronary artery disease, myocardial infarction, stable angina, unstable angina, stroke or intermittent claudication (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE‐2 2006; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008), one trial included participants without any history of cardiovascular disease (CSPPT 2015); a further trial explicitly included participants with a history of non‐disabling cerebral infarction (VISP 2004).
Fourteen trials included participants with at least one of the following known cardiovascular risk factors: diabetes mellitus, hypertension, elevated total cholesterol, current smoking, or low high‐density lipoprotein (HDL) cholesterol (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). This aspect was unclear for CHAOS 2002. One trial (WAFACS 2008) included participants with three or more coronary risk factors. One trial explicitly excluded participants with previously known hyperhomocysteinaemia (total plasma homocysteine > 18 μmol/L) (FOLARDA 2004).
BVAIT 2009 included participants with hyperhomocysteinaemia without diabetes and cardiovascular disease. HOPE‐2 2006 included participants without a history of coronary heart disease (CHD). WAFACS 2008 only included female participants. Li 2015a included hypertensive females with hyperhomocysteinaemia.
Eleven trials included more than 1000 participants (B‐PROOF 2015; CSPPT 2015; CHAOS 2002; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Two trials only included elderly participants (B‐PROOF 2015; Li 2015a).
Ten trials were compared with placebo (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008), and two with standard care (FOLARDA 2004; GOES 2003), while two trials were randomised controlled trials (Li 2015a; VISP 2004), which compared doses of homocysteine‐lowering interventions. One trial compared antihypertensive medication plus a homocysteine‐lowering intervention versus antihypertensive medication alone (CSPPT 2015).
The intervention assessed by most of the trials was a combination of vitamins B6, B9 and B12 (B‐PROOF 2015; BVAIT 2009; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Five trials only included vitamin B9 as intervention (CSPPT 2015; CHAOS 2002; FOLARDA 2004; GOES 2003; Li 2015a). SU.FOL.OM3 2010 used 5‐methyltetrahydrofolate instead of folic acid.
FOLARDA 2004, GOES 2003, HOPE‐2 2006, NORVIT 2006, SEARCH 2010, WAFACS 2008 and WENBIT 2008 described lipid‐lowering drugs used as concomitant medications. SU.FOL.OM3 2010 reported omega 3 polyunsaturated fatty acids used as concomitant medications. B‐PROOF 2015 reported vitamin D3 use as a concomitant medication. Li 2015a reported restriction of salt intake and administration of vitamin B12 as a concomitant medication. CSPPT 2015 described the use of antihypertensive medications, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, β‐Blockers, lipid‐lowering medications, glucose‐lowering medications and antiplatelet medications concomitantly.
Three trials were conducted in a "fortified" population (BVAIT 2009; VISP 2004; WAFACS 2008). The programme was described as a "...nutritional intervention programme with a specifically defined target, and fortified food products are expected to become a main source of the specific added nutrient" (Wirakartakusumah 1998). Two trials were performed in a mixed population (HOPE‐2 2006; VITATOPS 2010), and 10 were carried out in non‐fortified populations (B‐PROOF 2015; CSPPT 2015; CHAOS 2002; FOLARDA 2004; GOES 2003; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; WENBIT 2008).
Twelve trials used composite outcomes in their analyses (B‐PROOF 2015; CSPPT 2015; CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Four trials included revascularisation or other vascular procedures (CHAOS 2002; GOES 2003; WAFACS 2008; WENBIT 2008). Fourteen trials had stroke as the endpoint (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Fourteen trials assessed the impact of the intervention on myocardial infarction rates (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). One trial included angina pectoris as a component of composite outcomes (B‐PROOF 2015).
Thirteen studies reported the sample size calculation (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). The trials used 80% or 90% power to detect between a 20% and 50% reduction in endpoints.
Concentrations of total homocysteine blood levels at baseline were reported in 13 trials (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; CHAOS 2002; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008WENBIT 2008). Six trials reported the total homocysteine blood levels at the end of follow‐up (B‐PROOF 2015; CHAOS 2002; HOPE‐2 2006; NORVIT 2006; VISP 2004; WAFACS 2008). WENBIT 2008 described total homocysteine blood levels after one year of the intervention. CHAOS 2002 did not report total homocysteine blood levels at baseline or at the end of follow‐up in the control arm. GOES 2003 reported total homocysteine blood levels at baseline and at the end follow‐up, but only for the intervention arm and not for the control arm. FOLARDA 2004 and Li 2015a did not report the circulating total homocysteine blood levels in either group.
Definitions used for defining myocardial infarction, stroke, unstable angina and death (all‐cause) are described in Appendix 6.
Excluded studies
This review has 66 references excluded (44 in the prior versions and 22 in this update), which are described in the table of Characteristics of excluded studies. These studies were mainly systematic reviews, RCTs with a follow‐up of less of one year, and non‐RCTs.
Risk of bias in included studies
The risk of bias in the included trials is summarised in Figure 2 and Figure 3, and detailed in the Characteristics of included studies tables. See Appendix 5 for details.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The risk of bias arising from the method of generation of the allocation sequence was low in 10 trials (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; GOES 2003; HOPE‐2 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008). Five trials had an unclear risk for this domain (CHAOS 2002; FOLARDA 2004; Li 2015a; NORVIT 2006; WENBIT 2008).
Allocation concealment
We rated the risk of bias arising from the method of allocation concealment as low in 10 trials (B‐PROOF 2015; BVAIT 2009; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Five trials showed an unclear risk for this domain (CHAOS 2002; CSPPT 2015; FOLARDA 2004; GOES 2003; Li 2015a).
Blinding
We rated the risk of bias arising from lack of blinding of participants and personnel as low in 11 trials (B‐PROOF 2015; BVAIT 2009; CSPPT 2015; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). The risk of bias from blinding was unclear in two trials (CHAOS 2002; Li 2015a). We rated the risk of bias arising from lack of blinding as high in two trials (FOLARDA 2004; GOES 2003).
Blinding of outcome assessment (detection bias)
We rated the risk of bias arising from lack of blinding of outcome assessment as low in 12 trials (BVAIT 2009; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). The risk of bias from unblinding was unclear in three trials (B‐PROOF 2015; CHAOS 2002; Li 2015a).
Incomplete outcome data
We rated the risk of attrition bias as low in eight trials (BVAIT 2009; CSPPT 2015; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010). We rated the risk of attrition bias as high in three trials (B‐PROOF 2015; FOLARDA 2004; WENBIT 2008). We rated the risk of bias as unclear in four trials (CHAOS 2002; Li 2015a; VISP 2004; WAFACS 2008).
Selective reporting
Fourteen trials had a low risk of bias in this domain. One trial was rated as having high risk of bias for selective reporting (Li 2015a) due to lack of information on adverse events.
Other potential sources of bias
Ten trials had a low risk of bias due to other sources of bias not identified (B‐PROOF 2015; BVAIT 2009; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Four trials had an unclear risk of bias (CHAOS 2002; FOLARDA 2004; GOES 2003; Li 2015a). One trial was rated as having high risk of bias (CSPPT 2015).
Overall risk of bias
Nine trials were rated as having low risk of bias (BVAIT 2009; CSPPT 2015; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008).
Effects of interventions
See: Table 1; Table 2; Table 3
The results are based on 71,422 participants in 15 randomised clinical trials (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; CSPPT 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; Li 2015a; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VISP 2004; VITATOPS 2010; WAFACS 2008; WENBIT 2008). See Table 1; Table 2 and Table 3 for details.
Primary outcomes
Non‐fatal or fatal myocardial infarction
Homocysteine‐lowering interventions compared with placebo or conventional care
A meta‐analysis of 12 randomised clinical trials (46,699 participants) showed uncertainty in the effect on non‐fatal or fatal myocardial infarction between homocysteine‐lowering interventions and placebo or conventional care (1788/25,051 (7.14%) versus 1290/21,648 (5.96%); risk ratio (RR) 1.02, 95% confidence interval (CI) 0.95 to 1.10; P value = 0.56, I2 = 0%; high‐quality evidence) (B‐PROOF 2015; BVAIT 2009; CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008) (Analysis 1.1). The Bayes factor was 1.04, which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other. Trial sequential analysis for myocardial infarction suggested that no more trials are needed to disprove a 10% relative risk reduction with the intervention. Smaller risk reductions might still require further trials (Figure 4). There was a low risk of publication bias (P value = 0.88, Harbord test; P value = 0.86, Peters test). Figure 5 and Figure 6 show funnel and contour‐enhanced funnel plots, respectively.
1.1. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 1: Myocardial infarction
4.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on myocardial infarction. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 5.95% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.
5.
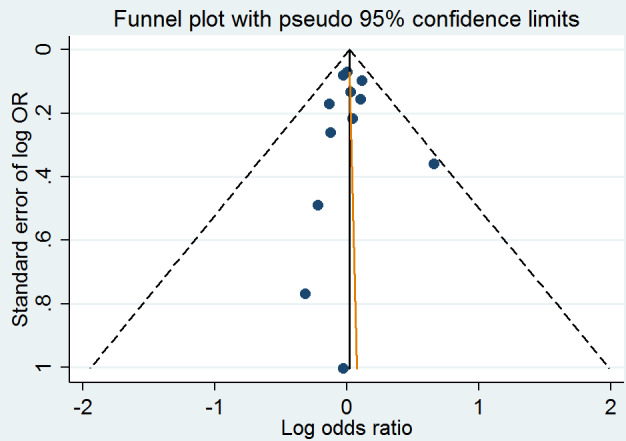
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.1 Myocardial infarction.
6.
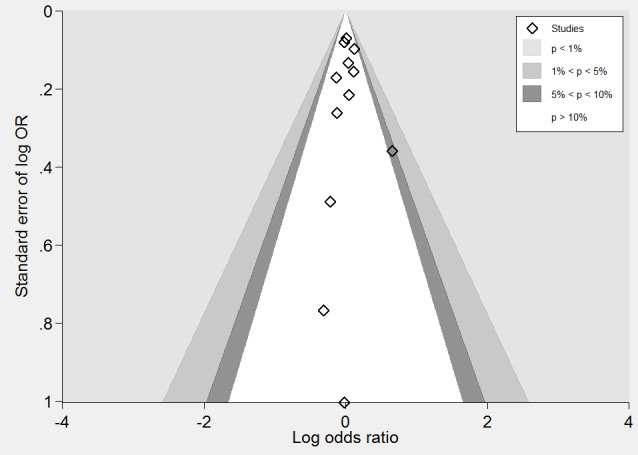
Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.1 Myocardial infarction.
Subgroup trials with a low risk of bias
A meta‐analysis of six trials (37,442 participants) found uncertainty over the effect of intervention in non‐fatal or fatal myocardial infarction rates (1517/19,649 (7.72%) versus 1161/17,793 (6.53%); RR 1.01, 95% CI 0.94 to 1.09, P value = 0.79, I2 = 0%) (HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008). (Analysis 2.1).
2.1. Analysis.

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 1: Myocardial infarction
Subgroup analysis comparing trials including participants without or with history of cardiovascular disease
One trial (490 participants) including participants without cardiovascular disease found uncertainty between intervention and placebo groups regarding non‐fatal or fatal myocardial infarction (2/248 (0.81%) versus 2/242 (0.83%); RR 0.98, 95% 0.14 to 6.87, P value = 0.98) (BVAIT 2009). A meta‐analysis of 11 trials (46,209 participants) including participants with a history of cardiovascular disease showed that there was no difference in non‐fatal or fatal myocardial infarction between intervention and placebo groups (1786/24,803 (7.20%) versus 1288/21,406 (6.02%); RR 1.02, 95% CI 0.95 to 1.10, I2 = 9%) (B‐PROOF 2015; FOLARDA 2004; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Testing for subgroup differences found no significant difference (P value = 0.96 and I2 = 0%). Analysis 3.1.
3.1. Analysis.

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 1: Myocardial Infarction
Homocysteine‐lowering interventions (high dose) compared with homocysteine‐lowering interventions (low dose)
One trial (3649 participants) found a lower proportion had non‐fatal or fatal myocardial infarctions in participants assigned to a high dose of homocysteine‐lowering interventions compared with those receiving a low dose of homocysteine‐lowering interventions (72/1814 (3.97%) versus 81/1835 (4.41%); RR 0.90, 95% CI 0.66 to 1.23, P value = 0.50; moderate‐quality evidence) (VISP 2004) (Analysis 1.1). The Bayes factor was 1.06, which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other.
Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril)
One trial (20,702 participants) found uncertainty in the rates of non‐fatal or fatal myocardial infarction between intervention and control groups (25/10,348) (0.24%) versus 24/10,354 (0.23%); RR 1.04, 95% CI 0.60 to 1.82, P value = 0.88; moderate‐quality evidence) (Analysis 1.1). The Bayes factor was 0.97 which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other.
Subgroup analysis for missing data
One trial (20,635 participants) comparing the combination of folic acid plus enalapril with enalapril alone showed inconsistent results in terms of non‐fatal or fatal myocardial infarction, according to per protocol analysis (25/10,316 (0.24%) versus 24/10,319 (0.23%); RR 1.04, 95% CI 0.60 to 1.82, P value = 0.89), best‐worst case scenario (25/10,348 (0.24%) versus 59/10,354 (0.57%); RR 0.42, 95% CI 0.27 to 0.68, P value = 0.0003) and worst‐best case scenario (57/10,348 (0.55%) versus 24/10,354 (0.23%); RR 2.38, 95% CI 1.48 to 3.83, P value = 0.0004). Testing for subgroup differences found a significant difference (P value <0.0001 and I2 = 92%) (CSPPT 2015). Analysis 4.1.
4.1. Analysis.

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 1: Myocardial infarction
Non‐fatal or fatal stroke
Homocysteine‐lowering interventions compared with placebo
A meta‐analysis of ten trials (44,224 participants) showed a risk reduction in non‐fatal or fatal stroke in participants assigned to homocysteine‐lowering interventions compared with placebo (1014/23,809 (4.26%) versus 1034/20,415 (5.06%); RR 0.90, 95% CI 0.82 to 0.99, P value = 0.03, I2 = 8%, high‐quality evidence) (B‐PROOF 2015; BVAIT 2009; FOLARDA 2004; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008) (Analysis 1.2). The Bayes factor was 5.84 which means that it is 5.84 times more likely that homocysteine‐lowering interventions reduce non‐fatal or fatal stroke compared with placebo. Trial sequential analysis for stroke suggested that no more trials are needed to disprove a 10% relative risk reduction with intervention. Smaller risk reductions might still require further trials (Figure 7). There was a low risk of publication bias (P value = 0.368, Harbord test; P value = 0.393, Peters test). Figure 8 and Figure 9 show funnel and contour‐enhanced funnel plots, respectively.
1.2. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 2: Stroke
7.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on stroke. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 5% with an alpha of 5% and beta of 20%.
8.
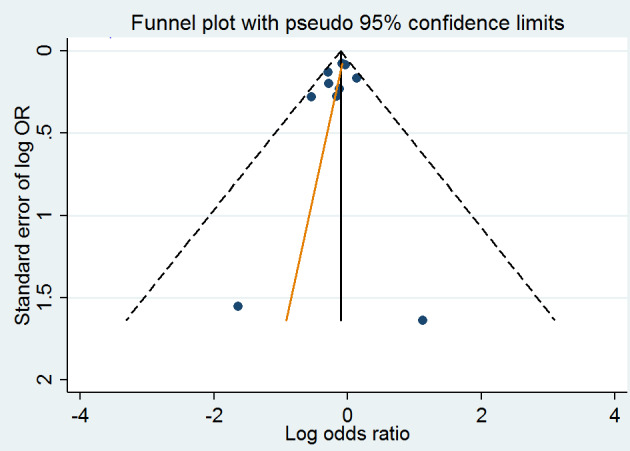
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.2 Stroke.
9.
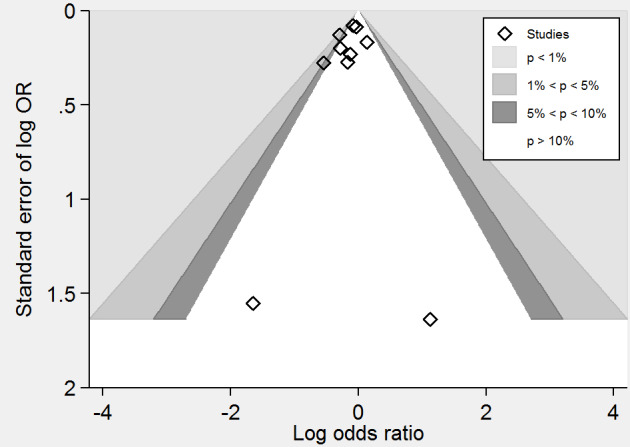
Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.2 Stroke.
Subgroup trials with a low risk of bias
A meta‐analysis of six trials (37,442 participants) found uncertainty in differences between non‐fatal or fatal stroke rates between intervention and placebo groups (919/19,649 (4.68%) versus 953/17,793 (5.36%); RR 0.90, 95% CI 0.80 to 1.02, P value = 0.10, I2 = 32%) (HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008). (Analysis 2.2).
2.2. Analysis.

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 2: Stroke
Subgroup analysis comparing trials including participants without or with history of cardiovascular disease
One trial (490 participants) including participants without cardiovascular disease found uncertainty between intervention and placebo groups regarding the rates of non‐fatal or fatal stroke (0/248 (0%) versus 2/242 (0.83%); RR 0.20, 95% 0.01 to 4.04, P value = 0.29) (BVAIT 2009). A meta‐analysis of nine trials (43,734 participants) including participants with history of cardiovascular disease showed evidence of effect favouring intervention group versus placebo group in terms of non‐fatal or fatal stroke rates (1014/23,561 (4.30%) versus 1032/20,173 (5.12%); RR 0.90, 95% CI 0.82 to 0.99, I2 = 9%) (B‐PROOF 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Testing for subgroup differences found no significant difference (P 0.32 and I2 = 0%). Analysis 3.2.
3.2. Analysis.

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 2: Stroke
Homocysteine‐lowering interventions (high dose) compared with homocysteine‐lowering interventions (low dose)
A meta‐analysis of two trials (3929 participants) showed uncertainty between the effects of high dose versus low dose of homocysteine‐lowering interventions with regards to non‐fatal or fatal stroke (211/1958 (10.78%) versus 221/1971 (11.21%); RR 0.90, 95% CI 0.66 to 1.22; I2 = 72%; very low‐quality evidence) (Li 2015a; VISP 2004) (Analysis 1.2). The Bayes factor was 1.06, which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other.
We detected high statistical heterogeneity, as conveyed by the I2 value (72%), and therefore we further explored by type of planned intervention.
One trial (3649 participants) comparing a combination of homocysteine‐lowering interventions (folic acid, vitamin B6 and vitamin B12), either at high dose (2.5 mg folic acid; 0.4 mg vitamin B12; 25 mg vitamin B6), or low dose (20 micrograms folic acid; 6 micrograms vitamin B12; 200 micrograms vitamin B6) found uncertainty over the effects on non‐fatal or fatal stroke rates (152/1814 (8.38%) versus 148/1835 (8.07%); RR 1.04, 95% CI 0.84 to 1.29; P value = 0.73) (VISP 2004). Analysis 5.1
5.1. Analysis.

Comparison 5: Homocysteine‐lowering treatment at high dose versus low dose (Subgoup analysis), Outcome 1: Stroke
One trial (280 participants) conducted only with elderly female participants, compared folic acid at high dose (0.8 mg) plus vitamin B12 (500 μg) versus folic acid at low dose (0.4 mg) plus vitamin B12 (500 μg). It found a lower proportion of non‐fatal or fatal strokes in participants assigned to high‐dose folic acid than those receiving a low‐dose folic acid (59/144 (40.97%) versus 73/136 (53.68%); RR 0.76, 95% CI 0.59 to 0.98; P value = 0.03) (Li 2015a). Analysis 5.1
Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril)
One trial (20,702 participants) found a reduced risk of non‐fatal or fatal stroke in participants receiving enalapril plus folic acid compared with participants receiving enalapril as monotherapy (281/10348 (2.72%) versus 354/10354 (3.42%); RR 0.79, 95% CI 0.68 to 0.93, P value = 0.003; NNTB 143, 95% CI 85 to 428, high‐quality evidence) (Analysis 1.2). The Bayes factor was 31.9 which means that it is 31.9 times more likely that homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) alone reduces non‐fatal or fatal stroke. The fragility Index was 23.
Subgroup analysis for missing data
The overall incidence of non‐fatal or fatal stroke seemed to be reduced in people assigned to a combination of folic acid plus enalapril versus those allocated to enalapril alone: per protocol analysis (281/10,316 (2.72%) versus 354/10,319 (3.43%); RR 0.79, 95% CI 0.68 to 0.93; P value = 0.003), best‐worst case scenario (281/10,348 (2.72%) versus 389/10,354 (3.76%); RR 0.72, 95% CI 0.62 to 0.84; P value = 0.0001) and worst‐best case scenario (313/10,348 (3.02%) versus 354/10,354 (3.42%); RR 0.88, 95% CI 0.76 to 1.03; P value = 0.11). Test for subgroup differences: P = 0.18, I² = 42.5%. (CSPPT 2015). Analysis 4.2.
4.2. Analysis.

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 2: Stroke
Secondary outcomes
First unstable angina pectoris episode requiring hospitalisation
Homocysteine‐lowering interventions compared with placebo
A meta‐analysis of four trials (12,644 participants) showed uncertainty between the effects intervention compared with placebo on the rate of unstable angina requiring hospitalisation (910/8015 (11.35%) versus 468/4629 (10.11%); RR 0.98, 95% CI 0.80 to 1.21, P value = 0.87, I2 = 66%) (FOLARDA 2004; HOPE‐2 2006; NORVIT 2006; WENBIT 2008) (Analysis 1.3.
1.3. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 3: First unstable angina pectoris episode requiring hospitalisation
Hospitalisation for heart failure
One trial found an uncertain effect in the hospitalisation for heart failure rates between intervention and placebo groups (202/2758 (7.32%) versus 174/2764 (6.30%); RR 1.16, 95% CI 0.96 to 1.41, P value = 0.13) (HOPE‐2 2006).
Death from any cause
Homocysteine‐lowering interventions compared with placebo
A meta‐analysis of 11 trials (44,817 participants) found uncertainty between the effects of intervention versus placebo on the rates of death from any cause (2821/24,109 (11.70%) versus 2544/20,708 (12.29%); RR 1.01, 95% CI 0.96 to 1.06, P value = 0.68, I2 = 0%, high‐quality evidence) (B‐PROOF 2015; BVAIT 2009; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008) (Analysis 1.4). The Bayes factor was 1.05, which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other. Trial sequential analysis for stroke suggested that no more trials are needed to disprove a 10% relative risk reduction with intervention. Smaller risk reductions might still require further trials (Figure 10). There was a low risk of publication bias (P value = 0.95, Harbord test; P value = 0.82, Peters test). Figure 11 and Figure 12 show funnel and contour‐enhanced funnel plots, respectively.
1.4. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 4: Death from any cause
10.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on death from any cause. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 12% from proportion event in control (Pc) group of 11.7% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.
11.
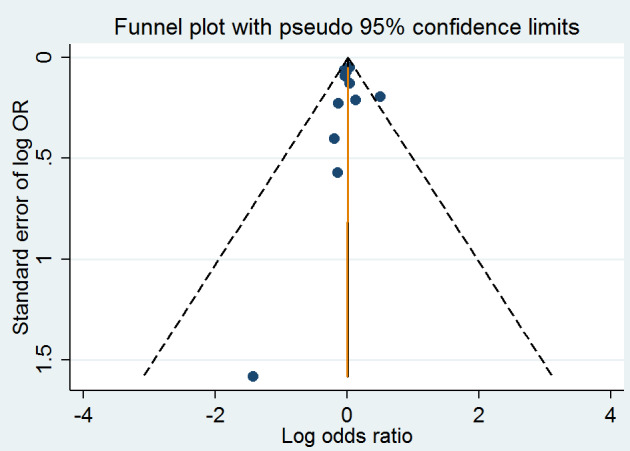
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.4 Death from any cause.
12.
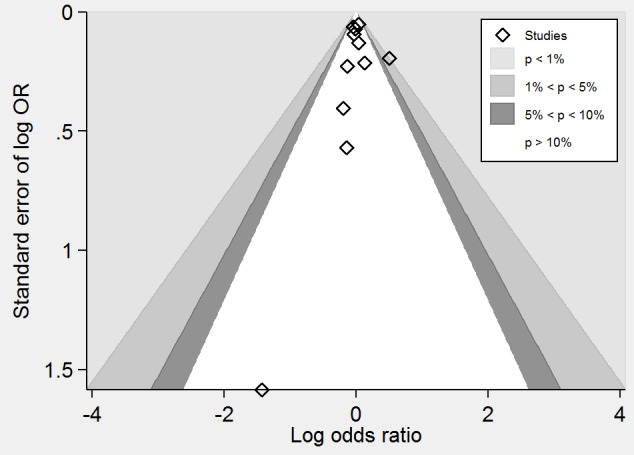
Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.4 Death from any cause.
Subgroup trials with a low risk of bias
A meta‐analysis of seven trials (37,932 participants) found uncertainty between the effects of intervention versus placebo in rates of death from any cause (2145/19,897 (10.78%) versus 1923/18,035 (10.66%); RR 1.03, 95% CI 0.95 to 1.12; P value = 0.48; I2 = 41%) (BVAIT 2009; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008). (Analysis 2.3).
2.3. Analysis.

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 3: Death from any cause
Subgroup analysis comparing trials including participants without or with history of cardiovascular disease
One trial (490 participants) including participants without cardiovascular disease found uncertainty between the effects of intervention versus placebo in rates of death from any cause (0/248 (0%) versus 2/242 (0.83%); RR 0.20, 95% 0.01 to 4.04, P value = 0.29) (BVAIT 2009). A meta‐analysis of 10 trials (44,327 participants) including participants with history of cardiovascular disease showed conclusive evidence that there was no difference in rates of death from any cause between intervention and placebo groups (2821/23,861 (11.82%) versus 2542/20,466 (12.42%); RR 1.01, 95% CI 0.96 to 1.06, I2 = 0%) (B‐PROOF 2015; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008). Testing for subgroup differences found no significant difference (P 0.32 and I2 = 0%). Analysis 3.3.
3.3. Analysis.

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 3: Death
Homocysteine‐lowering interventions (high dose) compared with homocysteine‐lowering interventions (low dose)
One trial (3649 participants) found uncertainty in mortality from any cause between intervention and control groups (99/1814 (5.46%) versus 117/1835 (6.38%); RR 0.86; 95% CI 0.66 to 1.11; P value = 0.24; moderate‐quality evidence) (VISP 2004) (Analysis 1.4). The Bayes factor was 1.07 which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other.
Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril)
One trial (20,702 participants) found uncertainty between the effects of enalapril plus folic acid compared with participants receiving enalapril as monotherapy on mortality from any cause (302/10,348 (2.92%) versus 320/10,354 (3.09%); RR 0.94, 95% CI 0.81 to 1.10; P value = 0.47; high‐quality evidence) (Analysis 1.4). The Bayes factor was 1.08 which means that evidence is insensitive, the data are equally well predicted by both models and the evidence does not favour either model over the other.
The combination of folic acid and enalapril seemed not to affect the incidence of death from any cause compared with enalapril alone. Per protocol analysis (302/10,316 (2.93%) versus 320/10,319 (3.10%); RR 0.94, 95% CI 0.81 to 1.10; P value = 0.47), best‐worst case scenario (302/10,348 (2.92%) versus 355/10,354 (3.43%); RR 0.85, 95% CI 0.73 to 0.99; P value = 0.04) and (334/10,348 (3.23%) versus 320/10,354 (3.09%); RR 1.04, 95% CI 0.90 to 1.21; P value = 0.57). Test for subgroup differences: P = 0.17, I² = 43.3%. (CSPPT 2015). Analysis 4.3.
4.3. Analysis.

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 3: Death from any cause
Serious or non‐serious adverse events
Homocysteine‐lowering interventions compared with placebo
A meta‐analysis of eight trials (35,788 participants) assessing cancer incidence found uncertainty in the incidence of cancer in intervention and placebo groups (1621/19,591 (8.27%) versus 1376/16,197 (8.50%); RR 1.07, 95% CI 1.00 to 1.14, P value = 0.07, I2 = 0%, high‐quality evidence) (B‐PROOF 2015; BVAIT 2009; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; WAFACS 2008; WENBIT 2008) (Analysis 1.5). Trial sequential analysis for adverse events suggested that no more trials are needed to disprove a 10% relative risk reduction (Figure 13).
1.5. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 5: Serious adverse events (cancer)
13.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on adverse events (cancer). The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 8.49% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.
A meta‐analysis of three trials (13,802 participants) found uncertainty in terms of serious or non‐serious adverse events rather than cancer between intervention and placebo groups (322/6903 (4.66%) versus 312/6899 (4.52%); RR 1.02, 95% 0.88 to 1.19; P value = 0.77, I2 = 0%, high‐quality evidence) (BVAIT 2009; SEARCH 2010; SU.FOL.OM3 2010). Analysis 1.6.
1.6. Analysis.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 6: Adverse events (serious and non‐serious) excluding cancer
Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril)
One trial (20,243 participants) found uncertainty in terms of cancer incidence between participants receiving enalapril plus folic acid compared with participants receiving enalapril as monotherapy (79/10,119 (0.78%) versus 82/10,124 (0.81%); RR 0.96, 95% CI 0.71 to 1.31; P value = 0.81; moderate‐quality evidence) (Analysis 1.5).
Discussion
Summary of main results
This updated Cochrane review of homocysteine‐lowering interventions (B vitamins) for preventing cardiovascular events identified 15 randomised controlled trials incorporating 71,422 participants. Trials reported different combinations of homocysteine‐lowering interventions compared with different control interventions (placebo: B‐PROOF 2015; BVAIT 2009; CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE‐2 2006; NORVIT 2006; SEARCH 2010; SU.FOL.OM3 2010; VITATOPS 2010; WAFACS 2008; WENBIT 2008, homocysteine‐lowering interventions at low dose: Li 2015a; VISP 2004, and antihypertensive medication (enalapril): (CSPPT 2015)). Overall, the trials had a low risk of bias and were adequately powered. Participants differed somewhat in cardiovascular risk levels (some with established cardiovascular disease (CVD), others at high risk of CVD), baseline total homocysteine blood levels, access to foods fortified with folic acid or not, different dosages of vitamins and different control groups, with treatment periods varying from two to seven years.
1. This review found no reduction of the incidence of either myocardial infarction (fatal or non‐fatal) and death from any cause or an increasing risk of adverse events (cancer).
2. With regard to stroke (fatal or non‐fatal), a meta‐analysis of homocysteine‐lowering interventions compared with placebo, and one mega‐trial comparing folic acid plus enalapril with enalapril alone found a reduction of risk stroke in those treated. A meta‐analysis of two trials comparing high dose versus low dose of homocysteine‐lowering interventions did not find a difference in the rates of fatal or non‐fatal strokes.
Overall completeness and applicability of evidence
This updated review found evidence suggesting that homocysteine‐lowering interventions (vitamins B6, B12 and folic acid (B9)) are not useful for preventing myocardial infarction (fatal or non‐fatal) or death from any cause. We conducted a sensitivity analysis restricted to trials with low risk of bias for myocardial infarction and death from any cause. These results show consistency and are based on data from trials that included a broad range of participants with different co‐morbidities who received different treatment approaches. Although these aspects could be considered as a threat to applicability, the consistency in the results derived from our analyses showed that the included trials may represent a broad picture of participants with a high risk of cardiovascular events.
With reference to stroke (non‐fatal or fatal stroke), this update found that homocysteine‐lowering interventions reduce the incidence of stroke compared with placebo or enalapril alone (Analysis 1.2). However, this result should be viewed with caution. In the trial sequential analysis graph called 'Trial sequential analysis on stroke in 10 trials investigating homocysteine‐lowering interventions versus placebo' (Figure 7), it was observed that after 44,224 participants the Z curve crossed the upper conventional alpha of 5%, but also the cumulative Z‐curve crossed no the trial sequential alpha‐spending monitoring boundaries also called as monitoring efficacy boundary (Roshanov 2017). This positive result appears to be weak, due to the 95% confidence interval reduction of risk of any stroke ranging between 1% and 18% and the very low basal risk. As is shown into Table 1, in the control group 51 people out of 1000 had a stroke (non‐fatal or fatal) over 1 to 7.3 years, compared with 46 (95% CI 42 to 50) out of 1000 for the active treatment group.
CSPPT 2015 also found a positive result of enalapril plus folic acid compared with enalapril on incidence of any stroke. The trial had a duration of follow‐up of five years. The absolute risk reduction in this trial was very low (0.7%), which explains the high number needed to treat for an additional beneficial outcome in this trial of 143 (95% CI 85 to 428). During five years between 85 and 428 hypertensive participants would need to take enalapril plus folic acid for to prevent one stroke. According to the Table 3, in the control group 34 people out of 1000 had a stroke (non‐fatal or fatal) over five years, compared to 27 (95% CI 23 to 32) out of 1000 in the active treatment group. Therefore, the clinical difference is small between the groups, which is reflected in the sensitivity analysis shown into Analysis 4.2. We estimated the fragility index of CSPPT 2015 as 23, which denotes that if 23 patients in the experimental group were converted from not having the primary endpoint to having the primary endpoint, the study would lose statistical significance (P > 0.05). Furthermore, it is unknown whether such treatment would benefit a non‐Chinese population. In both cases (meta‐analysis with more than 40,000 and a trial with 20,000 participants) shows a highly significant statistical result, but it "may not represent a clinically important effect when treating patients in our daily lives." (Fuster 2015).
In conclusion, this updated version showed the same findings as the previous version (Martí‐Carvajal 2015). It showed that supplementary vitamin B6, B12 and folic acid administration did not prevent cardiovascular events in participants with or without pre‐existing cardiovascular disease. The trial sequential analysis for the same outcomes suggested that no further randomised trials were needed to assess the benefits and harms of homocysteine‐lowering interventions to preventing cardiovascular events (Figure 4; Figure 7; Figure 10). Martí‐Carvajal 2013and Martí‐Carvajal 2015 found no effect of vitamin B‐complex supplementation on rates of cancer (Figure 13). Bayes factors give prominence to these findings. There is a likelihood for reducing the stroke rate.
Quality of the evidence
We conducted GRADE assessments on outcomes using the meta‐analysed trials.
Table 1 shows the quality of evidence for homocysteine‐lowering interventions compared with placebo or standard care for preventing cardiovascular events. The evidence available in this setting can be considered high quality due to the consistency of the results of the 12 trials for the main outcomes assessed (myocardial infarction, stroke and death from any cause), the precision in the pooled estimates, and the design and execution of these trials, which can be judged to be free of major threats to their validity.
Table 2 shows the quality of evidence for homocysteine‐lowering interventions (high dose) compared with homocysteine‐lowering interventions (low dose) for preventing cardiovascular events. The evidence was rated as moderate or very low due to imprecision i.e. low number of events, for risk of bias as one trial (Li 2015a) for having unclear risk of selection, conduction and detection biases, and for inconsistency (I2 = 72%).
Table 3 shows the quality of evidence for enalapril plus folic acid compared with enalapril for adults with hypertension. The evidence for stroke was rated as high.
Potential biases in the review process
In a systematic review process, there are a group of biases called significance‐chasing biases, such as publication bias and selective outcome reporting bias (Ioannidis 2010). Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to study publication bias in that 'negative' results remain unpublished (Ioannidis 2010). This Cochrane review found that overall, the included randomised trials had a low risk of attrition bias and a low risk of selective outcome reporting bias (Figure 2; Figure 3). This review might have a limitation due to paucity of data in terms of trials comparing 'head‐to‐head' homocysteine‐lowering interventions. A strength of this new updated Cochrane review is to have shown an absence of asymmetry in almost all funnel plots and to discard publication bias using appropriate statistical methodology i.e. Harbord and Peters tests.
Agreements and disagreements with other studies or reviews
Our results are similar to other non‐Cochrane reviews (Clarke 2010; Huang 2012; Huo 2012; Ji 2013). These four reviews differed in their eligibility criteria, i) resulting in the inclusion by Clarke 2010, Huang 2012, Huo 2012 and Ji 2013 of the HOST trial (Jamison 2007), designed to assess the effects of homocysteine in participants with kidney or renal disease, which is beyond our scope; ii) Clarke 2010 and Huo 2012 included all the trials in their pooled analysis (whereas we preferred to present the results from trials controlled with placebo separately from the results of the trials that compared different doses of homocysteine‐lowering drugs (VISP 2004)); iii) it can be concluded from the Clarke 2010 publication that the authors had access to some additional data from CHAOS 2002, which we had to extract from an abstract; and finally iv) our systematic review included five additional trials not considered in Clarke 2010, with 12,031 more participants, that allowed us to obtain more accurate estimates for our outcomes of interest (BVAIT 2009; FOLARDA 2004; GOES 2003; SU.FOL.OM3 2010; VITATOPS 2010). Lai 2015b and colleagues reported any affect on the risk of cardiovascular disease such as suggested this Cochrane review.
Two randomised controlled trials (Jamison 2007; Vianna 2007), and two systematic reviews, (Jardine 2012; Pan 2012) involving participants with end‐stage renal disease, found no effect of homocysteine‐lowering interventions in preventing cardiovascular events.
Regarding cancer, this Cochrane review showed similar results to a recent meta‐analysis involving data on 50,000 individuals (Vollset 2013). Both meta‐analyses found no increased risk of cancer associated with homocysteine‐lowering interventions.
Authors' conclusions
Implications for practice.
In this third update of the review, there were no differences in effects of homocysteine‐lowering interventions in the form of supplements of vitamins B6, B9 or B12 given alone or in combination comparing with placebo on myocardial infarction, death from any cause or adverse events. In terms of stroke, this review found a small difference in effect favouring homocysteine‐lowering interventions in the form of supplements of vitamins B6, B9 or B12 given alone or in combination compared with placebo. There were uncertain effects of folic acid compared with enalapril plus folic acid on stroke; approximately 143 (95% CI 85 to 428) people would need to be treated for 5.4 years to prevent 1 stroke, this evidence emerged from one mega‐trial. Trial sequential analyses showed that additional trials are unlikely to increase the certainty about the findings of this issue regarding homocysteine‐lowering interventions versus placebo.
Implications for research.
The association between both the lack of clinical effectiveness and harm of homocysteine‐lowering interventions might require further investigation into other homocysteine pathways. There is the need for additional trials comparing homocysteine‐lowering interventions combined with antihypertensive medication versus antihypertensive medication, and homocysteine‐lowering interventions at high doses versus homocysteine‐lowering interventions at low doses. Potential trials should be large and co‐operative.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2021 | Review declared as stable | This review topic is considered not to be a priority for the current scope of the Heart Group. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 1 June 2017 | New citation required and conclusions have changed | There is new information on stroke. |
| 1 June 2017 | New search has been performed | We updated the searches to June 2017. We found three new trials. This updated Cochrane Review now has four authors. |
| 15 October 2014 | New citation required but conclusions have not changed | We found no new trials for inclusion. |
| 9 July 2014 | New search has been performed | We updated the searches to February 2014. This updated Cochrane Review now has only three authors. |
| 7 March 2012 | New citation required but conclusions have not changed | This new updated version includes four additional RCTs and the conclusions are not changed. |
| 21 February 2012 | New search has been performed | We updated the searches to 21 February 2012. |
Acknowledgements
We express our gratitude to the Cochrane Heart Group and peer referees for the suggestions made to enhance the quality of this review. In addition, we acknowledge Carmen Verônica Abdala from BIREME/OPS/OMS for her help in developing the search strategy for LILACS. In addition, we want express our deep gratitude to Georgia Salanti for teaching us how to conduct the first version of this Cochrane review.
We want express our gratitude to Dr. Zoltan Dienes for helping us to conduct the Bayes factor estimation.
Appendices
Appendix 1. Search strategies 2008
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees #2 ”vitamin b*“ #3 folic next acid in Title, Abstract or Keywords #4 folate* in Title, Abstract or Keywords #5 (homocyst* near/6 lower*) #6 (homocyst* near/6 reduc*) #7 pyridoxin* #8 cobalamin* #9 cyanocobalamin* #10 pyridoxol* #11 MeSH descriptor Vitamins this term only #12 (vitamin* and homocyst*) #13 multivitamin* #14 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 MeSH descriptor Cardiovascular Diseases this term only #16 MeSH descriptor Myocardial Ischemia explode all trees #17 MeSH descriptor Brain Ischemia explode all trees #18 MeSH descriptor Cerebrovascular Disorders this term only #19 (coronary near/6 disease) #20 angina #21 myocardial next infarct* #22 heart next infarct* #23 (stroke or strokes) #24 (cerebr* near/6 accident*) #25 (cerebr* near/6 infarct*) #26 (brain near/6 infarct*) #27 apoplexy #28 cardiovascular next disease* #29 (cardiovascular near/6 event*) #30 MeSH descriptor Hyperhomocysteinemia explode all trees #31 hyperhomocyst* #32 cva #33 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) #34 (#26 or #27 or #28 or #29 or #30 or #31 or #32) #35 (#33 or #34) #36 (#14 and #35)
LILACS (accessed through Biblioteca Virtual em Saúde)
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and MH Vitamina B 12 OR Cobamidas OR Hidroxocobalamina OR Complejo Vitamínico B OR Ácido Fólico OR Ácidos Pteroilpoliglutámicos OR Tetrahidrofolatos OR Formiltetrahidrofolatos OR Vitamina B 6 OR Piridoxal OR Fosfato de Piridoxal OR Piridoxamina OR Piridoxina OR Homocisteína OR Vitaminas or TW vitamin$ or tw cobalamin$ or tw cianocobalamin$ or tw cyanocobalam$ or tw cobamid$ or tw hidroxocobalam$ or tw Hydroxocobalam$ or ((tw complejo or tw complex$) and tw vitamin$ and tw b) or (tw acid$ and (tw folic$ or tw ptero$)) or tw Tetrahidrofolatos or tw Formiltetrahidrofolatos or (tw vitamin$ or (tw b or tw b6 or tw b12)) or tw Piridoxal or tw Pyridoxal or ((tw Fosfat$ or tw phosphate$) and (tw Piridoxal or tw pyridoxal)) or tw Piridox$ or tw Pyridox$ or tw Homocisteína or tw Homocysteine) AND (MH Enfermedades Cardiovasculares or Isquemia Miocárdica or Ex C14.280.647$ or Isquemia Encefálica or Ex C10.228.140.300.150$ or Trastornos Cerebrovasculares or hiperhomocisteinemia or Accidente Cerebrovascular or ((tw apoplexia or tw derrame or tw trastorno$ or tw accident$ or tw acidente or tw stroke$ or tw disease$ or tw enfermedad$ or tw doenca$ or tw event$ or tw infart$ or tw isquemia or tw disorder$) and (tw miocardio or tw myocard$ or tw cerebr$ or tw cardiovascul$ or tw heart or tw cardiovascul$ or tw encefal$)) or tw hyperhomocyst$ or tw hiperhomocisteinemia) [Palavras]
MEDLINE
1 exp Vitamin B Complex/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp Myocardial Ischemia/ 14 exp Brain Ischemia/ 15 Cerebrovascular Disorders/ 16 (coronary adj3 disease$).tw. 17 angina.tw. 18 myocardial infarct$.tw. 19 heart infarct$.tw. 20 heart attack$.tw. 21 (stroke or strokes).tw. 22 (cerebr$ adj3 (accident$ or infarct$)).tw. 23 (brain adj3 infarct$).tw. 24 apoplexy.tw. 25 (cardiovascular adj2 (disease$ or event$)).tw. 26 Hyperhomocysteinemia/ 27 hyperhomocyst?in?emi$.tw. 28 or/12‐27 29 11 and 28 30 randomized controlled trial.pt. 31 controlled clinical trial.pt. 32 Randomized controlled trials/ 33 random allocation/ 34 double blind method/ 35 single‐blind method/ 36 or/30‐35 37 exp animal/ not humans/ 38 36 not 37 39 clinical trial.pt. 40 exp Clinical Trials as Topic/ 41 (clin$ adj25 trial$).ti,ab. 42 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 43 placebos/ 44 placebo$.ti,ab. 45 random$.ti,ab. 46 research design/ 47 or/39‐46 48 47 not 37 49 38 or 48 50 49 and 29
Embase
1 exp Vitamin B Group/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp ischaemic heart disease/ 14 exp Coronary Artery Disease/ 15 exp Brain Ischemia/ 16 cerebrovascular disease/ 17 stroke/ 18 cerebrovascular accident/ 19 (coronary adj3 disease$).tw. 20 angina.tw. 21 myocardial infarct$.tw. 22 heart infarct$.tw. 23 heart attack$.tw. 24 (stroke or strokes).tw. 25 (cerebr$ adj3 (accident$ or infarct$)).tw. 26 (brain adj3 infarct$).tw. 27 apoplexy.tw. 28 (cardiovascular adj2 (disease$ or event$)).tw. 29 Hyperhomocysteinemia/ 30 hyperhomocyst?in?emi$.tw. 31 or/12‐30 32 11 and 31 33 controlled clinical trial/ 34 random$.tw. 35 randomized controlled trial/ 36 follow‐up.tw. 37 double blind procedure/ 38 placebo$.tw. 39 placebo/ 40 factorial$.ti,ab. 41 (crossover$ or cross‐over$).ti,ab. 42 (double$ adj blind$).ti,ab. 43 (singl$ adj blind$).ti,ab. 44 assign$.ti,ab. 45 allocat$.ti,ab. 46 volunteer$.ti,ab. 47 Crossover Procedure/ 48 Single Blind Procedure/ 49 or/33‐48 50 32 and 49
Web of Science
# 11 TS=(#10 and (random* or blind* or placebo* or comparative or comparison or prospective or controlled or trial or evaluation or rct)) # 10 #7 or #8 or #9 # 9 TS=(#6 and (”cerebrovascular accident*“ or hyperhomocyst*)) # 8 TS=(#6 and (angina or stroke or strokes or cva or infarction*)) # 7 TS=(#6 and (cardiovascular or myocardial or coronary or cardiac or ”heart disease*“)) # 6 #1 or #2 or #3 or #4 or #5 # 5 TS=(homocyst* same (lower* or reduc*)) # 4 TS=(vitamin* and homocyst*) # 3 TS=folate* # 2 TS=”vitamin B“ # 1 TS=(pyridoxin* or cobalamin* or cyanocobalamin* or pyridoxol* or ”folic acid“)
Appendix 2. Search strategies 2012
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees #2 (vitamin b) #3 folic acid #4 folate* #5 ((homocystein* or homocystin*) near/3 (low* or reduc*)) #6 (pyridoxin*) #7 (cobalamin*) #8 (cyanocobalamin*) #9 (pyridoxol*) #10 MeSH descriptor Vitamins, this term only #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Cardiovascular Diseases, this term only #13 MeSH descriptor Myocardial Ischemia explode all trees #14 MeSH descriptor Brain Ischemia explode all trees #15 MeSH descriptor Cerebrovascular Disorders, this term only #16 (coronary near/3 disease*) #17 (angina) #18 (myocardial infarct*) #19 (heart infarct*) #20 (heart attack*) #21 (stroke or strokes) #22 (cerebr* near/3 (accident* or infarct*)) #23 (brain near/3 infarct*) #24 (apoplexy) #25 (cardiovascular near/2 (disease* or event*)) #26 MeSH descriptor Hyperhomocysteinemia, this term only #27 hyperhomocyst?in?emi* #28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29 (#11 AND #28)
MEDLINE
1 exp Vitamin B Complex/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp Myocardial Ischemia/ 14 exp Brain Ischemia/ 15 Cerebrovascular Disorders/ 16 (coronary adj3 disease$).tw. 17 angina.tw. 18 myocardial infarct$.tw. 19 heart infarct$.tw. 20 heart attack$.tw. 21 (stroke or strokes).tw. 22 (cerebr$ adj3 (accident$ or infarct$)).tw. 23 (brain adj3 infarct$).tw. 24 apoplexy.tw. 25 (cardiovascular adj2 (disease$ or event$)).tw. 26 Hyperhomocysteinemia/ 27 hyperhomocyst?in?emi$.tw. 28 or/12‐27 29 11 and 28 30 randomized controlled trial.pt. 31 controlled clinical trial.pt. 32 randomized.ab. 33 placebo.ab. 34 drug therapy.fs. 35 randomly.ab. 36 trial.ab. 37 groups.ab. 38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39 exp animals/ not humans.sh. (3663238) 40 38 not 39 41 29 and 40 42 (200808* or 200809* or 20081* or 2009* or 2010* or 2011* or 2012*).ed. 43 41 and 42
Embase
1 exp Vitamin B Complex/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp Myocardial Ischemia/ 14 exp Brain Ischemia/ 15 Cerebrovascular Disorders/ 16 (coronary adj3 disease$).tw. 17 angina.tw. 18 myocardial infarct$.tw. 19 heart infarct$.tw. 20 heart attack$.tw. 21 (stroke or strokes).tw. 22 (cerebr$ adj3 (accident$ or infarct$)).tw. 23 (brain adj3 infarct$).tw. 24 apoplexy.tw. 25 (cardiovascular adj2 (disease$ or event$)).tw. 26 Hyperhomocysteinemia/ 27 hyperhomocyst?in?emi$.tw. 28 or/12‐27 29 11 and 28 30 random$.tw. 31 factorial$.tw. 32 crossover$.tw. 33 cross over$.tw. 34 cross‐over$.tw. 35 placebo$.tw. 36 (doubl$ adj blind$).tw. 37 (singl$ adj blind$).tw. 38 assign$.tw. 39 allocat$.tw. 40 volunteer$.tw. 41 crossover procedure/ 42 double blind procedure/ 43 randomized controlled trial/ 44 single blind procedure/ 45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 46 (animal/ or nonhuman/) not human/ 47 45 not 46 48 29 and 47 49 (200808* or 200809* or 20081* or 2009* or 2010* or 2011* or 2012*).dd. 50 48 and 49
Web of Science
#24 #23 AND #22 #23 Topic=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) #22 #21 AND #9 #21 #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 #20 Topic=(hyperhomocyst$in$emi*) #19 Topic=((cardiovascular near/2 (disease* or event*))) #18 Topic=(apoplexy) #17 Topic=((brain near/3 infarct*)) #16 Topic=((cerebr* near/3 (accident* or infarct*))) #15 Topic=((stroke or strokes)) #14 Topic=(heart attack*) #13 Topic=(heart infarct*) #12 Topic=(myocardial infarct*) #11 Topic=(angina) #10 Topic=((coronary near/3 disease*)) #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 Topic=(pyridoxol*) #7 Topic=(cyanocobalamin*) #6 Topic=(cobalamin*) #5 Topic=(pyridoxin*) #4 Topic=(((homocystein*) near/3 (low$ or reduc*))) OR Topic=(((homocystin*) near/3 (low or reduc*))) #3 Topic=(folate*) #2 Topic=("folic acid") #1 Topic=("vitamin b")
Appendix 3. Search strategies 2014
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees #2 (vitamin b) #3 folic acid #4 folate* #5 ((homocystein* or homocystin*) near/3 (low* or reduc*)) #6 (pyridoxin*) #7 (cobalamin*) #8 (cyanocobalamin*) #9 (pyridoxol*) #10 MeSH descriptor Vitamins, this term only #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Cardiovascular Diseases, this term only #13 MeSH descriptor Myocardial Ischemia explode all trees #14 MeSH descriptor Brain Ischemia explode all trees #15 MeSH descriptor Cerebrovascular Disorders, this term only #16 (coronary near/3 disease*) #17 (angina) #18 (myocardial infarct*) #19 (heart infarct*) #20 (heart attack*) #21 (stroke or strokes) #22 (cerebr* near/3 (accident* or infarct*)) #23 (brain near/3 infarct*) #24 (apoplexy) #25 (cardiovascular near/2 (disease* or event*)) #26 MeSH descriptor Hyperhomocysteinemia, this term only #27 hyperhomocyst?in?emi* #28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29 (#11 AND #28)
MEDLINE
1 exp Vitamin B Complex/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp Myocardial Ischemia/ 14 exp Brain Ischemia/ 15 Cerebrovascular Disorders/ 16 (coronary adj3 disease$).tw. 17 angina.tw. 18 myocardial infarct$.tw. 19 heart infarct$.tw. 20 heart attack$.tw. 21 (stroke or strokes).tw. 22 (cerebr$ adj3 (accident$ or infarct$)).tw. 23 (brain adj3 infarct$).tw. 24 apoplexy.tw. 25 (cardiovascular adj2 (disease$ or event$)).tw. 26 Hyperhomocysteinemia/ 27 hyperhomocyst?in?emi$.tw. 28 or/12‐27 29 11 and 28 30 randomized controlled trial.pt. 31 controlled clinical trial.pt. 32 randomized.ab. 33 placebo.ab. 34 drug therapy.fs. 35 randomly.ab. 36 trial.ab. 37 groups.ab. 38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39 exp animals/ not humans.sh. (3663238) 40 38 not 39 41 29 and 40 42 (2012* or 2013* or 2014*).ed. 43 41 and 42
Embase
1 exp Vitamin B Complex/ 2 vitamin b.tw. 3 folic acid.tw. 4 folate$.tw. 5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. 6 pyridoxin$.tw. 7 cobalamin$.tw. 8 cyanocobalamin$.tw. 9 pyridoxol$.tw. 10 Vitamins/ 11 or/1‐10 12 Cardiovascular Diseases/ 13 exp Myocardial Ischemia/ 14 exp Brain Ischemia/ 15 Cerebrovascular Disorders/ 16 (coronary adj3 disease$).tw. 17 angina.tw. 18 myocardial infarct$.tw. 19 heart infarct$.tw. 20 heart attack$.tw. 21 (stroke or strokes).tw. 22 (cerebr$ adj3 (accident$ or infarct$)).tw. 23 (brain adj3 infarct$).tw. 24 apoplexy.tw. 25 (cardiovascular adj2 (disease$ or event$)).tw. 26 Hyperhomocysteinemia/ 27 hyperhomocyst?in?emi$.tw. 28 or/12‐27 29 11 and 28 30 random$.tw. 31 factorial$.tw. 32 crossover$.tw. 33 cross over$.tw. 34 cross‐over$.tw. 35 placebo$.tw. 36 (doubl$ adj blind$).tw. 37 (singl$ adj blind$).tw. 38 assign$.tw. 39 allocat$.tw. 40 volunteer$.tw. 41 crossover procedure/ 42 double blind procedure/ 43 randomized controlled trial/ 44 single blind procedure/ 45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 46 (animal/ or nonhuman/) not human/ 47 45 not 46 48 29 and 47 49 (2012* or 2013* or 2014*).dd. 50 48 and 49
Web of Science
#24 #23 AND #22 #23 Topic=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) #22 #21 AND #9 #21 #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 #20 Topic=(hyperhomocyst$in$emi*) #19 Topic=((cardiovascular near/2 (disease* or event*))) #18 Topic=(apoplexy) #17 Topic=((brain near/3 infarct*)) #16 Topic=((cerebr* near/3 (accident* or infarct*))) #15 Topic=((stroke or strokes)) #14 Topic=(heart attack*) #13 Topic=(heart infarct*) #12 Topic=(myocardial infarct*) #11 Topic=(angina) #10 Topic=((coronary near/3 disease*)) #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 Topic=(pyridoxol*) #7 Topic=(cyanocobalamin*) #6 Topic=(cobalamin*) #5 Topic=(pyridoxin*) #4 Topic=(((homocystein*) near/3 (low$ or reduc*))) OR Topic=(((homocystin*) near/3 (low or reduc*))) #3 Topic=(folate*) #2 Topic=("folic acid") #1 Topic=("vitamin b")
Appendix 4. Search strategies 2017
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 (vitamin b)
#3 folic acid
#4 folate*
#5 ((homocystein* or homocystin*) near/3 (low* or reduc*))
#6 (pyridoxin*)
#7 (cobalamin*)
#8 (cyanocobalamin*)
#9 (pyridoxol*)
#10 MeSH descriptor Vitamins, this term only
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Cardiovascular Diseases, this term only
#13 MeSH descriptor Myocardial Ischemia explode all trees
#14 MeSH descriptor Brain Ischemia explode all trees
#15 MeSH descriptor Cerebrovascular Disorders, this term only
#16 (coronary near/3 disease*)
#17 (angina)
#18 (myocardial infarct*)
#19 (heart infarct*)
#20 (heart attack*)
#21 (stroke or strokes)
#22 (cerebr* near/3 (accident* or infarct*))
#23 (brain near/3 infarct*)
#24 (apoplexy)
#25 (cardiovascular near/2 (disease* or event*))
#26 MeSH descriptor Hyperhomocysteinemia, this term only
#27 hyperhomocyst?in?emi*
#28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 (#11 AND #28)
MEDLINE OVID
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 exp animals/ not humans.sh. (3663238)
40 38 not 39
41 29 and 40
42 (2014* or 2015* or 2016*).ed.
43 41 and 42
Embase OVID
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 crossover procedure/
42 double blind procedure/
43 randomized controlled trial/
44 single blind procedure/
45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
49 (2014* or 2015* or 2016*).dd.
50 48 and 49
Web of Science
#26 #25 AND #24
#25 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#24 #23 AND #10
#23 #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11
#22 TS=(hyperhomocystein$emi*)
#21 TS=(hyperhomocystin$emi*)
#20 TS=(cardiovascular near/2 (disease* or event*))
#19 TS=(apoplexy)
#18 TS=((brain near/3 infarct*))
#17 TS=((cerebr* near/3 (accident* or infarct*)))
#16 TS=((stroke or strokes))
#15 TS=(heart attack*)
#14 TS=(heart infarct*)
#13 TS=(myocardial infarct*)
#12 TS=(angina)
#11 TS=((coronary near/3 disease*))
#10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#9 TS=(pyridoxol*)
#8 TS=(cyanocobalamin*)
#7 TS=(cobalamin*)
#6 TS=(pyridoxin*)
#5 TS=((homocystin*) near/3 (low or reduc*))
#4 TS=((homocystein*) near/3 (low$ or reduc*))
#3 TS=(folate*)
#2 TS=("folic acid")
#1 TS=("vitamin b")
LILACS (accessed through Biblioteca Virtual em Saúde)
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and MH Vitamina B 12 OR Cobamidas OR Hidroxocobalamina OR Complejo Vitamínico B OR Ácido Fólico OR Ácidos Pteroilpoliglutámicos OR Tetrahidrofolatos OR Formiltetrahidrofolatos OR Vitamina B 6 OR Piridoxal OR Fosfato de Piridoxal OR Piridoxamina OR Piridoxina OR Homocisteína OR Vitaminas or TW vitamin$ or tw cobalamin$ or tw cianocobalamin$ or tw cyanocobalam$ or tw cobamid$ or tw hidroxocobalam$ or tw Hydroxocobalam$ or ((tw complejo or tw complex$) and tw vitamin$ and tw b) or (tw acid$ and (tw folic$ or tw ptero$)) or tw Tetrahidrofolatos or tw Formiltetrahidrofolatos or (tw vitamin$ or (tw b or tw b6 or tw b12)) or tw Piridoxal or tw Pyridoxal or ((tw Fosfat$ or tw phosphate$) and (tw Piridoxal or tw pyridoxal)) or tw Piridox$ or tw Pyridox$ or tw Homocisteína or tw Homocysteine) AND (MH Enfermedades Cardiovasculares or Isquemia Miocárdica or Ex C14.280.647$ or Isquemia Encefálica or Ex C10.228.140.300.150$ or Trastornos Cerebrovasculares or hiperhomocisteinemia or Accidente Cerebrovascular or ((tw apoplexia or tw derrame or tw trastorno$ or tw accident$ or tw acidente or tw stroke$ or tw disease$ or tw enfermedad$ or tw doenca$ or tw event$ or tw infart$ or tw isquemia or tw disorder$) and (tw miocardio or tw myocard$ or tw cerebr$ or tw cardiovascul$ or tw heart or tw cardiovascul$ or tw encefal$)) or tw hyperhomocyst$ or tw hiperhomocisteinemia) [Palavras]
Appendix 5. Domains for assessing of risk of bias in included studies
Generation of the allocation sequence
Low risk of bias, if the allocation sequence was generated by a computer or random number table, drawing of lots, tossing of a coin, shuffling of cards or throwing dice.
Unclear, if the trial was described as randomised but the method used for the allocation sequence generation was not described.
High risk of bias, if a system involving dates, names or admittance numbers was used for the allocation of patients. These studies are known as quasi‐randomised and we excluded them from the review when assessing beneficial effects.
Allocation concealment
Low risk of bias, if the allocation of patients involved a central independent unit, on‐site locked computer, identical‐appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised but the method used to conceal the allocation was not described.
High risk of bias, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. We excluded the latter from the review when assessing beneficial effects.
Blinding (or masking)
We assessed each trial (as low, unclear or high risk) with regard to the following levels of blinding.
Blinding of clinician (person delivering treatment) to treatment allocation.
Blinding of participant to treatment allocation.
Blinding of outcome assessor to treatment allocation.
Incomplete outcome data
Low risk of bias, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals but this was not specifically stated.
High risk of bias, if the number or reasons for dropouts and withdrawals were not described.
We further examined the percentage of dropouts overall in each trial and per randomisation arm and we evaluated whether intention‐to‐treat analysis was performed or could be performed from the published information.
Selective outcome reporting
Low risk of bias, if pre‐defined or clinically relevant and reasonably expected outcomes were reported on.
Unclear, if not all pre‐defined or clinically relevant and reasonably expected outcomes were reported on or were not reported on fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias, if one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Other bias
Low risk of bias, the trial appeared to be free of other components that could put it at risk of bias.
Unclear, the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias, there were other factors in the trial that could put it at risk of bias.
Overall risk of bias
We considered studies to have an overall low risk of bias if they did not have high risk of bias in any of six individual domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data or selective reporting), and if a definitive risk of bias assessment could be made for the majority (at least five of six) of domains. We did not include ‘Other bias’ in our overall assessment.
Appendix 6. Definitions of myocardial infarction (MI), stroke, unstable angina and death
| Trial | Myocardial infarction | Stroke | Angina pectoris | Death | |
| B‐PROOF 2015 | Not available | Not available | Not available | Not available | |
| BVAIT 2009 | Not available | Not available | Not available | ||
| CSPPT 2015 | Criteria for ischaemic symptoms or corresponding electrocardiographic changes plus evidence of myocardial damage. | Medical records and imaging data | Not measured | Evidence for death included death certificates from hospitals or reports of home visit by investigators | |
| HOPE‐2 2006 | 2 of the following 3 criteria were met: typical symptoms, increased cardiac‐enzyme levels and diagnostic electrocardiographic changes. | Focal neurologic deficit lasting more than 24 hours. Computed tomography or magnetic resonance imaging was recommended to identify the type of stroke (ischaemic or haemorrhagic). When these tools were not available, the stroke was classified as of uncertain type | Not available | Cardiovascular causes were unexpected deaths presumed to be due to ischaemic cardiovascular disease and occurring within 24 hours after the onset of symptoms without clinical or postmortem evidence of another cause, deaths from myocardial infarction or stroke within 7 days after the event, deaths associated with cardiovascular interventions within 30 days after cardiovascular surgery or within 7 days after percutaneous interventions, and deaths from congestive heart failure, arrhythmia, pulmonary embolism or ruptured aortic aneurysm. Deaths from uncertain causes were presumed to be due to cardiovascular causes | Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined ‐ a consensus document of the joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959‐69. [Erratum, J Am Coll Cardiol 2001;37:973.]: source not available |
| Li 2015a | Not measured | Not available | Not measured | Not measured | ‐ |
| NORVIT 2006 | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | Definitions are too long to summarise in this table |
| SEARCH 2010 |
https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 |
https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 |
https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 |
https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 |
Definitions are too long to summarise in this table |
| SU.FOL.OM3 2010 | Myocardial infarction (ICD‐10 (International Classification of Diseases, 10th revision) codes I21.0–I21.9) was defined on the basis of 2 or more of the criteria: typical chest pain, electrocardiographic changes consistent with myocardial infarction and cardiac enzyme increase | An acute cerebral ischaemic event was defined as an ischaemic cerebrovascular accident based on clinical criteria confirmed by computed tomography or magnetic resonance imaging and a Rankin score 3 at inclusion (ICD‐10 codes I63.0–I63.9) | Acute coronary syndrome without myocardial infarction (ICD‐10 codes I20.0–I20.1) was initially defined by the presence of 3 criteria: typical chest pain, electrocardiographic changes consistent with coronary artery disease without myocardial infarction and evidence of coronary artery disease (myocardial infarction, angina with angiographic evidence of stenosis > 50% in one or more coronary arteries, or angina pectoris corroborated by coronary angiography or exercise testing, or coronary angioplasty or coronary artery bypass graft procedure). Suspected acute coronary syndrome without characteristic electrocardiographic evidence of myocardial infarction provided there was angiographic evidence of coronary artery disease | ||
| VISP 2004 | New ECG changes including Q waves or marked ST‐T changes plus abnormal cardiac enzymes, cardiac symptoms plus abnormal enzymes or symptoms plus hyperacute ECG changes resolving with thrombolysis | Evidence of sudden onset of focal neurologic deficit lasting at least 24 hours accompanied by an increased NIHSS Score in an area that was previously normal. When the sudden onset of symptoms lasting at least 24 hours was not accompanied by an increased NIHSS Score in an area that was previously normal, then recurrent stroke was diagnosed using cranial CT or MRI evidence of new infarction consistent with the clinical presentation | Not available | Not available | |
| WAFACS 2008 | According to World Health Organization criteria | A new neurologic deficit of sudden onset that persisted for more than 24 hours or until death within 24 hours | Not available | Death due to cardiovascular disease was confirmed by examinations of autopsy reports, death certificates, medical records and information obtained from the next kin or other family members. Death from any cause was confirmed by the endpoint committee on the basis of a death certificate | |
| WENBIT 2008 | According to the Joint European Society of Cardiology/American College of Cardiology Committee. Eur Heart J. 2000;21:1502‐13 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38:2114‐30 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001; 38:2114‐30 | If death occurred within 28 days after the onset of an event, the event was classified as fatal |
Data and analyses
Comparison 1. Homocysteine‐lowering treatment versus other (any comparisons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Myocardial infarction | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Homocysteine‐lowering versus placebo | 12 | 46699 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 1.1.2 Homocysteine‐lowering treatment at high dose versus low dose | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| 1.1.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.82] |
| 1.2 Stroke | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Homocysteine‐lowering treatment versus placebo | 10 | 44224 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 1.2.2 Homocysteine‐lowering treatment at high dose versus low dose | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 1.2.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 1.3 First unstable angina pectoris episode requiring hospitalisation | 4 | 12644 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.21] |
| 1.4 Death from any cause | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Homocysteine‐lowering treatment versus placebo | 11 | 44817 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 1.4.2 Homocysteine‐lowering treatments at high dose versus low dose | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.11] |
| 1.4.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 1.5 Serious adverse events (cancer) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Homocysteine‐lowering versus placebo | 8 | 35788 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.14] |
| 1.5.2 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20243 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.71, 1.31] |
| 1.6 Adverse events (serious and non‐serious) excluding cancer | 3 | 13802 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| 1.6.1 Homocysteine‐lowering versus placebo | 3 | 13802 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
Comparison 2. Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Myocardial infarction | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 2.1.1 Trials with low risk of bias | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 2.2 Stroke | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 2.2.1 Trials with low risk of bias | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 2.3 Death from any cause | 7 | 37932 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 2.3.1 Trials with low risk of bias | 7 | 37932 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
Comparison 3. Homocysteine‐lowering treatment versus placebo (Subgoup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Myocardial Infarction | 12 | 46699 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 3.1.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.87] |
| 3.1.2 With history of cardiovascular disease | 11 | 46209 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 3.2 Stroke | 10 | 44224 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 3.2.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.04] |
| 3.2.2 With history of cardiovascular disease | 9 | 43734 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 3.3 Death | 11 | 44817 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 3.3.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.04] |
| 3.3.2 With history of cardiovascular disease | 10 | 44327 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
Comparison 4. Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.82] |
| 4.1.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.27, 0.68] |
| 4.1.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [1.48, 3.83] |
| 4.2 Stroke | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 4.2.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 4.2.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 4.3 Death from any cause | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 4.3.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.99] |
| 4.3.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.21] |
Comparison 5. Homocysteine‐lowering treatment at high dose versus low dose (Subgoup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Stroke | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 5.1.1 Combined (folic acid, vitamin B6 and vitamin B12) | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 5.1.2 Folic acid alone | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
Comparison 6. Homocysteine‐lowering treatment (high dose) versus Homocysteine‐lowering treatment (low dose) (Sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Stroke | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 6.1.1 Trials with low risk of bias | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 6.1.2 Trials with high risk of bias | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
6.1. Analysis.

Comparison 6: Homocysteine‐lowering treatment (high dose) versus Homocysteine‐lowering treatment (low dose) (Sensitivity analysis), Outcome 1: Stroke
Characteristics of studies
Characteristics of included studies [ordered by study ID]
B‐PROOF 2015.
| Study characteristics | ||
| Methods | Parallel design (2 arms Multicentre study: yes (3 centres) Country: The Netherlands Follow‐up period (years): 2 |
|
| Participants |
Randomised: 2919 (Original group of 'B‐vitamins for the PRevention Of Osteoporotic Fractures')
The following data belong to subgroup analysis on cardiovascular events (named as 'Vascular subgroup' by trial authors). Randomised: 569
Age (Mean SD):
Gender (male %):
Homocysteine levels at baseline (median interquartile range ) (μmol/L):
Self‐reported cardiovascular medical history (intervention versus placebo):
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Treatment duration: two years |
|
| Outcomes | Outcomes related to vascular subgroup analysis:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The random allocation sequence and randomization were generated and performed using SAS 9.2 by an independent research dietician." (Page 402) |
| Allocation concealment (selection bias) | Low risk | Quote "The random allocation sequence and randomization were generated and performed using SAS 9.2 by an independent research dietician." (Page 402) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Intervention and placebo tablets were indistinguishable in taste, smell, and appearance. Both the participants and all researchers and research assistants were blinded to the study treatment" (Page 402) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information on blinding of outcome assessment to judge as 'high' or 'low' risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up: Experimental group: 94.8% (260/274) Control: 80.3% (237/295) Imbalance between comparison groups: 14.5% |
| Selective reporting (reporting bias) | Low risk | It is clear that the published reports relevant clinical outcomes |
| Other bias | Low risk | Other sources of bias not identified |
BVAIT 2009.
| Study characteristics | ||
| Methods | Parallel design Multicentre study: yes Country: USA Follow‐up period (years): B vitamins group (3.14 (0.48 to 4.56) versus placebo group (3.07 (0.46 to 5.0)) |
|
| Participants |
Eligibility: 5309 Randomised: 506 (254 vitamins versus 252 placebo) Age (years):
Gender (men):
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary: Rate of change in the right distal carotid artery intima media thickness Secondary: Changes in calcium in the coronary arteries and abdominal aorta Safety:
|
|
| Notes |
We sent an email to the main author of this trial in order to get the type cardiovascular event data by comparison group (4 March 2012) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random numbers were used to assign participants" (page 731) |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated random numbers were used to assign participants" (page 731) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, clinical staff, imaging specialists, and data monitors were masked to treatment assignment." (page 731) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...imaging specialists, ... were masked to treatment assignment." (page 731). & "Scans were analyzed without knowledge of treatment assignment using validated calcium scoring software" (for secondary outcome)" (page 731) Comments: the main outcomes were to assess the impact of the HLI on reduction of subclinical atherosclerosis progression |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
B vitamins group
Placebo group
Evaluable included in analysis:
Completed the initially planned (2.5‐year trial period): 8.1% (446/506): (88% (223/446) B vitamin; 88% (223/446) placebo) |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. We also checked www.clinicaltrials.gov and the ID number was: NCT00114400 |
| Other bias | Low risk | Other sources of bias not identified |
CHAOS 2002.
| Study characteristics | ||
| Methods | Parallel design Multicentre study Follow‐up period: mean of 1.7 years |
|
| Participants |
Randomised: 1882 participants randomised (folic acid: 942 versus placebo: 940 participants) Gender: not reported Age: not reported Homocysteine levels at baseline: (treatment group) (μmol/L): 11.2 (6.9 μmol/L) Inclusion criteria: (1 of the following):
Exclusion criteria: not reported |
|
| Interventions |
Treatment duration: 2 years |
|
| Outcomes | Composite outcome: MI, revascularisation, death from cardiovascular cause | |
| Notes |
Homocysteine levels were only collected in 2 participating centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' Data not yet fully published. Results in the table correspond to conference proceedings |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' Data not yet fully published. Results in the table correspond to conference proceedings |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded. However, the information was obtained from the final report (abstract) Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded. However, the information was obtained from the final report (abstract) Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of participants during trial was not reported. Data not yet fully published. Results in the table correspond to conference proceedings |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
CSPPT 2015.
| Study characteristics | ||
| Methods | Parallel design Multicentre study (32 communities in Jiangsu and Anhui provinces in China) Country: China Run‐in treatment: three weeks taken enalapril (10 mg) oral daily. Follow‐up period: 5 year |
|
| Participants |
Randomised: 20,702 adults with hypertension without history of stroke or MI
Age, mean (SD) years:
Gender (male):
Homocysteine, median (IQR), μmol/L:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Treatment duration (median 4.5 years) |
|
| Outcomes |
Primary:
Secondary:
Other outcome measures (Source:NCT00794885)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization was performed centrally by means of 4 randomization tables: 1was a randomization of drug code and treatment allocation, and the other 3 were MTHFR C677T genotype–specific randomized sequences with a fixed block size of 4." |
| Allocation concealment (selection bias) | Unclear risk | Quote "All study investigators and participants were blinded to the randomization procedure and the treatment assignments." Insufficient information to permit judgement of 'Low risk' or 'High risk' Trial authors did not describe procedure to guarantee an adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Both types of tablets were concealed in a single capsule formulation and were identical in appearance, size, color, and taste" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "Both types of tablets were concealed in a single capsule formulation and were identical in appearance, size, color, and taste" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Lost to follow‐up:
Trial authors described reasons |
| Selective reporting (reporting bias) | Low risk | No bias identified |
| Other bias | High risk | Industry bias |
FOLARDA 2004.
| Study characteristics | ||
| Methods | Parallel design Multicentre study Country: The Netherlands Follow‐up period: 1 year |
|
| Participants |
Randomised: 283 randomised participants (folic acid: 140 versus standard care: 143) Gender (% men): folic acid: 69% versus standard care: 70% Age (mean): folic acid: 59 years versus standard care: 59 Homocysteine levels at baseline: not reported Inclusion criteria (1 of the following):
Exclusion criteria:
|
|
| Interventions |
Intervention: Folic acid: 5 mg per day Treatment was initiated at least 1 day prior to hospital discharge, and no later of 14 days after the MI. The treatment continued for 1 year. participants in this group also received statin therapy (fluvastatin, 40 mg per day). The clinician had at their discretion the prescription of additional prophylactic medication (aspirin, beta‐blocking agents and/or ACE inhibitors) Control: Standard care: statin therapy (fluvastatin, 40 mg per day). The clinician had at their discretion the prescription of additional prophylactic medication (aspirin, beta‐blocking agents and/or ACE inhibitors) Treatment duration: 1 year |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised..." Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "... treatment with open label folic acid [...] or not" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An Independent Data and Safety Monitoring Committee adjudicated all major clinical events." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23 participants discontinued treatment and no information is given |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
GOES 2003.
| Study characteristics | ||
| Methods | Parallel design Single‐centre study Country: The Netherlands Follow‐up period: 1 year |
|
| Participants |
Rndomised: 593 randomised participants (folic acid: 300 versus standard care: 293) Gender (% men): folic acid: 76% versus standard care: 80% Age (mean SD): folic acid: 64.9 (9.9) versus standard care: 65.5 (9.7) Homocysteine levels at baseline: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary (composite):
Secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer program randomly allocated patients [...] to treatment" |
| Allocation concealment (selection bias) | Unclear risk | No information reported about this domain |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "... treatment with open label folic acid [...] or standard care." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Adjudication of all clinical events was performed by an independent end point monitoring committee unaware of treatment arm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, 12 patients per group withdrew from the study but were followed up and included in the final analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
HOPE‐2 2006.
| Study characteristics | ||
| Methods | Parallel design Multicentre international study (13 countries; 145 centres) Follow‐up period: 5 years |
|
| Participants |
Randomised: 5522 patients randomised (vitamin: 2758 versus placebo group: 2764 patients) Gender (% men): vitamin: 71.1% versus placebo: 72.4% Age (mean SD): vitamin: 68.8 (7.1) versus placebo: 68.9 ( 6.8) Homocysteine level at baseline: 12.2 μmol/L (1.6 mg/L) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control:
Treatment duration: 5 years |
|
| Outcomes |
Primary outcome (composite):
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study used central telephone randomization" |
| Allocation concealment (selection bias) | Low risk | Centralised telephone randomisation (accessible 24 hours a day) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study investigators, personnel, and participants were unaware of the randomization procedure and the treatment assignments." Vitamins manufactured to be indistinguishable in colour, weight or ability to be dissolved in water |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This trial assessed objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21 patients in the treatment group and 16 in the placebo group did not complete the study Vital status known for 99.3% of the sample |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Other sources of bias not identified |
Li 2015a.
| Study characteristics | ||
| Methods | Parallel design Multicentre study: not stated Country: China Follow‐up period: 5 years |
|
| Participants |
Age: ≥ 65 year Gender: only female. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised Insufficient information about the random sequence generation to permit judgement of 'Low risk' or 'High risk' Data not yet fully published. Results in the table correspond to conference proceedings |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised Insufficient information about the allocation concealment to permit judgement of 'Low risk' or 'High risk' Data not yet fully published. Results in the table correspond to conference proceedings |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk' |
| Selective reporting (reporting bias) | High risk | Trial reported no information clinical relevant outcomes such as MI, mortality or harms. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
NORVIT 2006.
| Study characteristics | ||
| Methods | Parallel design Multicentre study Country: Norway Follow‐up period: 3.5 years |
|
| Participants |
Randomised: 3749 patients randomised (folic acid, vitamins B6 and B12: 937 versus folic acid, vitamin B12: 935 versus vitamin B6: 934 versus placebo: 943) Gender (% men): Folic acid, vitamins B6 and B12: 73% Folic acid, vitamin B12: 74% Vitamin B6: 73% Placebo: 75% Age (mean SD, years): Folic acid, vitamins B6 and B12: 63.6 (11.9) Folic acid, vitamin B12: 63.2 (11.6) Vitamin B6: 62.5 (11.7) Placebo: 62.6 (11.4) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control: placebo Medication was delivered in single capsules taken once per day. For the first 2 weeks after study entry patients in groups 1 and 2 received an additional folic acid dose (5 mg) per day, whereas the other 2 groups received placebo Treatment duration: not clearly described |
|
| Outcomes |
Primary outcome (composite):
Secondary outcomes:
Incident cases of cancer |
|
| Notes |
The calculation of the sample size was based on data from previous Scandinavian trials, assuming the 3‐year rate of the primary endpoint would be 25% in the placebo group. The planned enrolment of 3500 patients, with an average follow‐up of 3.0 years, was expected to result in 750 primary events and give the study statistical power of more than 90% to detect a 20% relative reduction in the rate of the primary endpoint, given a 2‐sided alpha value of 0.05
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information reported about this domain |
| Allocation concealment (selection bias) | Low risk | The manufacturer provided central study sites with blocks of medication assigned in numerical order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel and participants were unaware of the treatment assignments Vitamins were manufactured to be indistinguishable in colour, weight or ability to be dissolved in water |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All end points were adjudicated by members of the end‐points committee, who were unaware of patients’ treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of patients stopped the medication 94% attended the final visit, but data on mortality were available for the entire sample. Incomplete outcome data for 20 patients Patients that had not completed the planned follow‐up were followed up by phone or consulted for vital status |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Other sources of bias not identified |
SEARCH 2010.
| Study characteristics | ||
| Methods | Parallel design Multicentre study (88 sites) Country: UK Follow‐up period: 6.7 ± 1.5 person‐years |
|
| Participants | Clinical condition: survivors of MI in secondary care hospitals
Gender (% men): Men: 10,012 Women: 2052
Age (at randomisation): Mean (SD) age of 64.2 (8.9) years Folic acid and vitamin B12:
Placebo:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Both medications were supplied in specially prepared calendar packs (and, separately, using a 2 x 2 factorial design, either 80 mg or 20 mg simvastatin daily) |
|
| Outcomes |
Primary outcome (composite):
Secondary outcomes:
Tertiary outcomes:
|
|
| Notes | Identifier: ISRCTN 74348595 Reason for a pre‐randomisation run‐in phase: to limit subsequent randomisation to those likely to take the randomly allocated study treatment for several years (page 2487) Conducted between September 1998 and June 2008 A priori sample estimation: yes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The central telephone randomization system used a minimization algorithm to balance the treatment groups with respect to major prognostic factors." (page 2487) |
| Allocation concealment (selection bias) | Low risk | Quote: "The central telephone randomization system used a minimization algorithm to balance the treatment groups with respect to major prognostic factors." (page 2487) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " All such information was reviewed by coordinating center clinicians who were unaware of the study treatment allocation and events coded according to prespecified criteria" (page 2487) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vitamin group: 98.9% (5970/6033) completed follow‐up Placebo group: 99.1% (5975/6031) completed follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | Other sources of bias not identified |
SU.FOL.OM3 2010.
| Study characteristics | ||
| Methods | Parallel design Multicentre study (257 sites) Country: France Follow‐up period: median: 4.7 years; mean 4.2 (1.0) years |
|
| Participants | Clinical condition: patients with a history of ischaemic heart disease or stroke
Gender (% men): Men: 1987 Women: 514
Age: Mean (SD) age of 60.9 (8.8) years.
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Furthermore: supplement containing omega 3 polyunsaturated fatty acids (600 mg of eicosapentaenoic acid and docosahexaenoic acid at a ratio of 2:1) |
|
| Outcomes |
Primary outcome (composite):
Secondary outcomes:
|
|
| Notes | Identifier: ISRCTN 41926726 Conducted between 1 February 2003 and 1 June 2007 A priori sample estimation: yes
Comment: assumptions were based on Galan et al (HSC 2002; SU.FOL.OM3 2010; Yusuf 2000)
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by means of a computerised block sequence stratified by three age groups (44 – 54, 55 – 64, and 65 – 80 years), sex, prior disease at enrolment (myocardial infarction, acute coronary syndrome, or ischaemic stroke) and recruitment centre. Permuted block randomisation (with block size randomly selected as 8) was used." (page 2) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by means of a computerised block sequence stratified by three age groups (44 – 54, 55 – 64, and 65 – 80 years), sex, prior disease at enrolment (myocardial infarction, acute coronary syndrome, or ischaemic stroke) and recruitment centre. Permuted block randomisation (with block size randomly selected as 8) was used." (page 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, clinicians, trial coordinators, and outcome investigators were blinded to treatment allocation." (page 2) Quote: "treatment capsules for one year (and repeated yearly) in an appropriately labelled package." (page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "... and outcome investigators were blinded to treatment allocation." (page 2) Quote: "All events were adjudicated by two independent committees of cardiologists or neurologists who were blinded to treatment allocation." (page 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comments: reasons for losses were reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have reported in the pre‐specified way. "This study is registered with Current Controlled Trials (No ISRCTN41926726" (page 3) |
| Other bias | Low risk | Other sources of bias not identified |
VISP 2004.
| Study characteristics | ||
| Methods | Parallel design Country: USA, Canada and Scotland Multicentre international study Follow‐up period: 2 years |
|
| Participants | 3680 randomised (high‐dose: 1827 versus low‐dose: 1853) Age (mean SD): high‐dose: 66.4 (10.8) versus low‐dose: 66.2 (10.8) Gender (% men): high‐dose: 62.3% versus low‐dose: 62.8% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
High‐dose multivitamin therapy 2.5 mg folic acid; 0.4 mg vitamin B12; 25 mg vitamin B6 per day Low‐dose multivitamin therapy 20 micrograms folic acid; 6 micrograms vitamin B12; 200 micrograms vitamin B6 per day Co‐interventions: 1. Risk factor control education 2. Aspirin (325 mg/day) Duration of treatment: not described |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation of participants was programmed by the statistical co‐ordinating centre, encrypted and entered into a data entry program installed on a study computer at each site |
| Allocation concealment (selection bias) | Low risk | Allocation programmed by the statistical co‐ordinating centre. All the information on assignment were encrypted an entered in computers in study sites After verification of eligibility participants were assigned in 1 of 20 medication codes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The drug distributor centre bottled and distributed the vitamins, which were manufactured to be indistinguishable in colour, weight or ability to be dissolved in water |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary endpoint was reviewed by a local neurologist and 2 external independent review neurologists |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 132 patients in the low‐dose group and 133 in the high‐dose group were lost to follow‐up. Of these 18 and 13 patients respectively had no contact after randomisation, and were not included in the analysis. 186 patients in the low‐dose group and 179 in the high‐dose group discontinued the assigned treatment Patients who had not completed the planned follow‐up were invited to an exit visit |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes,including those that were pre‐specified |
| Other bias | Low risk | Other sources of bias not identified |
VITATOPS 2010.
| Study characteristics | ||
| Methods | Parallel design Multicentre study: 123 medical centres (20 countries) from 4 continents Follow‐up period (median and interquartile range, years): 3.4 (2.0 to 5.5) |
|
| Participants | 8164 randomised
4089 received folic acid and vitamins B (B6 and B12)
4075 received placebo Age (mean SD years):
Gender (men):
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control: placebo Co‐interventions: not reported |
|
| Outcomes |
Primary outcome (composite): whichever occurred first
Secondary outcomes:
|
|
| Notes | Identifier numbers: NCT00097669 and ISRCTN74743444 Date of study: 19 November 1998 to 31 December 2008
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation was done by use of a central 24 hrs telephone service or an interactive website by use of random permuted blocks stratified by hospital" (page 856) |
| Allocation concealment (selection bias) | Low risk | Quote: "Random allocation was done by use of a central 24 hrs telephone service or an interactive website by use of random permuted blocks stratified by hospital" (page 856) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, clinicians, trial coordinators, and outcome investigators were masked to treatment allocation" (page 856) Quote: "had the same colour and coating" (page 856) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...and outcome investigators were masked to treatment allocation" (page 856) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to final follow‐up:
Global: 8.6% (702/8164)
B vitamins group: 8.5% (348/4089)
Placebo group: 8.7% (354/4075) Comment: reasons for losses were reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that published reports include all expect outcomes, including those that were pre‐specified. This trial is registered with ClinicalTrials.gov, NCT00097669 and Current Controlled Trials, ISRCTN74743444." (page 858) |
| Other bias | Low risk | Other sources of bias not identified |
WAFACS 2008.
| Study characteristics | ||
| Methods | Parallel design Multicentre study Country: USA Follow‐up period: 7.3 years |
|
| Participants | N: 5442 randomised patients (vitamin group: 2721 patients; placebo group: 2721 patients) Gender: women health professionals Age (mean (SD)) years: Active group: 62.8 (8.8) Control group: 62.8 (8.8) Inclusion criteria
Exclusion criteria:
|
|
| Interventions |
Intervention: Folic acid: 2.5 mg; vitamin B12: 1 mg; vitamin B6: 50 mg per day Control: Matching placebo per day Co‐interventions: vitamin C, vitamin E, ß‐carotene Treatment duration: not clearly reported |
|
| Outcomes |
Primary (composite):
Secondary:
|
|
| Notes |
The WACS study was a 2 x 2 x 2 factorial trial of 3 antioxidants, vitamins C, E and beta‐carotene. Randomisation of the 8171 participants into the 8 treatment groups took place from June 1995 to October 1996, and was conducted using blocks of size 16 within 5‐year age groups. The folate/B6/B12 arm was added in April 1998, and the 5442 participants who were willing and eligible were randomised (at one time) using blocks of size 8 within strata defined by age and the other treatment arms. Participants were sent yearly supplies of calendar packs containing the study medications or matching placebo pills that were identical in appearance. All medical records were reviewed by an Endpoints Committee that was blinded to treatment assignment
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with a block size of 8 generated by computer, stratified by age |
| Allocation concealment (selection bias) | Low risk | Central randomisation. Patients were sent yearly supplies of calendar packs containing their medication or matching placebos identical in appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study investigators, personnel and participants were unaware of the participants' treatment assignments
Patients were sent packs containing medication or matching placebos identical in appearance An independent committee monitored the "safety and overall quality and scientific integrity" of the trial, which was blinded to treatment assignment All the information was supplied by Nancy Cook (WACS statistician, 23 June 2008) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent committee monitored the "safety and overall quality and scientific integrity" of the trial, which was blinded to treatment assignment
All the information was supplied by Nancy Cook (WACS statistician, 23 June 2008) Comments: this trial had objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown vital status for 194 patients in the folic acid group and 207 patients in the placebo group. All the patients were included in the primary analysis, but how was not described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Other sources of bias not identified |
WENBIT 2008.
| Study characteristics | ||
| Methods | Parallel design Multicentre study Country: Norway Follow‐up period: 4 years |
|
| Participants | 3096 patients randomised (folic acid, vitamins B6 and B12: 772 versus folic acid, vitamin B12: 772 versus vitamin B6: 772 versus placebo: 780) Gender (% men):
Age (mean SD, years):
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control: placebo Co‐interventions: statins, insulin, aspirin, clopidogrel, beta‐blockers, ACE inhibitors/ARBs, calcium channel blockers, loop diuretics, oral antidiabetics, medication for chronic obstructive pulmonary disease Duration of treatment: not described |
|
| Outcomes |
Primary outcome (composite):
Secondary outcomes:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2 x 2 factorial design with block randomisation, with a block size of 20 |
| Allocation concealment (selection bias) | Low risk | Centralised independently by the manufacturer (Alpharma) Study nurses received coded boxes provided to participants in numerical order. The codes were kept by the manufacturer until eligibility data were complete |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vitamins were manufactured to be indistinguishable in colour, weight or ability to be dissolved in water. Endpoints adjudicated by an independent committee unaware of patient's assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "end‐points committees were unaware of the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 patients (0.2% from the sample) withdrew consent to participate in the trial and were excluded from the analysis. Due to the media impact of the NORVIT interim results 692 patients were asked to stop the medication; outcome data available for 86% of patients at the final visit |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Other sources of bias not identified |
ACE: angiotensin‐converting enzyme ARB: angiotensin receptor blockers CAD: coronary artery disease CHD: coronary heart disease CK‐MB: creatine kinase‐MB CVD: cardiovascular disease ECG: electrocardiogram HLI: homocysteine‐lowering interventions IQR: interquartile range ITT: intention‐to‐treat IU: international units MI: myocardial infarction RCT: randomised controlled trial SD: standard deviation t‐Hcy: total homocysteine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bahmani 2014 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Bailey 2015 | Observational study |
| Baszczuk 2014 | Narrative review |
| Baszczuk 2015 | Non randomised clinical trial |
| Bazzano 2006 | Systematic review |
| Bobak 2014 | Observational study |
| Clarke 2010 | Systematic review |
| Cui 2010 | Observational study |
| Debreceni 2014 | Narrative review |
| Dell'edera 2013 | Non randomised clinical trial |
| Deshmukh 2010 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Dong 2015 | Network meta‐analysis |
| Durga 2011 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Earnest 2012 | Randomised clinical trial with follow‐up of less than 1 year |
| Ebbing 2009 | Combined analyses of NORVIT 2006 and WENBIT 2008 |
| Ebbing 2009a | Combined analyses of NORVIT 2006and WENBIT 2008 |
| FINEST 2006 | Randomised clinical trial with follow‐up of less than 1 year |
| Goel 2015 | Comment on CSPPT 2015 |
| Green 2010 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Holmes 2011 | Meta‐analysis of genetic studies and randomised trials |
| Huang 2012 | Systematic review |
| Huang 2015 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Huo 2012 | Systematic review |
| Imasa 2009 | Randomised clinical trial with follow‐up of less than 1 year |
| Jardine 2012 | Systematic review in people with kidney disease |
| Ji 2013 | Systematic review of randomised clinical trials |
| Lange 2004 | Randomised clinical trial with follow‐up of less than 1 year |
| Lee 2010 | Systematic review |
| Li 2014 | Systematic review |
| Li 2015 | Systematic review |
| Liu 2014 | Systematic review |
| Lonn 2007 | Narrative review |
| Mager 2009 | Observational study |
| Manolescu 2010 | Narrative review |
| Mei 2010 | Systematic review of randomised clinical trials including pre‐existing cardio‐cerebrovascular or renal disease patients |
| Méndez‐González 2010 | Narrative review |
| Miller 2010 | Systematic review |
| Mishchenko 2015 | Pharmacoeconomic study |
| Moghaddasi 2010 | Case‐control study |
| Ntaios 2009 | Narrative review |
| Ntaios 2010 | Randomised clinical trial that did not assess patient‐oriented outcomes such as was pre‐defined for this Cochrane review |
| PACIFIC 2002 | Randomised clinical trial with follow‐up of less than 1 year |
| Pan 2012 | Systematic review |
| Park 2016 | Systematic review |
| Qin 2014 | Systematic review |
| Rautiainen 2010 | Observational study |
| Sharifi 2010 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Shidfar 2009 | Randomised clinical trial that evaluated the effects of folate supplementation on lowering homocysteine levels and changes in total antioxidant capacity in asymptomatic hypercholesteraemic adults under lovastatin treatment. It did not include the pre‐defined outcomes for this Cochrane review |
| Sudchada 2012 | Systematic review |
| Swiss 2002 | Randomised clinical trial with follow‐up of less than 1 year |
| Tighe 2011 | Randomised clinical trial that evaluated the effects of folate supplementation on lowering homocysteine levels. It did not include the pre‐defined outcomes for this Cochrane review |
| Vesin 2007 | Narrative review |
| Wang 2007 | Systematic review |
| Wang 2012 | Systematic review |
| Wang 2015a | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Wang 2015b | Systematic review |
| Wierzbicki 2007 | Narrative review |
| Wise 2015 | Comment on CSPPT 2015 |
| Yang 2012 | Systematic review |
| Yi 2014 | Systematic review |
| Zappacosta 2013 | Randomised clinical trial that did not assess patient‐oriented outcomes and excluded the pre‐defined outcomes for this Cochrane review |
| Zeng 2015 | Systematic review |
| Zhang 2009 | Systematic review |
| Zhang 2013 | Systematic review |
| Zhang 2014 | Systematic review |
| Zhou 2011 | Systematic review |
Characteristics of ongoing studies [ordered by study ID]
NCT01956786.
| Study name | Efficacy of amlodipine‐folic acid tablets on reduction of blood pressure and plasma homocysteine |
| Methods |
|
| Participants | Age: 18 years to 75 years. Gender: both. Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2013. |
| Contact information | Wang Jiguang, MD. Ruijin Hospital, Shanghai Jiao Tong University. |
| Notes |
|
Differences between protocol and review
Number needed to treat for an additional beneficial outcome if the risk reduction was significant (P value = < 0.05)
Harbord and Peters tests for estimation publication bias.
Bayes factors
Fragility Indices
Trials including participants without cardiovascular disease versus trials including participants with cardiovascular disease (considered post‐hoc).
We considered studies to have an overall low risk of bias if they did not have high risk of bias in any of six individual domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data or selective reporting), and if a definitive 'Risk of bias' assessment could be made for the majority (at least five of six) of domains. We did not include ‘Other bias’ in our overall assessment.
Second update (Martí‐Carvajal 2015): included trial sequential analyses.
First update (Martí‐Carvajal 2013): In the first version of the review (Martí‐Carvajal 2009), we searched the Allied and Complementary Medicine ‐ AMED database (accessed through Ovid) and the Cochrane Stroke Group Specialised Register. For this update, we did not search either database.
This review has been updated at each step to current recommendations of Cochrane, including updates to the Plain Language Summary and inclusion of the quality of the evidence assessed according to GRADE ('Summary of findings').
Contributions of authors
Arturo Marti‐Carvajal took the lead on writing up the review. Ivan Solà identified trials, extracted data, edited the 'Summary of findings' tables and drafted the review. Dimitris Lathyris extracted and checked the data and reviewed the review. Mark Dayer critically reviewed and amended the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
Iberoamerican Cochrane Centre, Spain
Academic
-
Cochrane Heart Group, UK
Academic
Declarations of interest
Arturo Marti‐Carvajal: In 2004, Arturo Martí‐Carvajal was employed by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with Cochrane or any Cochrane review. In 2007, Arturo Martí‐Carvajal was employed by Merck to run a four‐hour workshop 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with Cochrane or any Cochrane review. Ivan Solà: none known. Dimitris Lathyris: none known. Mark Dayer: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
B‐PROOF 2015 {published data only}
- Enneman AW, Swart KM, Wijngaarden JP, Dijk SC, Ham AC, Brouwer-Brolsma EM, et al. Effect of vitamin B12 and folic acid supplementation on bone mineral density and quantitative ultrasound parameters in older people with an elevated plasma homocysteine level: B-PROOF, a randomized controlled trial. Calcified Tissue International 2015;96(5):401-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk SC, Enneman AW, Swart KM, Wijngaarden JP, Ham AC, Brouwer-Brolsma EM, et al. Effects of 2-year vitamin B12 and folic acid supplementation in hyperhomocysteinemic elderly on arterial stiffness and cardiovascular outcomes within the B-PROOF trial. Journal of Hypertension 2015;33(9):1897-906; discussion 1906. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wijngaarden JP, Dhonukshe-Rutten RA, Schoor NM, Velde N, Swart KM, Enneman AW, et al. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatrics 2011;11:80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijngaarden JP, Swart KM, Enneman AW, Dhonukshe-Rutten RA, Dijk SC, Ham AC, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. American Journal of Clinical Nutrition 2014;100(6):1578-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
BVAIT 2009 {published data only}
- Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, BVAIT Research Group. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke 2009;40(3):730-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CHAOS 2002 {published data only}
- Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation 2002;106(Suppl II):741. [Google Scholar]
CSPPT 2015 {published data only}
- Huang X, Wang H, Qin XH, Yang WB, Jiang SQ, Liu LS, et al. Serum homocysteine lowering by long-term low-dose folic acid: new insight on MTHFR gene-folate interaction from China stroke primary prevention trial. Journal of the American College of Cardiology 2017;69(11):1770. [Google Scholar]
- Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313(13):1325-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Li H, Qin X, Xie D, Tang G, Zhang Y, Li J, et al. Effects of combined enalapril and folic acid therapy on the serum uric acid levels in hypertensive patients: a multicenter, randomized, double-blind, parallel-controlled clinical trial. Internal Medicine 2015;54(1):17-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Liu X, Shi M, Xia F, Han J, Liu Z, Wang B, et al. The China Stroke Secondary Prevention Trial (CSSPT) protocol: a double-blinded, randomized, controlled trial of combined folic acid and B vitamins for secondary prevention of stroke. International Journal of Stroke 2015;10(2):264-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Qin X, Li J, Spence JD, Zhang Y, Li Y, Wang X, et al. Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke 2016;47(11):2805-12. [PMID: 27729579 ] [DOI] [PubMed] [Google Scholar]
- Qin X, Li Y, Sun N, Wang H, Zhang Y, Wang J, et al. Elevated homocysteine concentrations decrease the antihypertensive effect of angiotensin-converting enzyme inhibitors in hypertensive patients. Arteriosclerosis, Thrombosis, and Vascular Biology 2017;37(1):166-72. [PMID: 27834686] [DOI] [PubMed] [Google Scholar]
- Qin X, Shen L, Zhang R, Li Y, Wang X, Wang B, et al. Effect of folic acid supplementation on cancer risk among adults with hypertension in China: A randomized clinical trial. International Journal of Cancer 2017;141(4):837-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wang M, Bie Z, Guo L, Wang J. A 3-year clinical study reveals that enalapril folic acid tablet effectively controls H-type hypertension and improves ventricular structure and function. Experimental and Clinical Cardiology 2014;20(6):3723-34.
- Xu B, Kong X, Xu R, Song Y, Liu L, Zhou Z, et al. Homocysteine and all-cause mortality in hypertensive adults without pre-existing cardiovascular conditions: Effect modification by MTHFR C677T polymorphism. Medicine 2017;96(8):e5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Kong X, Xu R, Zhao M, Song Y, Zhang C, et al. Impact of folic-acid intervention and MTHFRC677T gene polymorphism on all-cause mortality associated with elevated serum homocysteine levels in chinese adults with hypertension. FASEB Journal 2016;30(1 Suppl):422.3.
- Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, et al, investigators of the Renal Substudy of the China Stroke Primary Prevention Trial (CSPPT). Efficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease: the Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Internal Medicine 2016;176(10):1443-50. [DOI] [PubMed] [Google Scholar]
FOLARDA 2004 {published data only}
- Liem AH, Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, Tijssen JG, et al. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. International Journal of Cardiology 2004;93:175-9. [PMID: 14975544 ] [DOI] [PubMed] [Google Scholar]
GOES 2003 {published data only}
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. Journal of the American College of Cardiology 2003;41:2105-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005;91:1213-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HOPE‐2 2006 {published data only}
- Lonn E, Held C, Arnold JM, Probstfield J, McQueen M, HOPE-2 Investigators. Rationale, design and baseline characteristics of a large, simple, randomized trial of combined folic acid and vitamins B6 and B12 in high-risk patients: the Heart Outcomes Prevention Evaluation (HOPE)-2 trial. Canadian Journal of Cardiology 2006;22(1):47-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine 2006;354:1567-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saposnik G, Ray J, Sheridan P, McQueen M, Lonn E. Homocysteine lowering therapy and stroke risk, severity and disability: results from the HOPE-2 trial. Stroke 2009;40(4):e131. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E, Heart Outcomes Prevention Evaluation Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke 2009;40(4):1365-72. [PMID: ] [DOI] [PubMed]
Li 2015a {published data only}
- Li F, Hao YM, Zu XG, Liu JM. Prevention of cerebrovascular events by high-dose folic acid supplement in elderly female patients with hypertensive emergency and hyperhomocysteinemia. Journal of the American Geriatrics Society 2015;63(Suppl 2):S334. [Google Scholar]
NORVIT 2006 {published data only}
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. New England Journal of Medicine 2006;354:1578-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
SEARCH 2010 {published data only}
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303:2486-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Clinical Trial Service Unit & Epidemiological Studies Unit. SEARCH Study Protocol. https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view (accessed 7 January 2015):1-36.
- Group Search Collaborative. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised. Lancet 2011;377(9760):126. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SU.FOL.OM3 2010 {published data only}
- Andreeva VA, Latarche C, Hercberg S, Briançon S, Galan P, Kesse-Guyot E. B vitamin and/or n-3 fatty acid supplementation and health-related quality of life: ancillary findings from the SU.FOL.OM3 randomized trial. PLOS One 2014;9(1):e84844. [PMID: 24465438] [DOI] [PMC free article] [PubMed]
- Andreeva VA, Touvier M, Kesse-Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or ω-3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega-3 fatty acids (SU.FOL.OM3) randomized trial. Archives of Internal Medicine 2012;172(7):540-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Blacher J, Czernichow S, Paillard F, Ducimetiere P, Hercberg S, Galan P, on behalf of the SUFOLOM3 Study Research Group. Cardiovascular effects of B-vitamins and/or N-3 fatty acids: the Su.Fol.OM3 trial. International Journal of Cardiology 2013;167(2):508-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Briancon S, Blacher J, Czernichow S, Hercberg S. The scientific basis of the SU.FOL.OM3 study: a secondary intervention trial of folate, B6 and B12 vitamins and/or omega 3 fatty acid supplements in the prevention of recurrent ischemic events [Bases scientifiques de l’étude SUFOLOM3: essai de prévention secondaire visant à tester l’impact d’une supplémentation en folates, vitamines B6 et B12 et/ou acides gras oméga-3 dans la prévention de la récidive de pathologies ischémiques]. Sang Thrombose Vaisseaux 2009;21(4):207-13. [ISSN 0999-7385] [Google Scholar]
- Galan P, Briancon S, Blacher J, Czernichow S, Hercberg S. The SU.FOL.OM3 Study: a secondary prevention trial testing the impact of supplementation with folate and B-vitamins and/or Omega-3 PUFA on fatal and non fatal cardiovascular events, design, methods and participants characteristics. Trials 2008;9:35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Bree A, Mennen L, Potier de Courcy G, Preziozi P, Bertrais S, et al. Background and rationale of the SU.FOL.OM3 study: double-blind randomized placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. Journal of Nutrition, Health & Aging 2003;7(6):428-35. [PMID: ] [PubMed] [Google Scholar]
- Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, SUFOLOM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:1-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, SUFOLOM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2011;341:c6273. [ISSN 0959-8146] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo de Edelenyi F, Vergnaud AC, Ahluwalia N, Julia C, Hercberg S, Blacher J, et al. Effect of B-vitamins and n-3 PUFA supplementation for 5 years on blood pressure in patients with CVD. British Journal of Nutrition 2012;107(6):921-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Vesin C, Galan P, Gautier B, Czernichow S, Hercberg S, Blacher J. Control of baseline cardiovascular risk factors in the SU-FOL-OM3 study cohort: does the localization of the arterial event matter? European Journal of Cardiovascular Prevention and Rehabilitation 2010;17(5):541-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
VISP 2004 {published data only}
- Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004;291(5):565-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
VITATOPS 2010 {published data only}
- Cavalieri M, Schmidt R, Chen C, Mok V, Freitas GR, Song S, et al. B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: the VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke 2012;43(12):3266-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dusitanond P, Eikelboom JW, Hankey GJ, Thom J, Gilmore G, Loh K, et al. Homocysteine-lowering treatment with folic acid, cobalamin, and pyridoxine does not reduce blood markers of inflammation, endothelial dysfunction, or hypercoagulability in patients with previous transient ischemic attack or stroke: a randomized substudy of the VITATOPS trial. Stroke 2005;36(1):144-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Group Vitatops Study. The VITATOPS (VITAmins TO Prevent Stroke) trial: rationale, design and progress of an international, large simple, randomised trial of homocysteine-lowering multivitamin therapy in patients with recent transient attack or stroke [Abstract]. In: Internal Medicine Journal. Vol. Suppl 4. 2007:A110.
- Hankey GJ, Eikelboom JW, Yi Q, Lees KR, Chen C, Xavier D, et al. Treatment with B vitamins and incidence of cancer in patients with previous stroke or transient ischemic attack: results of a randomized placebo-controlled trial. Stroke 2012;43(6):1572-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hankey GJ. The vitamins to prevent stroke (VITATOPS) trial: results of a double-blind, placebo-controlled, randomised trial of B-vitamin therapy in 8,164 patients with recent transient ischaemic attack or stroke. In: Cerebrovascular Diseases. Vol. 29. 2010:11. [DOI: 10.1159/000321266] [DOI] [PubMed]
- Potter K, Hankey GJ, Green DJ, Eikelboom J, Jamrozik K, Arnolda LF. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis. BMC Cardiovascular Disorders 2008;8:24. [PMID: 18803866] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K, Lenzo N, Eikelboom JW, Arnolda LF, Beer C, Hankey GJ. Effect of long-term homocysteine reduction with B vitamins on arterial wall inflammation assessed by fluorodeoxyglucose positron emission tomography: a randomised double-blind, placebo-controlled trial. Cerebrovascular Diseases 2009;27(3):259-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saposnik G. The role of vitamin B in stroke prevention: a journey from observational studies to clinical trials and critique of the VITAmins TO Prevent Stroke (VITATOPS). Stroke 2011;42(3):838-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
- The VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial:a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurology 2010;9(9):855–65. [PMID: ] [DOI] [PubMed] [Google Scholar]
- VITATOPS Trial Study Group, Hankey GJ, Algra A, Chen C, Wong MC, Cheung R, et al. VITATOPS, the VITAmins TO prevent stroke trial: rationale and design of a randomised trial of B-vitamin therapy in patients with recent transient ischaemic attack or stroke (NCT00097669) (ISRCTN74743444). International Journal of Stroke 2007;2(2):144-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
WAFACS 2008 {published data only}
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008;299(17):2027-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn RJ, Manson JE, Women’s Antioxidant and Folic Acid Cardiovascular Study Group. Effect of folic acid and B vitamins on the occurrence of venous thromboembolism: a randomized trial in women at high cardiovascular risk. Circulation 2008;118:S1137. [Google Scholar]
- Redbeg RF, Block PC. A randomized trial of folic acid and B-vitamins in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS). ACC Cardiosource Review Journal 2006;16(7):52-8. [Google Scholar]
- Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes 2009;58(8):1921-8. [PMID: 19491213] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic acid and B vitamins on risk of type 2 diabetes mellitus in women: a randomized controlled trial. In: Circulation. Vol. 119. 2009:E273. [ISSN 0009-7322] [DOI] [PMC free article] [PubMed]
- Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA 2008;300(17):2012-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WENBIT 2008 {published data only}
- Bleie Ø, Strand E, Ueland PM, Vollset SE, Refsum H, Igland J, et al. Coronary blood flow in patients with stable coronary artery disease treated long term with folic acid and vitamin B12. Coronary Artery Disease 2011;22(4):270-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ding YP, Pedersen EK, Johansson S, Gregory JF 3rd, Ueland PM, Svingen GF, et al. B vitamin treatments modify the risk of myocardial infarction associated with a MTHFD1 polymorphism in patients with stable angina pectoris. Nutrition, Metabolism, and Cardiovascular Diseases 2016;26(6):495-501. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008;300:795-804. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ebbing M, Bonaa KH, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. Journal of Internal Medicine 2010;268(4):367-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Loland KH, Bleie O, Blix AJ, Strand E, Ueland PM, Refsum H, et al. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: a Western Norway B Vitamin Intervention Trial (WENBIT) substudy. American Journal of Cardiology 2010;105(11):1577-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bahmani 2014 {published data only}
- Bahmani F, Karamali M, Shakeri H, Asemi Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clinical Endocrinology 2014;81(4):582-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bailey 2015 {published data only}
- Bailey RL, Fakhouri TH, Park Y, Dwyer JT, Thomas PR, Gahche JJ, et al. Multivitamin-mineral use is associated with reduced risk of cardiovascular disease mortality among women in the United States. Journal of Nutrition 2015;145(3):572-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baszczuk 2014 {published data only}
- Baszczuk A, Kopczynski Z. [Hyperhomocysteinemia in patients with cardiovascular disease] [Hiperhomocysteinemia u chorych na schorzenia ukladu krazenia]. Postepy Higieny i Medycyny Doswiadczalnej 2014;68:579-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Baszczuk 2015 {published data only}
- Baszczuk A, Kopczynski Z, Kopczynski J, Cymerys M, Thielemann A, Bielawska L, et al. Impact of administration of folic acid on selected indicators of inflammation in patients with primary arterial hypertension. Postepy Higieny i Medycyny Doswiadczalnej 2015;69:429-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bazzano 2006 {published data only}
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006;296:2720-6. [DOI] [PubMed] [Google Scholar]
Bobak 2014 {published data only}
- Bobak M, Pajak A, Tamosiunas A, Kubinova R, Gardiner J, Jansen E. Plasma concentrations of folate and vitamin B12 and risk of fatal and non-fatal cardiovascular disease: A nested case-control study nested in a population-based cohort. European Society of Cardiology annual meeting (http://congress365.escardio.org/Search-Results?vgnextkeyword=C365PRESENTATION101078#.VsOczh0X3IU) 2014;(4170).
Clarke 2010 {published data only}
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JAE, for the B-Vitamin Treatment Trialists’ Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Archives of Internal Medicine 2010;170(18):1622-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cui 2010 {published data only}
- Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A. Dietary folate and vitamin b6 and B12 intake in relation to mortality from cardiovascular diseases: Japan Collaborative Cohort Study. Stroke 2010;41(6):1285-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Debreceni 2014 {published data only}
- Debreceni B, Debreceni L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovascular Therapeutics 2014;32(3):130-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dell'edera 2013 {published data only}
- Dell'edera D, Tinelli A, Milazzo GN, Malvasi A, Domenico C, Pacella E, et al. Effect of multivitamins on plasma homocysteine in patients with the 5,10 methylenetetrahydrofolate reductase C677T homozygous state. Molecular Medicine Reports 2013;8(2):609-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deshmukh 2010 {published data only}
- Deshmukh US, Joglekar CV, Lubree HG, Ramdas LV, Bhat DS, Naik SS, et al. Effect of physiological doses of oral vitamin B12 on plasma homocysteine: a randomized, placebo-controlled, double-blind trial in India. European Journal of Clinical Nutrition 2010;64(5):495-502. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dong 2015 {published data only}
- Dong H, Pi F, Ding Z, Chen W, Pang S, Dong W, et al. Efficacy of supplementation with B vitamins for stroke prevention: A network meta-analysis of randomized controlled trials. PLOS One 2015;10(9):e0137533. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durga 2011 {published data only}
- Durga J, Bots ML, Schouten EG, Grobbee DE, Kok FJ, Verhoef P. Effect of 3 y of folic acid supplementation on the progression of carotid intima-media thickness and carotid arterial stiffness in older adults. American Journal of Clinical Nutrition 2011;93(5):941-9. [PMID: 21430116] [DOI] [PubMed] [Google Scholar]
Earnest 2012 {published data only}
- Earnest CP, Kupper JS, Thompson AM, Guo W, Church T. Complementary effects of multivitamin and omega-3 fatty acid supplementation on indices of cardiovascular health in individuals with elevated homocysteine. International Journal for Vitamin and Nutrition Research 2012;82(1):41-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebbing 2009 {published data only}
- Ebbing M, Bonaa KH, Nordrehaug JE, Ueland PM, Arnesen E, Refsum H, et al. Combined results and long-term follow-up in NORVIT and WENBIT with 6837 coronary artery disease patients: homocysteine-lowering B-vitamin treatment does not prevent major cardiovascular events. Journal of Internal Medicine 2009;30(4):184. [PMID: 20698927]20698927 [Google Scholar]
Ebbing 2009a {published data only}
- Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009;302(19):2119-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
FINEST 2006 {published data only (unpublished sought but not used)}
- Nevado J. Folate intervention in non-ST elevation myocardial infarction and unstable angina: a randomised placebo-controlled trial [2006]. http://controlled-trials.com/ISRCTN30249553/homocysteine (accessed 12 October 2007).
Goel 2015 {published data only}
Green 2010 {published data only}
- Green TJ, Skeaff CM, McMahon JA, Venn BJ, Williams SM, Devlin AM, et al. Homocysteine-lowering vitamins do not lower plasma S-adenosylhomocysteine in older people with elevated homocysteine concentrations. British Journal of Nutrition 2010;103(11):1629-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Holmes 2011 {published data only}
- Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 2011;378(9791):584-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clinical Nutrition 2012;31(4):448-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Huang T, Li D. Reply to Fang SK--Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clinical Nutrition 2013;32(2):315-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zhou C, Wu J, Fang S. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clinical Nutrition 2013;32(2):314. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang T, Li K, Asimi S, Chen Q, Li D. Effect of vitamin B-12 and n-3 polyunsaturated fatty acids on plasma homocysteine, ferritin, C-reaction protein, and other cardiovascular risk factors: a randomized controlled trial. Asia Pacific Journal of Clinical Nutrition 2015;24(3):403-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huo 2012 {published data only}
- Huo Y, Qin X, Wang J, Sun N, Zeng Q, Xu X, et al. Efficacy of folic acid supplementation in stroke prevention: new insight from a meta-analysis. International Journal of Clinical Practice 2012;66(6):544-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Imasa 2009 {published data only}
- Imasa MS, Gomez NT, Nevado JB. Folic acid-based intervention in non-ST elevation acute coronary syndromes. Asian Cardiovascular & Thoracic Annals 2009;17(1):13-21. [PMID: 19515873] [DOI] [PubMed] [Google Scholar]
Jardine 2012 {published data only}
- Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ 2012;344:e3533. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2013 {published data only}
- Ji Y, Tan S, Xu Y, Chandra A, Shi C, Song B, et al. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: a meta-analysis. Neurology 2013;81(15):1298-307. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lange 2004 {published data only}
- Lange H, Suryapranata H, De Luca G, Börner C, Dille J, Kallmayer K, et al. Folate therapy and in-stent restenosis after coronary stenting. New England Journal of Medicine 2004;350:2673-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke 2010;41(6):1205-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine lowering therapy with folic acid in stroke prevention: a meta-analysis of randomized controlled trials. In: Stroke. Vol. 41. 2010:e291. [ISSN: 0039-2499] [DOI] [PMC free article] [PubMed]
Li 2014 {published data only}
- Li WF, Zhang DD, Xia JT, Wen SF, Guo J, Li ZC. The association between B vitamins supplementation and adverse cardiovascular events: a meta-analysis. International Journal of Clinical and Experimental Medicine 2014;7(8):1923-30. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li J, Li B, Qi J, Shen B. [Meta-analysis of clinical trials of folic acid, vitamin B12 and B6 supplementation on plasma homocysteine level and risk of cardiovascular disease]. Zhonghua Xin Xue guan Bing Za Zhi 2015;43(6):554-61. [PMID: ] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu Y, Tian T, Zhang H, Gao L, Zhou X. The effect of homocysteine-lowering therapy with folic acid on flow-mediated vasodilation in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Atherosclerosis 2014;235(1):31-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lonn 2007 {published data only}
- Lonn E. Homocysteine in the prevention of ischemic heart disease, stroke and venous thromboembolism: therapeutic target or just another distraction? Current Opinion in Hematology 2007;14(5):481-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mager 2009 {published data only}
- Mager A, Orvin K, Koren-Morag N, Lev IE, Assali A, Kornowski R, et al. Impact of homocysteine-lowering vitamin therapy on long-term outcome of patients with coronary artery disease. American Journal of Cardiology 2009;104(6):745-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manolescu 2010 {published data only}
- Manolescu BN, Oprea E, Farcasanu IC, Berteanu M, Cercasov C. Homocysteine and vitamin therapy in stroke prevention and treatment: a review. Acta Biochimica Polonica 2010;57(4):467-77. [PMID: ] [PubMed] [Google Scholar]
Mei 2010 {published data only}
- Mei W, Rong Y, Jinming L, Yongjun L, Hui Z. Effect of homocysteine interventions on risk of cardiocerebrovascular events: a meta-analysis of randomized controlled trials. Circulation 2010;122(2):e140-1. [DOI] [PubMed] [Google Scholar]
- Mei W, Rong Y, Jinming L, Yongjun L, Hui Z. Effect of homocysteine interventions on the risk of cardiocerebrovascular events: a meta-analysis of randomised controlled trials. International Journal of Clinical Practice 2010;64(2):208-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Méndez‐González 2010 {published data only}
- Méndez-González J, Rodríguez-Millán E, Julve J, Blanco-Vaca F. Vitamin treatments that lower homocysteine concentration: can they decrease cerebrovascular disease in primary prevention? [Tratamientos vitamínicos para disminuir la concentración de homocisteína: reducen el riesgo de enfermedad cerebrovascular en prevención primaria?]. Revista de Neurologia 2010;50(4):235-44. [PMID: ] [PubMed] [Google Scholar]
Miller 2010 {published data only}
- Miller ER 3rd, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. American Journal of Cardiology 2010;106:517-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mishchenko 2015 {published data only}
- Mishchenko O, Iakovlieva L, Kyrychenko O, Gerasymova O, Bezditko N, Tkachova O. Pharmacoeconomic analysis of the combined homocysteine-lowering and standard therapy versus standard therapy of patients with CHD, postpercutaneous coronary intervention (Pci) and B12 deficiency. Value in Health 2015;18(7):A404. [PMID: 26532279] [Google Scholar]
Moghaddasi 2010 {published data only}
- Moghaddasi M, Mamarabadi M, Mirzadeh S, Freydoonnejad AA, Razjouyan H. Homocysteine, vitamin B12 and folate levels in Iranian patients with ischemic stroke. Neurological Research 2010;32(9):953-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Moghaddasi M, Mamarabad M. Homocysteine, vitamin B12 and folic acid levels in cerebrovascular accident: a case-control study. Journal of Neurology 2009;256(Suppl 2):S171. [Google Scholar]
Ntaios 2009 {published data only}
- Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: an update. Archives of Cardiovascular Diseases 2009;102(12):847-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ntaios 2010 {published data only}
- Ntaios G, Savopoulos C, Karamitsos D, Economou I, Destanis E, Chryssogonidis I, et al. The effect of folic acid supplementation on carotid intima-media thickness in patients with cardiovascular risk: a randomized, placebo-controlled trial. International Journal of Cardiology 2010;143(1):16-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
PACIFIC 2002 {published data only}
- Neal B, MacMahon S, Ohkubo T, Tonkin A, Wilcken D, PACIFIC Study Group. Dose-dependent effects of folic acid on plasma homocysteine in a randomized trial conducted among 723 individuals with coronary heart disease. European Heart Journal 2002;23:1509-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pan 2012 {published data only}
- Pan Y, Guo LL, Cai LL, Zhu XJ, Shu JL, Liu XL, et al. Homocysteine-lowering therapy does not lead to reduction in cardiovascular outcomes in chronic kidney disease patients: a meta-analysis of randomised, controlled trials. British Journal of Nutrition 2012;108(3):400-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park JH, Saposnik G, Ovbiagele B, Markovic D, Towfighi A. Effect of B-vitamins on stroke risk among individuals with vascular disease who are not on antiplatelets: A meta-analysis. International Journal of Stroke 2016;11(2):206-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qin 2014 {published data only}
- Qin X, Fan F, Cui Y, Chen F, Chen Y, Cheng X, et al. Folic acid supplementation with and without vitamin B6 and revascularization risk: a meta-analysis of randomized controlled trials. Clinical Nutrition 2014;33(4):603-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rautiainen 2010 {published data only}
- Rautiainen S, Akesson A, Levitan EB, Morgenstern R, Mittleman MA, Wolk A. Multivitamin use and the risk of myocardial infarction: a population-based cohort of Swedish women. American Journal of Clinical Nutrition 2010;92(5):1251-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharifi 2010 {published data only}
- Sharifi F, Fakhrzadeh H, Mirarefin M, Pourebrahim R, Nouri M, Forouzanfar MH, et al. The effects of high-dose folic acid on blood pressure of hypertensive adults with hyperhomocysteinemia: a randomized double-blind placebo controlled clinical trial (Tehran homocysteine survey). Iranian Journal of Diabetes and Lipid Disorders 2010;9:13-20. [Google Scholar]
Shidfar 2009 {published data only}
- Shidfar F, Homayounfar R, Fereshtehnejad SM, Kalani A. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: a double-blind randomized controlled clinical trial. Archives of Medical Research 2009;40(5):380-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sudchada 2012 {published data only}
- Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Research and Clinical Practice 2012;98(1):151-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Swiss 2002 {published data only}
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA 2002;288:973-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tighe 2011 {published data only}
- Tighe P, Ward M, McNulty H, Finnegan O, Dunne A, Strain J, et al. A dose-finding trial of the effect of long-term folic acid intervention: implications for food fortification policy. American Journal of Clinical Nutrition 2011;93(1):11-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vesin 2007 {published data only}
- Vesin C, Mairesse S, Iaria P, Blacher J. Efficacy of folic acid in prevention of cerebrovascular disease [Efficacite de l’acide folique dans la prevention cerebrovasculaire]. Medecinedes Maladies Metaboliques 2007;1:50-2. [EMBASE: 2007490543] [Google Scholar]
Wang 2007 {published data only}
- Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007;369:876-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang ZM, Zhou B, Nie ZL, Gao W, Wang YS, Zhao H, et al. Folate and risk of coronary heart disease: a meta-analysis of prospective studies. Nutrition, Metabolism, and Cardiovascular Diseases 2012;22(10):890-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2015a {published data only}
- Wang L, Li H, Zhou Y, Jin L, Liu J. Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. European Journal of Nutrition 2015;54(3):455-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2015b {published data only}
- Wang LP, Cui WW, Nan GX, Yu Y. Meta-analysis reveals protective effects of vitamin B on stroke patients. Translational Neuroscience 2015;6(1):150-6. [DOI] [PMC free article] [PubMed]
Wierzbicki 2007 {published data only}
- Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diabetes & Vascular Disease Research 2007;4(2):143-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wise 2015 {published data only}
Yang 2012 {published data only}
- Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. European Journal of Internal Medicine 2012;23(8):745-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yi 2014 {published data only}
- Yi X, Zhou Y, Jiang D, Li X, Guo Y, Jiang X. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: A meta-analysis of randomized controlled trials. Experimental and Therapeutic Medicine 2014;7(5):1100-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zappacosta 2013 {published data only}
- Zappacosta B, Mastroiacovo P, Persichilli S, Pounis G, Ruggeri S, Minucci A, et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients 2013;5(5):1531-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2015 {published data only}
- Zeng R, Xu CH, Xu YN, Wang YL, Wang M. The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: a meta-analysis. Public Health Nutrition 2015;18(8):1514-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang C, Zhu HL. Effect of B vitamins supplementation on cardiovascular and cerebrovascular disease by lowering plasma homocysteine concentration: a meta-analysis [Chinese]. Chinese Journal of Evidence-Based Medicine 2009;1:55-62. [ISSN: 1672-2531] [Google Scholar]
Zhang 2013 {published data only}
- Zhang C, Chi FL, Xie TH, Zhou YH. Effect of B-vitamin supplementation on stroke: a meta-analysis of randomized controlled trials. PLOS One 2013;8(11):e81577. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang C, Wang ZY, Qin YY, Yu FF, Zhou YH. Association between B vitamins supplementation and risk of cardiovascular outcomes: a cumulative meta-analysis of randomized controlled trials. PLOS One 2014;9(9):e107060. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, et al. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLOS One 2011;6(9):e25142. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01956786 {published data only}
- Efficacy of amlodipine-folic acid tablets on reduction of blood pressure and plasma homocysteine. Ongoing study. September 2013.. Contact author for more information.
Additional references
Bansilal 2015
- Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. International Journal of Cardiology 2015;201 Suppl 1:S1-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barquera 2015
- Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Archives of Medical Research 2015;46(5):328-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Benziger 2016
- Benziger CP, Roth GA, Moran AE. The Global burden of disease study and the preventable burden of NCD. Global Heart 2016;11(4):393-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bowman 2007
- Bowman L, Armitage J, Bulbulia R, Parish S, Collins R, SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. American Heart Journal 2007;154(5):815-23, 823.e1-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive--trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
CAPRIE 1996
- No authors listed. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348(9038):1329-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Casas 2005
- Casas JP, Bautista LE, Smeeth L, Sharma, P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet 2005;365:224-32. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke R, Lewington S, Sherliker P, Armitage J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Current Opinion in Clinical Nutrition and Metabolic Care 2007;10:32-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Crider 2011
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3(3):370-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTU 2011 [Computer program]
- TSA - Trial Sequential Analysis. Copenhagen Trial Unit. http://ctu.dk/tsa/, 2011 (accessed 29 September 2011).
Cybulska 2015
- Cybulska B, Klosiewicz-Latoszek L. Homocysteine--is it still an important risk factor for cardiovascular disease? Kardiologia Polska 2015;73(11):1092-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Danesh 1998
- Danesh J, Lewington S. Plasma homocysteine and coronary heart disease: systematic review of published epidemiological studies. Journal of Cardiovascular Risk 1998;5:229-32. [PMID: ] [PubMed] [Google Scholar]
Dienes 2008
- Dienes Z. Understanding Psychology as a Science: An Introduction to Scientific and Statistical Inference. First edition. London: Palgrave Macmillan, 2008. [9780230542303] [Google Scholar]
Dienes 2014
- Dienes Z. Using Bayes to get the most out of non-significant results. Frontiers in Psychology 2014;5:781. [DOI: 10.3389/fpsyg.2014.00781] [PMC4114196] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dienes 2017
- Dienes Z, Mclatchie N. Four reasons to prefer Bayesian analyses over significance testing. Psychonomic Bulletin and Review 2017;Mar 28:Epub ahead of print. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
di Salvo 2011
- di Salvo ML, Contestabile R, Safo MK. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochimica et Biophysica Acta 2011;1814(11):1597-608. [PMID: ] [DOI] [PubMed] [Google Scholar]
di Salvo 2012
- di Salvo ML, Safo MK, Contestabile R. Biomedical aspects of pyridoxal 5'-phosphate availability. Frontiers in Bioscience 2012;4:897-913. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eikelboom 1999
- Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Annals of Internal Medicine 1999;131:363-75. [DOI] [PubMed] [Google Scholar]
Fedosov 2012
- Fedosov SN. Physiological and molecular aspects of cobalamin transport. Sub-cellular Biochemistry 2012;56:347-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ford 2002
- Ford ES, Smith SJ, Stroup DF, Steinberg KK, Mueller PW, Thacker SB. Homocyst(e)ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. International Journal of Epidemiology 2002;31:59-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Friso 2012
- Friso S, Lotto V, Corrocher R, Choi SW. Vitamin B6 and cardiovascular disease. Sub-cellular Biochemistry 2012;56:265-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fuster 2015
- Fuster V. Unraveling the complexities of statistical presentation: Why it is important. Journal of the American College of Cardiology 2015;66(25):2909-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ganguly 2015
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutrition Journal 2015;14:6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goff 2014
- Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014;63(25 Pt B):2935-59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1999
- Goodman SN. Toward evidence-based medical statistics. 2: The Bayes factor. Annals of Internal Medicine 1999;130(12):1005-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodman 2005
- Goodman SN. Introduction to Bayesian methods I: measuring the strength of evidence. Clinical Trials 2005;2(4):282-90; discussion 301-4, 364-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gori 2007
- Gori AM, Sofi F, Marcucci R, Giusti B, Franco Gensini G, Abbate R. Association between homocysteine, vitamin B(6) concentrations and inflammation. Clinical Chemistry and Laboratory Medicine 2007;45:1728-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- The GRADE Working Group GRADEpro. Brozek J, Oxman A, Schünemann H, Version 3.2 for Windows. The GRADE Working Group, 2008.
Guthikonda 2006
- Guthikonda S, Haynes WG. Homocysteine: role and implications in atherosclerosis. Current Atherosclerosis Reports 2006;8:100-6. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013
Hannibal 2016
- Hannibal L, Blom HJ. Homocysteine and disease: Causal associations or epiphenomenons? Molecular Aspects of Medicine 2016;Nov 19:in press. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herrmann 2012
- Herrmann W, Obeid R. Cobalamin deficiency. Sub-cellular Biochemistry 2012;56:301-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
HLTC 2005
- Homocysteine Lowering Trialists' Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. American Journal of Clinical Nutrition 2005;82:806-12. [DOI] [PubMed] [Google Scholar]
HSC 2002
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002;288:2015-22. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Committee on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulations & International Conference on Harmonisation Guidelines. Philadelphia, US: Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannidis 2010
- Ioannidis JP. Meta-research: the art of getting it wrong. Research Synthesis Methods 2010;10(3-4):169-84. [DOI] [PubMed] [Google Scholar]
Jacobsen 2005
- Jacobsen DW, Catanescu O, Dibello PM, Barbato JC. Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clinical Chemistry & Laboratory Medicine 2005;43:1076-83. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamison 2007
- Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA 2007;298(10):1163-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Joseph 2013
- Joseph J, Loscalzo J. Methoxistasis: integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013;5(8):3235-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kardesoglu 2011
- Kardesoglu E, Uz O, Isilak Z, Cebeci BS. Homocysteine as a new risk factor for cardiovascular events in heart failure. International Journal of Cardiology 2011;146(1):126-7. [PMID: 21074871] [DOI] [PubMed] [Google Scholar]
Kräutler 2012
- Kräutler B. Biochemistry of B12-cofactors in human metabolism. Sub-cellular Biochemistry 2012;56:323-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kwan 2016
- Kwan GF, Mayosi BM, Mocumbi AO, Miranda JJ, Ezzati M, Jain Y, et al. Endemic cardiovascular diseases of the poorest billion. Circulation 2016;133(24):2561-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lackland 2015
Lai 2015b
- Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Annals of Nutrition & Metabolism 2015;67(1):1-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laupacis 1988
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318(26):1728-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S(editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Li 2016
- Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, et al. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. Journal of the American College of Cardiology 2016;68(8):818-33. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maredza 2015
- Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurology 2015;15:54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maron 2006
- Maron BA, Loscalzo J. Homocysteine. Clinics in Laboratory Medicine 2006;26:591-609. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maron 2009
- Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annual Review of Medicine 2009;60:39-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCully 2015a
- McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Review of Clinical Pharmacology 2015;8(2):211-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
McCully 2015b
- McCully KS. Homocysteine metabolism, atherosclerosis, and diseases of aging. Comprehensive Physiology 2015;6(1):471-505. [PMID: ] [DOI] [PubMed] [Google Scholar]
Molloy 2012
- Molloy AM. Genetic aspects of folate metabolism. Sub-cellular Biochemistry 2012;56:105-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mudd 2000
- Mudd SH, Finkelstein JD, Refsum H, Ueland PM, Malinow MR, Lentz SR, et al. Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arteriosclerosis, Thrombosis, and Vascular Biology 2000;20:704-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mukherjee 2011
- Mukherjee T, Hanes J, Tews I, Ealick SE, Begley TP. Pyridoxal phosphate: biosynthesis and catabolism. Biochimica et Biophysica Acta 2011;1814(11):1585-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohrvik 2011
- Ohrvik VE, Witthoft CM. Human folate bioavailability. Nutrients 2011;3(4):475-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2015
- Oliveira GB, Avezum A, Roever L. Cardiovascular disease burden: evolving knowledge of risk factors in myocardial infarction and stroke through population-based research and perspectives in global prevention. Frontiers in Cardiovascular Medicine 2015;2:32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perk 2012
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European Heart Journal 2012;33(13):1635-701. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Prabhakaran 2016
- Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation 2016;133(16):1605-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pushpakumar 2014
- Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Current Medicinal Chemistry 2014;21(32):3662-72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Refsum 1998
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annual Review of Medicine 1998;49:31-62. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roshanov 2017
- Roshanov PS, Dennis BB, Pasic N, Garg AX, Walsh M. When is a meta-analysis conclusive? A guide to Trial Sequential Analysis with an example of remote ischemic preconditioning for renoprotection in patients undergoing cardiac surgery. Nephrology, Dialysis, Transplantation 2017;32(Suppl 2):23-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roth 2015a
- Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. New England journal of Medicine 2015;372(14):1333-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2015b
- Roth GA, Nguyen G, Forouzanfar MH, Mokdad AH, Naghavi M, Murray CJ. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation 2015;132(13):1270-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Selhub 1993
- Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993;270:2693-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shelhub 2008
- Shelhub J. Public health significance of elevated homocysteine. Food and Nutrition Bulletin 2008;29 Suppl 2:116-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2015
- Singh GK, Siahpush M, Azuine RE, Williams SD. Widening socioeconomic and racial disparities in cardiovascular disease mortality in the United States, 1969-2013. International Journal of MCH and AIDS 2015;3(2):106-18. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Skovierova 2016
- Skovierova H, Vidomanova E, Mahmood S, Sopkova J, Drgova A, Cervenova T, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. International Journal of Molecular Sciences 2016;17(10):E1733. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2012
- Smith SC Jr, Collins A, Ferrari R, Holmes DR Jr, Logstrup S, McGhie DV, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Global Heart 2012;7(4):297-305. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sofi 2008
- Sofi F, Marcucci R, Bolli P, Giambene B, Sodi A, Fedi S, et al. Low vitamin B6 and folic acid levels are associated with retinal vein occlusion independently of homocysteine levels. Atherosclerosis 2008;198:223-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Splaver 2004
- Splaver A, Lamas GA, Hennekens CH. Homocysteine and cardiovascular disease: biological mechanism, observational epidemiology, and the need for randomised trials. American Heart Journal 2004;148:34-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
SSSS 1994
- No authors listed. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-9. [PMID: ] [PubMed] [Google Scholar]
Stampfer 1992
- Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 1992;268:877-81. [PMID: ] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of clinical epidemiology 2001;54(10):1046-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011a
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed.) 2011;343:d4002. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011b
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). Available from www.cochrane-handbook.org. Wiley-Blackwell, The Cochrane Collaboration, 2011. [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? International Journal of Epidemiology 2009;38(1):276-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clinical Epidemiology 2010;2:57-66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 30 April 2012).
Tzoulaki 2016
- Tzoulaki I, Elliott P, Kontis V, Ezzati M. Worldwide exposures to cardiovascular risk factors and associated health effects: current knowledge and data gaps. Circulation 2016;133(23):2314-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vianna 2007
- Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodialysis International 2007;11:210-6. [PMID: 17403173] [DOI] [PubMed] [Google Scholar]
Vollset 2013
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, B-Vitamin Treatment Trialists' Collaboration. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013;381(9871):1029-36. [PMID: 23352552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2002
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002;325:1202-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2014
- Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. Journal of Clinical Epidemiology 2014;67(6):622-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2005
- Wang H, Tan H, Yang F. Mechanisms in homocysteine-induced vascular disease. Drug Discovery Today: Disease Mechanisms 2005;2:25-31. [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2010
- Williams KT, Schalinske KL. Homocysteine metabolism and its relation to health and disease. Bifactors 2010;36(1):19-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wirakartakusumah 1998
- Wirakartakusumah MA, Hariyadi P. Technical aspects of food fortification. In: Food and Nutrition Bulletin. Vol. 19. United Nations University Press, Tokyo, Japan, 1998. [Google Scholar]
Wu 2013
- Wu Y, Huang Y, Hu Y, Zhong J, He Z, Li W, et al. Hyperhomocysteinemia is an independent risk factor in young patients with coronary artery disease in southern China. Herz 2013;38(7):779-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeates 2015
- Yeates K, Lohfeld L, Sleeth J, Morales F, Rajkotia Y, Ogedegbe O. A global perspective on cardiovascular disease in vulnerable populations. Canadian Journal of Cardiology 2015;31(9):1081-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yetley 2011
- Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. American Journal of Clinical Nutrition 2011;94(1):322S-31S. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yusuf 2000
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):145-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martí‐Carvajal 2007
- Martí-Carvajal AJ, Salanti G, Hidalgo R, Ciapponi A. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006612. [DOI: 10.1002/14651858.CD006612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martí‐Carvajal 2009
- Martí-Carvajal AJ, Sola I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD006612. [DOI: 10.1002/14651858.CD006612.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martí‐Carvajal 2013
- Martí-Carvajal AJ, Solà I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD006612. [DOI: 10.1002/14651858.CD006612.pub3] [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2015
- Martí-Carvajal AJ, Sola I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD006612. [DOI: 10.1002/14651858.CD006612.pub4] [DOI] [PubMed] [Google Scholar]


