Abstract
Background
People with hemophilia A or B with inhibitors are at high risk of bleeding complications. Infusion of bypassing agents, such as recombinant activated FVII (rFVIIa) and plasma‐derived activated prothrombin complex concentrate, are suggested as alternative therapies to factor VIII (haemophilia A) or IX (haemophilia B) for individuals who no longer respond to these treatments because they develop inhibitory antibodies. The ultimate goal of treatment is to preserve the individual's joints, otherwise destroyed by recurrent bleeds.
Objectives
To assess the effects of bypassing agent prophylaxis to prevent bleeding in people with hemophilia A or B and inhibitors.
Search methods
We searched for relevant studies from the Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register, comprising of references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. We also searched trial registries (16 February 2017) and bibliographic references of retrieved studies were reviewed for potential articles to be included in the review.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Coagulopathies Trials Register: 12 December 2016.
Selection criteria
We included randomized and quasi‐randomized controlled studies (cross‐over or parallel design) evaluating the effect of prophylaxis treatment with bypassing agents compared with on‐demand treatment, or studies evaluating the effects of high‐dose compared with low‐dose prophylaxis in males of any age with hemophilia with inhibitors.
Data collection and analysis
Two authors independently selected studies and extracted data and assessed the risk of bias according to standard Cochrane criteria. They assessed the quality of the evidence using the GRADE criteria.
Main results
We included four randomized studies (duration 7 to 15 months) involving 116 males. Risk of bias was judged to be high in two studies due to the open‐label study design and in one study due to attrition bias.
Two studies compared on‐demand treatment to prophylaxis with bypassing agents. In one study (34 males) prophylaxis significantly reduced mean overall bleeding rates, MD ‐ 7.27 (95% CI ‐9.92 to ‐4.62) (low quality evidence), mean number of overall bleeding events per month, MD ‐1.10 (95% CI ‐1.54 to ‐0.66), mean number of hemarthrosis, MD ‐6.60 (95% CI ‐9.32 to ‐3.88) (low quality evidence) and mean number of joints that had hemarthrosis, MD ‐0.90 (95% CI ‐1.36 to ‐0.44). The meta‐analysis did not conclusively demonstrate significant benefit of prophylaxis on health‐related quality of life as measured by Haem‐A‐QoL score, EQ‐5D total score and utility score, EQ‐5D VAS and SF‐36 physical summary and mental summary score (low quality evidence for all health‐related quality of life analyses).
The remaining two studies compared dose regimens. The results from one study (22 males) did not conclusively demonstrate benefit or harm of high‐dose versus low‐dose recombinant activated factor VIIa (rFVIIa) as a prophylaxis for overall bleeding rate, MD ‐0.82 (95% CI ‐2.27 to 0.63) (moderate quality evidence), target joint bleeding rate, MD ‐3.20 (95% CI ‐7.23 to 0.83) (moderate quality evidence) and serious adverse events, RR 9.00 (95% CI, 0.54 to 149.50) (moderate quality evidence).
The overall quality of evidence was moderate to low due to imprecision from limited information provided by studies with small sample sizes and incomplete outcome data in one study.
Authors' conclusions
The evidence suggests that prophylaxis with bypassing agents may be effective in reducing bleeding in males with hemophilia with inhibitors. However, there is a lack of evidence for the superiority of one agent over the other or for the optimum dosage regimen. Further studies are needed to evaluate the benefits and harms of prophylaxis treatment on health‐related quality of life, as well as the effects of dose of bypassing agents on the outcomes.
Plain language summary
The use of bypassing agents for preventing bleeding in people with hemophilia with inhibitors
Backgound
Infusion of bypassing agents, such as recombinant activated FVII (rFVIIa) and plasma‐derived activated prothrombin complex concentrate (APCC), are suggested as alternative therapies to factor VIII (haemophilia A) or IX (haemophilia B) for individuals who no longer respond to these treatments because they develop inhibitory antibodies. The ultimate goal of treatment is to preserve the individual's joints, otherwise destroyed by recurrent bleeds. We therefore evaluated the effectiveness and safety of bypassing agents when used to prevent, as compared to treat, bleeds. We also compared different doses of bypassing agents in men with hemophilia A or B with inhibitors as a preventative (prophylactic) therapy.
Search date
The evidence is current to 12 December 2016.
Study characteristics
We searched the scientific databases for clinical studies evaluating the effects of bypassing agents in men with hemophilia A or B with inhibitors. We included four studies (duration 7 to 15 months), involving 116 individuals. Two studies compared the prophylactic infusion with bypassing agent to on‐demand treatment (treatment given only after the bleeding occurred) and two studies compared high‐dose to low‐dose preventative therapies.
Key Results
Limited evidence showed that prophylactic use of bypassing agents reduced bleeding events, joint bleeding events and number of affected joints. There was no evidence for improved quality of life amongst those who received prophylaxis as compared to those who received on‐demand therapy. There was no evidence for a difference in benefits or harms between high‐ and low‐dose rFVIIa for prophylaxis.
Quality of evidence
The overall quality of evidence of these studies was moderate to low as the included studies were small and provided limited information. Also, in one of the studies, up to 24% of the men recruited were not included in the analysis of the results, which further increases imprecision of results.
Summary of findings
Summary of findings for the main comparison. Prophylaxis therapy compared with on‐demand therapy with FEIBA for hemophilia A or B with inhibitors.
| Prophylaxis therapy compared with on‐demand therapy with FEIBA for hemophilia A or B with inhibitors | ||||||
|
Population: adults and children with hemophilia A or B with inhibitors Settings: outpatients Intervention: prophylaxis therapy (FEIBA) Comparison: on‐demand therapy (FEIBA) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| On‐demand | Prophylaxis | |||||
|
Overall bleeding rates: total number of bleeding events Follow‐up: 6 months |
Not estimable (see comment) |
The mean total number of bleeding events in the prophylaxis group was 7.27 lower than the on‐demand group (9.92 lower to 4.62 lower). | Not estimable (see comment) |
26 (1 study)1 | ⊕⊕⊝⊝ low2,3 | Corresponding risk (mean difference between groups) was estimated taking account of the cross‐over design of the study. Assumed risk in the on‐demand group cannot be directly calculated. |
|
Annualised bleeding rate Follow‐up: 12 months |
Median 28.7 (IQR, 32.3) | The median annualised bleeding rate was 7.9 (IQR 8.1), which was 72.5% lower than on‐demand group. | Not estimable (see comment) |
34 (1 study) |
⊕⊕⊕⊝ moderate2 | Data presented as median values and could not be entered into analysis. |
|
Target joint bleeding rate: number of hemarthrosis Follow‐up: 6 months |
Not estimable (see comment) |
The mean number of hemarthrosis in the prophylaxis group was 6.60 lower than the on‐demand group (9.32 lower to 3.88 lower). | Not estimable (see comment) |
26 (1 study)1 | ⊕⊕⊝⊝ low2,3 | Corresponding risk (mean difference between groups) was estimated taking account of the cross‐over design of the study. Assumed risk in the on‐demand group cannot be directly calculated. |
|
AJBR Follow‐up: 12 months |
Median 22.9 (IQR, 32.8) | The median AJBR was 6.0 (IQR 7.1), which was 73.8% lower than on‐demand group. | Not estimable (see comment) |
34 (1 study) |
⊕⊕⊕⊝ moderate2 | Data presented as median values and could not be entered into analysis. |
|
Quality of life Follow‐up: 6 to 12 months |
Not estimable (see comment) |
There were also no significant differences between the prophylaxis and on‐demand treatment for health‐related quality of life. | Not estimable (see comment) |
up to 58 (2 studies)1 |
⊕⊕⊝⊝ low2,3 | The health‐related quality of life was measured using Haem‐A‐QoL, Haemo‐QoL, EQ‐5D, and general pain visual analog scale (VAS) and the Short‐Form (SF)‐36 Health survey. |
|
Safety of bypassing agents Follow‐up: 6 to 12 months |
Not estimable | There were no thromboembolic event and serious complications reported in participants who received treatment. | Not estimable (see comment) |
up to 67 (2 studies)1 |
⊕⊕⊝⊝ low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AJBR: annualised joint bleeding rate; CI: confidence interval; FEIBA: factor eight inhibitor bypassing activity; IGR: interquartile range | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. One study is of a cross‐over design. 34 participants were randomized in the study but up to 24% of participants were excluded from results. 2. Downgraded once due to imprecision; limited information available from one or two small studies. 3. Downgraded once due to risk of bias: incomplete outcome data, up to 24% of participants are excluded from analyses.
Summary of findings 2. High‐dose compared with low‐dose rFVIIa for hemophilia A or B with inhibitors.
| High‐dose compared with low‐dose rFVIIa for hemophilia A or B with inhibitors | ||||||
|
Population: adults and children with hemophilia A or B with inhibitors Settings: outpatients Intervention: high‐dose therapy (rFVIIa) Comparison: low‐dose therapy (rFVIIa) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low dose | High dose | |||||
|
Overall bleeding rates: number of bleeds per month Follow‐up: 3 months |
The mean number of bleeds per month in the low‐dose group was 2.18 | The mean number of bleeds per month in the high‐dose group was 0.82 lower than the low‐dose group (2.27 lower to 0.63 higher). | Not estimable | 22 (1 study) |
⊕⊕⊕⊝ moderate1 | An additional study recruited participants to 200 μg/kg, 100 μg/kg and 25 μg/kg. Results showed reduction of overall bleeding rates for all 3 groups during prophylaxis as compared to on‐demand period. (Data could not be entered into analysis as SDs not presented.) |
|
Annualised bleeding rate Follow‐up: 3 months |
Not estimable (see comment) |
Not estimable (see comment). |
Not estimable (see comment) |
24 (1 study) |
NA | Results showed reduction of overall bleeding rates for all 3 groups during prophylaxis as compared to on‐demand period. (Data could not be entered into analysis as SDs not presented.) |
|
Target joint bleeding rate Follow‐up: 3 months |
The mean number of joint bleeds per month in the low‐dose group was 4.7 | The mean number of bleeds per month in the high‐dose group was 3.20 lower than the low‐dose group (7.23 lower to 0.83 higher). | Not estimable | 22 (1 study) |
⊕⊕⊕⊝ moderate1 | |
|
AJBR Follow‐up: 3 months |
Outcome not reported. | NA | ||||
|
Quality of life Follow‐up: NA |
Outcome not reported. | NA | ||||
| Safety of bypassing agents | There were no significant differences between doses in terms of adverse events or serious adverse events (see comment). | Not estimable | 46 (2 studies) |
⊕⊕⊕⊝ moderate1 | Doses compared were: 270 μg/kg and 90 μg/kg 200 μg/kg and 100 μg/kg 200 μg/kg and 25 μg/kg 100 μg/kg and 25 μg/kg. |
|
| *The basis for the assumed risk is the event rate in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AJBR: annualised joint bleeding rate; CI: confidence interval; NA: not applicable; rFVIIa: recombinant factor VIIa; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to imprecision; limited information available from one or two small studies.
Background
Description of the condition
Congenital deficiency of factor (F) VIII or FIX results in the formation of alloantibodies which inhibit the activity of infused clotting factor concentrates (CFCs) in approximately 30% of people with severe (defined as less than 1 international unit (IU) dL⁻¹ baseline clotting factor activity) hemophilia A (FVIII deficiency) and 2% to 5% of those with severe hemophilia B (FIX deficiency) (Iorio 2010a).
In an analysis of the UK Haemophilia Centre Doctors' Organisation database, the cumulative risk of inhibitor formation was 16% and 36% at five years and 75 years of age (respectively) for the 6078 males with hemophilia A and 6% and 8% (respectively) for the 1172 males with hemophilia B (Darby 2004). The rate of inhibitor formation is dependent on the residual or baseline circulating FVIII or FIX level, being greatest in those people with severe disease (Hay 1998). Among people with non‐severe disease, those with certain mutations, particularly in the A2 and C2 domains of the F8 gene, are more likely to develop inhibitory antibodies.
Development of inhibitors is a complex process, but likely includes factors related to the individual being treated, the environment and the treatment provided. Patient‐related or genetically determined factors include ethnicity, race, the severity of the hemophilia, hemophilia causing mutation, major histocompatibility class, and immunogenotype (Astermark 2010a). Environmental or non‐genetic factors are ones perceived by the immune system as danger signals (Matzinger 1994; Matzinger 2012) and may include the reason for the first infusion at a young age and the intensity of treatment (Astermark 2010b).
Description of the intervention
Once the diagnosis of an inhibitor is made, treatment options include the management of acute bleeding, prevention of bleeding, and immune tolerance induction (ITI), the latter being the primary treatment option (Collins 2013). One randomized prospective trial of ITI in children with favorable risk factors has been completed (Hay 2012). Although 70% of participants had a complete response to ITI, six of 37 participants relapsed after a median of 9.5 months. Those in whom ITI failed or who relapsed after successful ITI, along with those in whom ITI was not attempted or those on ITI but still with bleeding, remain at risk for bleeding and hence are candidates for prophylaxis with a bypassing agent.
Recombinant activated FVII (rFVIIa) and plasma‐derived activated prothrombin complex concentrate (APCC) are currently the only two bypassing agents available for use in people with hemophilia with inhibitors. Two randomized trials of acute bleeding management with bypassing agents have been completed, one with rFVIIa (Young 2008) and one with APCC (Astermark 2007) but neither involved a comparison of the prophylactic use of the two drugs. These trials were reviewed in 2010 and superiority of one treatment over the other in terms of hemostatic control or thrombosis risk could not be demonstrated in participants with acute bleeding (Iorio 2010b). Similarly, several non‐randomized studies failed to demonstrate superiority of one agent over the other (Chuansumrit 2000; Kavakli 2006; Lusher 1998; Pruthi 2007; Santagostino 2006; Seremetis 1994; Shapiro 1998).
Frequent bleeding, especially joint bleeding, is a common disease manifestation in people with hemophilia and inhibitors and impacts overall health, joint health, and quality of life (Scalone 2006). Prophylaxis is the use of treatment on a regular basis to prevent or reduce bleeding episodes. The choice of prophylactic infusion requires the medication to be effective for bleeding management. Therefore, prophylaxis in people with hemophilia with inhibitors that do not respond to routine factor concentrates must rely on the use of bypassing agents. In people without inhibitors, prophylaxis is regarded as standard of care and is associated with a reduction in musculoskeletal disease burden and with a good quality of life (Iorio 2011). However, experience with prophylaxis is limited in people with inhibitors.
In 2013, Collins recommended that prophylaxis with a bypassing agent should be considered in children with inhibitors after the first hemarthrosis in an effort to prevent joint damage and in older people with recurrent bleeding (Collins 2013). Regarding the choice of bypassing agent, it was recommended that this decision be individualized based on prior response to treatment, logistics of administration and cost. In October 2013, the Medical and Scientific Advisory Council (MASAC) of the National Hemophilia Foundation recommended that prophylaxis with bypassing agents should be considered in people with inhibitors (MASAC 2013). No specific guidelines were provided to guide clinicians who wished to prescribe prophylaxis.
The optimal treatment for people with hemophilia without inhibitors is the prophylactic administration of factor VIII (hemophilia A) or factor IX (hemophilia B) concentrates (Iorio 2011). The development of neutralizing antibodies makes treatment of bleeding with CFCs more difficult and when the concentration of the antibody is above a certain level (≥ 5 Bethesda units dL⁻¹ (BU)), replacement therapy is no longer effective (Berntorp 2006). In these cases with high titres of antibody, treatment with bypassing agents, either rFVIIa or APCC, is necessary to control acute bleeding (Shapiro 2003).
Prevention of bleeding or prophylaxis in cases with high titres of antibody requires use of rFVIIa or APCC.
How the intervention might work
The development of anti‐factor VIII or anti‐factor IX antibodies makes the administration of substitution therapy with factor VIII or IX (respectively) ineffective. Bypassing agents are treatments that are able to activate the coagulation cascade independently of factor VIII and IX; thus they are unaffected by the presence of factor VIII or factor IX inhibitors. Bypassing agents, including rFVIIa and APCC, have different mechanisms of action by which they drive coagulation. It is known that rFVIIa activates factor Xa on an activated platelet surface or when, it is bound to tissue factor, it can directly activate thrombin. Alternatively, APCC mainly acts by providing factors IX and X which are able to bypass the need for FVIII to drive thrombin generation (Hedner 2000).
Prophylaxis with bypassing agents may prevent bleeding in people with hemophilia with inhibitors and specifically reduce overall bleeding rates and joint bleeding rates without excess thrombotic and infectious risks. Ideally, prophylaxis with bypassing agents in people with inhibitors will ensure long‐term joint protection, in the way that the prophylactic administration of factor VIII and IX does in people without inhibitors.
Why it is important to do this review
The rationale for this review is that prophylaxis with bypassing agents may improve the quality of life for people with hemophilia A or B with inhibitors and reduce the economic burden of treatment. This information may help to inform and guide clinicians in decision making when managing people with congenital hemophilia and inhibitors.
Objectives
To assess the effects of bypassing agent prophylaxis to prevent bleeding in people with hemophilia A or B and inhibitors.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled studies and quasi‐randomized controlled studies (cross‐over or parallel design).
Types of participants
Males of any age with severe congenital hemophilia A or B complicated by high‐responding inhibitors to FVIII or FIX, respectively, requiring a bypassing agent as prophylaxis to control or prevent bleeding.
Types of interventions
Prophylaxis, at any dose, any dosing frequency, and any regimen, of rFVIIa or APCC for preventing bleeding versus each other or no prophylaxis.
Types of outcome measures
Primary outcomes
Overall bleeding events (per month), as defined by study authors
Secondary outcomes
Annualised bleeding rate
Target joint bleeding rate
Annualised joint bleeding rate (AJBR)
Quality of life (QoL) (generic and specific validated scales including EQ‐5D, Haem‐A‐QoL, Haemo‐QoL)
Safety of the bypassing agents including adverse events, serious adverse events, or thromboembolic events
Cost and resource utilization when comparing prophylaxis to on‐demand treatment regimens, including overall drug utilization
Search methods for identification of studies
There were no restrictions regarding language or publication status.
Electronic searches
We identified relevant studies from the Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register using the terms: (Factor VIII Inhibitors) OR (factor inhibitors).
The Coagulopathies Register was compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE and prospective hand‐searching of one specialized journal, Haemophilia. Unpublished work was identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the International Society of Haemostasis and Thrombosis Congresses; and the International Congresses of World Federation of Haemophilia. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Coagulopathies Trials Register: 12 December 2016.
We also searched trial registries such as ClinicalTrials.gov (www.clinicaltrials.gov) and the International Clinical Trials Registry Platform (www.who.int/ictrp/en/) in an attempt to identify relevant studies for inclusion (Appendix 1). Date of most recent search: 16 February 2017.
Searching other resources
The bibliographic references of retrieved studies were reviewed for additional references to be included in this review.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the abstracts from the identified articles to select those that were potentially eligible to be included in the review. The authors retrieved the full text reports of studies that were deemed potentially relevant and linked together multiple reports of the same study. The authors independently examined the full text of the studies for compliance with the eligibility criteria; and if necessary contacted the study investigators to determine study eligibility. The authors resolved any disagreements on study inclusion by discussion in order to reach a consensus.
Data extraction and management
A pair of authors (CC, SJN and MS) independently reviewed the identified articles and extracted data on the following (a third author arbitrated any differences).
Inclusion criteria for the study
Location and timeframe of the study
Participant number and demographics
Study methods
Study design
Type, characteristics and duration of the intervention and control groups if applicable
Outcome measures and description
Information on limitations or bias (or both)
Assessment of risk of bias in included studies
A pair of authors (CC, SJN and MS) independently assessed the risk of bias of the included studies. The risk of bias was assessed using Cochrane's tool for assessing the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For each study, the risk of bias was noted as being either 'high risk', 'low risk', or 'unclear risk' for the following criteria.
Sequence generation
Allocation concealment
Blinding of participant and personnel
Blinding of outcome assessment
Incomplete outcome data
Selected outcome reporting
Other issues
Measures of treatment effect
For the primary outcome, overall bleeding events were represented as a mean number of bleeding events per month. Secondary outcomes of target joint bleeding events and annualised joint bleeding events were represented as a mean number of bleeding events per month. We analyzed the data as continuous data, and reported mean differences (MD) with the corresponding 95% confidence intervals (CIs).
For safety outcomes, i.e. adverse events related to treatment, the effect was presented as the proportion of males presenting the event, and a risk ratio (RR). We calculated a pooled estimate of the treatment effect for each outcome using the pooled RR and 95% CIs.
With respect to health‐related quality of life (HRQoL), the mean and standard deviation (SD) of scores from scales were presented. We calculated the mean change from baseline for each group or the mean post‐intervention values and SD for each group. We converted standard errors (SE) to SDs. We produced a pooled estimate of treatment effect by calculating the MD and 95% CIs. If economic data are reported in updates of this review, we will analyse these data in the same way.
If for a future update, continuous scores measure the same outcome but in a variety of ways (e.g. different scales to measure knowledge or quality of life), we plan to standardize the outcomes to a uniform scale using the standardized MD (SMD).
Where studies reported multiple measures for the same outcome, the review authors considered absolute changes in the measure in the context of comparable data being available for each participant before and after the intervention (i.e. change from baseline).The authors recorded continuous data, such as joint score change, as either mean change from baseline for each group or mean post‐treatment values (if change from baseline was not reported) and SD for each group.
Unit of analysis issues
No cluster‐randomized studies were identified. If cluster‐randomized studies are identified for updates of the review, we will check these studies for unit of analysis errors and perform analysis based on the advice given in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
When conducting a meta‐analysis combining results from cross‐over studies, we used the methods recommended by Elbourne (Elbourne 2002). We extracted (or calculated) MDs and SEs adjusted for the paired design of cross‐over studies. If this had not been possible, we would have considered whether data were presented by treatment period and include only first‐period data in the analysis. If neither of these options were possible, we would have included data from cross‐over studies narratively in the review.
We included one cross‐over study in the review (Leissinger 2011). For the outcome of overall bleeding events, we were able to extract individual participant data from a graph and calculate a MD and adjusted SE. We were also able to estimate that the correlation between treatment arms was around 0.4 from these data. Therefore, we have used this correlation estimate to adjust estimates of SE for other continuous outcomes measured in this study.
Dealing with missing data
If reports were incomplete, we attempted to contact the original investigators in an effort to obtain the necessary data or information. Where the original investigators could not provide additional information, we examined the proportion and distribution of missing data (e.g. proportion of missing outcome data, demographic data, missing information regarding study design methods etc.). We also considered, where possible, whether data were likely to be missing at random or not and whether the missing data were likely to have had an impact on the results of the study. We judged the risk of bias due to incomplete outcome data accordingly and performed sensitivity analyses, excluding studies with large proportions of missing data, if appropriate.
Assessment of heterogeneity
We assessed heterogeneity through visual examination of the combined data presented in the forest plots, and by considering the I² statistic together with Chi² values (significance level P < 0.1) (Deeks 2011). The I² statistic reflects the likelihood that variation of results across studies are due to heterogeneity rather than by chance, and is interpreted as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We intended to assess publication bias by constructing, then visually inspecting, the funnel plot (where a minimum of 10 studies could be included), and investigated outcome reporting bias by comparing the methods and results sections of the published papers.
Data synthesis
Where meta‐analysis could be conducted, we employed a fixed‐effect model in the first instance. For future updates, when moderate or higher heterogeneity is identified (I² of around 30% or higher), a random‐effects model will be employed. For dichotomous outcomes (adverse events related to treatment), we employed the Mantel‐Haenszel method of meta‐analysis and present pooled RRs and 95% CIs. For continuous outcomes (overall bleeding events, annualised bleeding rate, target joint bleeding events, annualised bleeding events, HRQoL, economic data, joint score change), we employed the inverse variance method of meta‐analysis and present pooled MD or SMD (as appropriate, see Measures of treatment effect) and 95% CIs.
Subgroup analysis and investigation of heterogeneity
If we had identified moderate or higher heterogeneity (I² of around 30% or higher), we intended to investigate this by subgroup analyses based on:
diagnosis (e.g. hemophilia A or B);
age of the participants (boys or adult males).
Sensitivity analysis
We intended to explore the impact of including studies with high levels of missing data on the overall treatment effect, if appropriate.
Summary of findings and quality of the evidence (GRADE)
In a post hoc change from protocol, we have presented a summary of findings tables for each comparison in the review (Table 1; Table 2).
The following outcomes were reported in the tables (chosen based on relevance to clinicians and consumers): overall bleeding events; annualised bleeding rate; target joint bleeding rate; AJBR; quality of life; safety of the bypassing agents.
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious (Balshem 2011; Guyatt 2008).
Results
Description of studies
Results of the search
See Figure 1.
1.
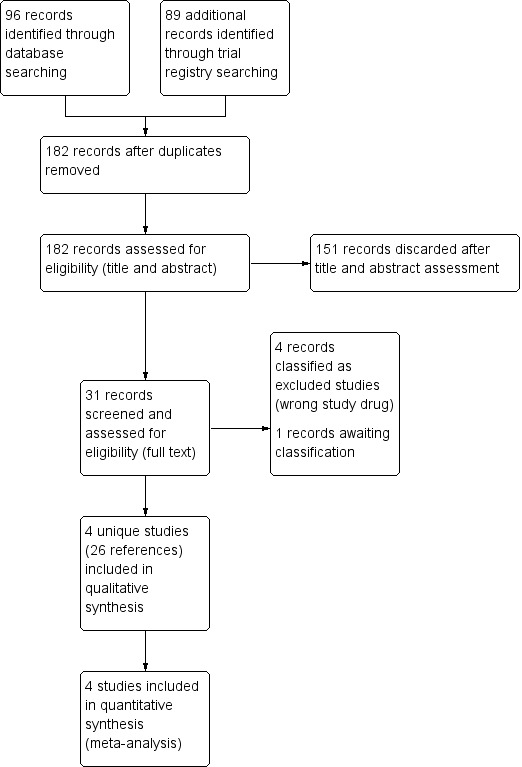
Study flow diagram.
We identified 96 citations from the database searches. We identified a further 89 references from searches of trial registries. Of the total 185 references, we removed three duplicates. After we reviewed the remaining titles and abstracts, 151 were discarded as not being relevant and we retrieved the full‐text references (where possible) of nine studies (31 references). Of these, four unique studies (reported in 26 references) were eligible randomized controlled studies and were included in the review (Antunes 2014; Konkle 2007; Leissinger 2011; Ljung 2013). We excluded four studies (NCT02622321; NCT02795767; NCT02847637; NCT03020160) and classified one study as awaiting further assessment (NCT01105546).
Included studies
SeeCharacteristics of included studies.
Four studies are included in the review (Antunes 2014; Konkle 2007; Leissinger 2011; Ljung 2013).
The Antunes study was a multicenter, parallel, randomized controlled study conducted in 17 centers (Antunes 2014). Participants were enrolled if they were hemophilia A or B with a history of high‐titre inhibitor (> 5 BU/mL) or low‐titre inhibitor (≤ 5 BU/mL) with refractory to increased dosing of factor replacement therapy. A total of 17 participants were randomized to prophylaxis group and 19 were randomized to the on‐demand therapy group. Prophylaxis regimen was nanofiltered FVIII inhibitor bypassing activity (FEIBA NF) 85 +/‐ 15 units/kg bolus infusion every other day. Participants who were in the on‐demand therapy group received FEIBA NF administration when they experienced a bleeding episode, dosing depended on type of bleeding. The study lasted for 12 months. The primary outcome was annualised bleeding rate. Secondary outcomes were AJBR, overall bleeding events, target joint bleeding events, occurrence of new target joints, hemostatic efficacy, total FEIBA NF utilization, safety and QoL.
The Konkle study was a multicenter, parallel, randomized controlled study conducted in 20 centers (Konkle 2007). Participants were enrolled if they had severe hemophilia A or B with a high inhibitor titre (> 2 BU/mL), required treatment of bleeds with a bypassing agent and had had at least four bleeds requiring hemostatic drug treatment within the previous month prior to enrolling. There were three study periods (pre‐prophylaxis, prophylaxis and post‐prophylaxis). Each period was for three consecutive months. Eleven participants were randomized to rFVIIa 270 μg/kg once daily and 11 were randomized to rVFIIa 90 μg/kg once daily. The primary outcome was the number of bleeds per month. Secondary outcomes were site‐specific bleeding rates, safety, HRQoL and orthopedic joint scores.
The Leissinger study was a multicenter, cross‐over study conducted in 16 centers (Leissinger 2011). Eligible participants were males with severe hemophilia A and a history of high‐titre inhibitor (> 5 BU/mL), who were older than two years of age, being treated with bypassing agents and had six or more episodes of bleeding requiring treatment in the six months prior to study enrollment. Prophylaxis regimen was anti‐inhibitor coagulant complex (AICC)‐FEIBA at a target dose of 85 (+/‐15%) units/kg. During the on‐demand therapy period, participants received FEIBA at a dose of 85 (+/‐15%) units/kg if they experienced bleeding episodes. A total of 17 participants were randomized to prophylaxis first (six months of prophylaxis, followed by a three‐month washout period and six months of an on‐demand period). A total of 17 participants were randomized to on‐demand therapy first (six months of on‐demand therapy, followed by three months of washout period and six months of prophylaxis). The primary outcome was total bleeding events (prophylaxis versus on‐demand periods). Secondary outcomes were the number of joint bleeds, the number of target joint bleeds, HRQoL and safety.
The Ljung study was a multicenter, parallel study conducted in 19 centers (Ljung 2013). Eligible participants had hemophilia A or B with high titre inhibitor (≥ 5 BU/mL, frequent bleeds, age 12 to 65 years), had at least two bleeding episodes within the last month or 12 bleeding episodes within the last six months prior to enrolling. There were three study periods (three months of observation, followed by three months of prophylaxis and one month of observation). The prophylaxis regimen was 40K glycoPEGylated recombinant FVIIa bypassing agent (N7‐GP) administered at target doses of 25 μg/kg, 100 μg/kg and 200 μg/kg intravenously every second day. During the on‐demand period, participants received rFVIIa for the treatment of bleeding episodes. The primary outcome was a reduction in the annualised bleeding rate (prophylaxis versus on‐demand therapy periods). Secondary outcomes were the number of specific bleeds (stratified by sites) and causes of bleeding.
Excluded studies
We identified four studies which were excluded from the review (NCT02622321, NCT02795767, NCT02847637, NCT03020160) as the study drug was neither rFVIIa or APCC.
Studies awaiting assessment
We identified one study which we are currently awaiting further information on, once obtained, we will assess this for eligibility (NCT01105546).
Risk of bias in included studies
2.
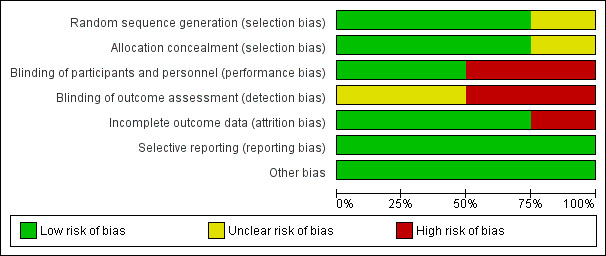
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
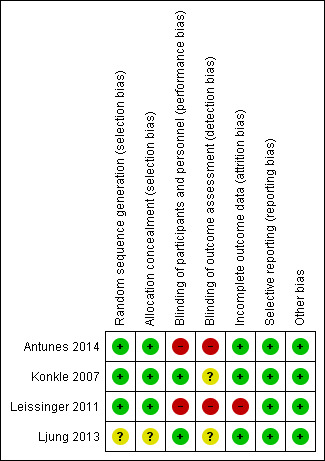
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The Antunes study used centralised stratified, block randomization (Antunes 2014), the Konkle study used centralised computer‐generated randomization (Konkle 2007) and the Leissinger study conducted the randomization and treatment allocation by using centralized call center (telephone randomization) (Leissinger 2011). Risk of selection bias was judged as 'low risk' of bias for these three studies. The Ljung study described that the participants were randomized and stratified by age (Ljung 2013). There was no further information regarding random sequence generation and allocation concealment; the risk of bias for this study was judged as 'unclear'.
Blinding
Two studies were open‐label and there was no information regarding the blinding of outcome assessors; therefore the risk of bias was judged as 'high risk' (Antunes 2014; Leissinger 2011). The Konkle and Ljung studies described the blinding procedure as providing an equal volume of trial drug to be injected in both groups (Konkle 2007; Ljung 2013). There was no information regarding blinding of the outcome assessors; the risk of bias was judged as 'low risk' for the latter studies.
Incomplete outcome data
The Antunes study reported that two participants withdrew from the on‐demand group and one from the prophylaxis group (Antunes 2014). The Ljung study reported that three participants withdrew during the study period (one from the 100 μg/kg group and one from the 200 μg/kg group) (Ljung 2013). There were no withdrawals during the study period in the Konkle study (Konkle 2007). All participants were included in an intention‐to‐treat analysis. The participants were well balanced across groups. The risk of bias was judged to be 'low risk' for these three studies. The Leissinger study reported that one participant withdrew consent before receiving the study medication and a further seven did not complete the study; the risk of bias was judged as 'high risk' for this study (Leissinger 2011).
Selective reporting
All of the studies clearly specified primary and secondary outcomes. The data on all outcomes were reported. There was no evidence of selective reporting, therefore, the risk of bias was judged to be 'low risk' for all four studies.
Other potential sources of bias
All four studies were deemed to be at 'low risk' of bias from other source of bias.
Effects of interventions
Two comparisons were made in this review, prophylaxis compared to on‐demand therapy and low‐dose compared to high‐dose therapy.
Prophylaxis compared to on‐demand therapy
Two studies recruiting 70 participants contributed to this comparison (Antunes 2014; Leissinger 2011). Both studies compared prophylaxis to on‐demand therapy with FEIBA (AICC or NF).
Primary outcome
1. Overall bleeding rates (per month)
One study reported on the bleeding rate per month (over a six‐month period) on prophylaxis and on‐demand therapy (Leissinger 2011). At six months, prophylaxis with bypassing agent (FEIBA) significantly reduced mean overall bleeding rates, MD ‐7.27 (95% CI ‐9.92 to ‐4.62) (low quality evidence) (Analysis 1.1) and the mean number of overall bleeding per month, MD ‐1.10 (95% CI ‐1.54 to ‐0.66) compared with those who are on‐demand group (Analysis 1.2).
1.1. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 1 Overall bleeding rates: total number of bleeding events.
1.2. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 2 Overall bleeding rates: number of monthly bleeding events.
Secondary outcomes
1. Annualised bleeding rates
One study reported on annualised bleeding rates; however, data were presented as median values and cannot be entered into analysis (Antunes 2014). The median annualised bleeding rate was significantly lower among those who were allocated to prophylaxis as compared to on‐demand treatment (7.9 versus 28.7) (moderate quality evidence).
2. Joint bleeding rates
One study reported mean number of hemarthrosis (Leissinger 2011). At six months, prophylaxis with bypassing agent (FEIBA) significantly reduced mean number of hemarthrosis, MD ‐6.60 (95% CI ‐9.32 to ‐3.88) when compared to on‐demand treatment (low quality evidence) (Analysis 1.3). Mean number of joint hemorrhages was significantly lower among those who were on prophylaxis, MD ‐0.90 (95% CI ‐1.36 to ‐0.44) when compared to those with on‐demand therapy (Analysis 1.4).
1.3. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 3 Target joint bleeding rate: number of hemarthroses.
1.4. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 4 Target joint bleeding rate: number of joint hemorrhages.
3. AJBR
One study reported on annualised bleeding rates; however, data were presented as median values and cannot be entered into analysis (Antunes 2014). The median AJBR was significantly lower among those who were allocated to prophylaxis as compared to on‐demand treatment (6.0 versus 22.9) (moderate‐quality evidence).
4. Quality of life
Two studies reported outcome regarding to HRQoL (low‐quality evidence) (Antunes 2014; Leissinger 2011). The Antunes study evaluated HRQoL using the Haem‐A‐QoL, Haemo‐QoL, EQ‐5D, and general pain visual analog scale (VAS) at six and 12 months (Antunes 2014). Leissinger assessed HRQoL using the EQ‐5D questionnaire and the Short‐Form (SF)‐36 Health survey (Leissinger 2011).
After 12 months of study, mean change from baseline of Haem‐A‐QoL score was not significantly different but in favour of prophylaxis as compared with on‐demand treatment, MD 3.40 (95% CI, ‐5.53 to 12.33) (Analysis 1.5). Mean change from baseline of EQ‐5D scores were not significantly different but in favour prophylaxis, measuring at six months, MD 0.09 (95% CI ‐0.09 to 0.27) and 12 months, MD 0.07 (95% CI ‐0.13 to 0.27) (Analysis 1.6). Mean change from baseline of EQ‐5D VAS was not significantly different but in favour prophylaxis, measuring at six months, MD 4.11 (95% CI, ‐3.66 to 11.88) and 12 months, MD 9.90 (95% CI, ‐5.93 to 25.73) (Analysis 1.7). Mean change from baseline of EQ‐5D utility score were not statistically different between prophylaxis and on‐demand group, MD 0.00 (95% CI ‐0.12 to 0.12) (Analysis 1.8). The mean change from baseline of SF36‐physical summary score was not significantly different comparing between prophylaxis and on‐demand treatment at six months, MD 2.90 (95% CI ‐1.53 to 7.33) (Analysis 1.17). Likewise, the mean change from baseline of SF36‐mental summary score was not significantly different comparing between prophylaxis and on‐demand treatment at six months, MD 1.20 (95% CI ‐2.75 to 5.15) (Analysis 1.18). There were also no significant differences between the treatments for any of the individual SF‐36 physical or mental domains (Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16).
1.5. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 5 Health‐related QoL: change from baseline in total Haem‐A‐QoL score.
1.6. Analysis.
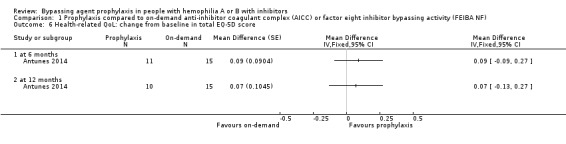
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 6 Health‐related QoL: change from baseline in total EQ‐5D score.
1.7. Analysis.
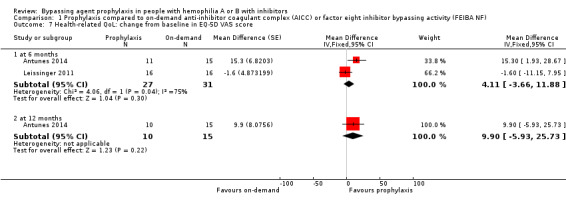
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 7 Health‐related QoL: change from baseline in EQ‐5D VAS score.
1.8. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 8 Health‐related QoL: change from baseline in EQ‐5D Utility score.
1.17. Analysis.
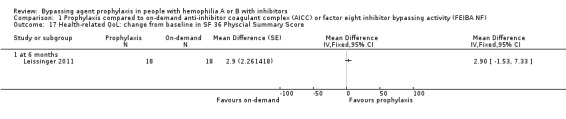
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 17 Health‐related QoL: change from baseline in SF 36 Physcial Summary Score.
1.18. Analysis.
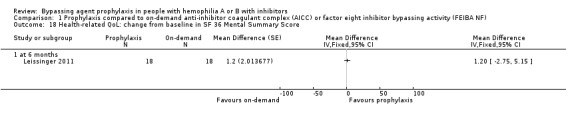
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 18 Health‐related QoL: change from baseline in SF 36 Mental Summary Score.
1.9. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 9 Health‐related QoL: change from baseline in SF 36 Physical Functioning Score.
1.10. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 10 Health‐related QoL: change from baseline in SF 36 Physical Score.
1.11. Analysis.
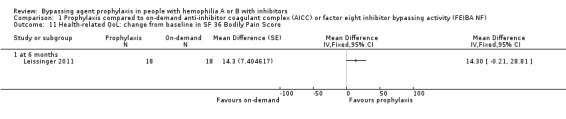
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 11 Health‐related QoL: change from baseline in SF 36 Bodily Pain Score.
1.12. Analysis.
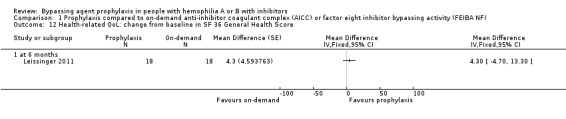
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 12 Health‐related QoL: change from baseline in SF 36 General Health Score.
1.13. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 13 Health‐related QoL: change from baseline in SF 36 Vitality / Energy Score.
1.14. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 14 Health‐related QoL: change from baseline in SF 36 Social Functioning Score.
1.15. Analysis.

Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 15 Health‐related QoL: change from baseline in SF 36 Emotional Score.
1.16. Analysis.
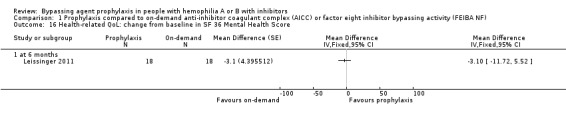
Comparison 1 Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF), Outcome 16 Health‐related QoL: change from baseline in SF 36 Mental Health Score.
5. Safety of the bypassing agents
Two studies reported on the safety of the bypassing agents (low quality evidence) (Antunes 2014;Leissinger 2011). Antunes reported 36 (28.8%) of treated participants in two groups experienced serious adverse effects (Antunes 2014). No study reported thromboembolic events or major safety issues. Leissinger reported that one participant had an allergic reaction to the study drug during prophylaxis and three participants had events related to central venous access during prophylaxis and on‐demand therapy (Leissinger 2011).
6. Cost and resource utilization
Neither study reported this outcome (Antunes 2014; Leissinger 2011)
High‐dose compared to low‐dose prophylaxis therapy
Two studies recruiting 46 participants contributed to this comparison (Konkle 2007; Ljung 2013). Both compared doses of rFVIIa; Konkle compared 270 μg/kg to 90 μg/kg and Ljung compared three doses of 200 μg/kg, 100 μg/kg and 25 μg/kg (Konkle 2007; Ljung 2013).
Primary outcome
1. Overall bleeding rate (per month)
One study reported the overall bleeding rates comparing high‐dose (270 μg/kg) versus low‐dose (90 μg/kg) rFVIIa (Konkle 2007). High‐dose rFVIIa reduced the mean number of bleeds per month when compared to low‐dose rFVIIa, but the difference between the groups was not statistically significant, MD ‐0.82 (95% CI ‐2.27 to 0.63) (moderate quality evidence) (Analysis 2.1).
2.1. Analysis.
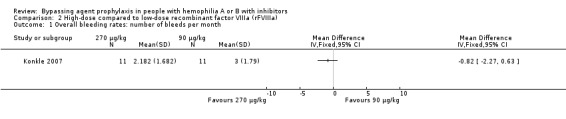
Comparison 2 High‐dose compared to low‐dose recombinant factor VIIIa (rFVIIIa), Outcome 1 Overall bleeding rates: number of bleeds per month.
Ljung reported the mean annualised bleeding rate per month, comparing three doses of rFVIIa (200 μg/kg, 100 μg/kg and 25 μg/kg) (Ljung 2013). The mean annualised bleeding rates per month were reduced during prophylaxis as compared to the on‐demand treatment period for all three doses of rFVIIa. The bleeding rate could not be analysed between the groups due to the inadequate outcome reports.
Secondary outcomes
1. Annualised bleeding rates
Ljung reported annualised bleeding among those who received three different doses of rFVIIa (Ljung 2013). As compared to on‐demand treatment period, the annualised bleeding rate during prophylaxis were reduced by 36%, 45% and 52% in participants who received rFVIIa 200 μg/kg, 100 μg/kg and 25 μg/kg, respectively. The bleeding rate could not be analysed between the groups due to the inadequate outcome reports.
2. Joint bleeding rate
One study reported target joint bleeding rates comparing high‐dose (270 μg/kg) versus low‐dose (90 μg/kg) rFVIIa (Konkle 2007). At three months, high‐dose rFVIIa reduced mean joint bleeding rates when compared to low‐dose rFVIIa but the difference between the groups was not statistically significant, MD ‐3.20 (95% CI ‐7.23 to 0.83) (moderate quality evidence) (Analysis 2.2).
2.2. Analysis.
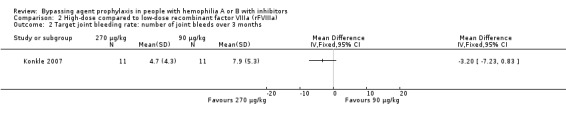
Comparison 2 High‐dose compared to low‐dose recombinant factor VIIIa (rFVIIIa), Outcome 2 Target joint bleeding rate: number of joint bleeds over 3 months.
3. AJBR
Neither study reported this outcome (Konkle 2007; Ljung 2013).
4. Quality of life
Neither study reported this outcome (Konkle 2007; Ljung 2013).
5. Safety of the bypassing agents
Two studies reported adverse events comparing high‐dose versus low‐dose rFVIIa (Ljung 2013; Konkle 2007). There was no statistical difference of the risk of adverse events between doses, RR 1.00 (95% CI 0.57 to 1.76) for 200 μg/kg compared to 100 μg/kg, RR 1.50 (95% CI 0.67 to 3.34) for 200 μg/kg compared to 25 μg/kg, RR 1.50 (95% CI 0.67 to 3.34) for 100 μg/kg compared to 25 μg/kg and RR 0.89 (95% CI 0.56 to 1.40) for 270 μg/kg compared to 90 μg/kg (moderate‐quality evidence) (Analysis 2.3).
2.3. Analysis.
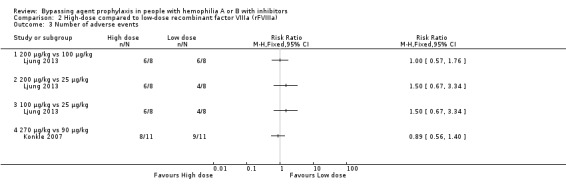
Comparison 2 High‐dose compared to low‐dose recombinant factor VIIIa (rFVIIIa), Outcome 3 Number of adverse events.
One study reported serious adverse events comparing high‐dose versus low‐dose rFVIIa (Konkle 2007). Four participants who received high‐dose rFVIIa (270 μg/kg) reported serious adverse events compared to no participants who received low‐dose (90 μg/kg). However, this difference between groups was not statistically significant, RR 9.00 (95% CI 0.54 to 149.50) (Analysis 2.4).
2.4. Analysis.
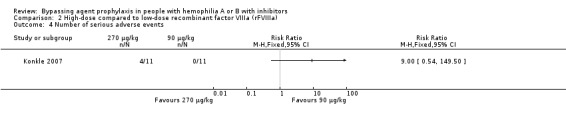
Comparison 2 High‐dose compared to low‐dose recombinant factor VIIIa (rFVIIIa), Outcome 4 Number of serious adverse events.
6. Cost and resource utilization
Neither study reported this outcome (Konkle 2007; Ljung 2013)
Discussion
Summary of main results
Four studies were found to be eligible for the review (Antunes 2014; Konkle 2007; Leissinger 2011; Ljung 2013). Two studies compared prophylaxis versus on‐demand treatment (Antunes 2014; Leissinger 2011) and two studies compared high to low doses of bypassing agents for prophylaxis (Konkle 2007; Ljung 2013). The main findings of this Cochrane Review and meta‐analysis in people with hemophilia with inhibitors suggested that prophylaxis with bypassing agents reduces overall bleeding rates and joint bleeding rates as compared to on‐demand treatment. No statistically significant differences in change from baseline for HRQoL were found and this review did not conclusively rule out benefit or harm of high‐dose compared with low‐dose rFVIIa.
Overall completeness and applicability of evidence
All of the included studies enrolled people with hemophilia A or hemophilia B with inhibitors. Two studies compared prophylaxis with on‐demand treatment (Antunes 2014; Leissinger 2011). However, the remaining two studies did not include participants who were given on‐demand treatment as a control (Konkle 2007; Ljung 2013). The prophylactic durations ranged from three months to 12 months. The data were presented differently (e.g. crude bleeding rate, annualised bleeding rate or bleeding per month) and reported in mean or median. Consequently, meta‐analyses were prevented in some cases. Two studies evaluated HRQoL; however, the tools used in these studies varied (Antunes 2014; Leissinger 2011). Consequently, a pooled analysis could not be performed for the majority of HRQoL domains. Although we observed statistically significant differences of the clinical outcomes regarding to the reduction of overall bleeding rates and joint bleeding rates in those who were allocated to prophylaxis as compared to on‐demand treatment, the clinical relevance of this finding warrant further prospective studies with a greater number of participants and a longer duration of follow‐up.
Although the studies included in this review were conducted as multicenter, multinational studies; bypassing agents as a prophylaxis in people with hemophilia with inhibitors are not widely used in countries where the resources are limited. Hence, the applicability of this evidence to certain groups of people with hemophilia A or B is limited.
Quality of the evidence
When comparing between prophylaxis versus on‐demand treatment, the quality of the evidence was moderate for outcomes relating to annualised bleeding rates; we downgraded the evidence due to the imprecision of limited data from one or two small studies. Other outcomes (bleeding rates, HRQoL and safety) provided low quality evidence for this comparison; in addition to imprecision, evidence was also downgraded due to incomplete outcome data with up to 24% of participants excluded from one cross‐over study.
When comparing high‐dose to low‐dose rFVIIa, the quality of evidence was moderate for overall bleeding rates, target joint bleeding rate and safety of bypassing agents; we downgraded evidence due to imprecision of limited data from one or two small studies. Other outcomes were not reported for this comparison.
Potential biases in the review process
We performed extensive searches for this Cochrane Review. The methodology regarding study selection, data extraction, risk of bias assessment and analyses were rigorously conducted. We contacted the corresponding authors for clarification on data; however, there were some missing reported outcomes regarding overall bleeding rate, joint bleeding rate and health‐related outcomes. In addition, the small number of included studies in this review precluded us from conducting subgroup analyses.
Agreements and disagreements with other studies or reviews
Valentino conducted a systematic review of six observational studies, involving 34 hemophilia people with inhibitors who were treated with FEIBA for prophylaxis (Valentino 2010). The median prophylactic dose was 78.5 unit per kg. There was 63.9% reduction in overall bleeding events and 73% reduction in annual joint bleeding.
Authors' conclusions
Implications for practice.
The evidence suggests that prophylaxis with bypassing agent may be effective in people with hemophilia A or B with inhibitors for the reduction of overall bleeding rates and joint bleeding rates (low to moderate quality evidence). There is insufficient evidence to show that prophylaxis does affect health‐related quality of life (HRQoL) as compared to on‐demand treatment (low quality evidence). There is lack of evidence of superiority of one agent over the other as well as the dosage regimen.
Implications for research.
The small sample sizes and substantial attrition rates of the included studies limited the precision of the effect estimates. Larger prospective studies are warranted in order to evaluate the efficacy of prophylaxis compared to on‐demand treatment and high‐dose versus low‐dose regimen of bypassing agents. In addition, outcome measures (such as bleeding event and HRQoL) were non‐uniformly reported. Consequently, it is difficult to compare the results across the studies and to perform meta‐analyses. Hemophilia‐specific tools for assessing HRQoL may be more informative and uniform. The hemophilia research community needs to develop a consensus on measuring and reporting outcomes in hemophilia‐related literature.
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2020 | Amended | Clarification statement added from Alan Smyth, Co‐ordinating Editor on 19 March 2020: The published protocol and review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. The review will be updated by March 2021 with the majority of review authors free of conflicts conflicts. Current version (post‐publication): Chatree Chai‐Adisaksopha: no conflicts of interest. Sarah J Nevitt: no conflicts of interest. Mindy L Simpson: Rush University Medical Center receives grant support on behalf of Mindy Simpson from CSL Behring and Baxter Bioscience. Mindy Simpson has also been compensated for participation in advisory boards by Baxter Bioscience, Biogen Idec, CSL Behring, and Octapharm. Maissaa Janbain: once received consultant fees from CSL Behring and Bayer and Baxter for attending advisory board meetings. Barbara A Konkle: consults with industry partners in study design and receives payment for effort in specific clinical trials. |
Notes
Sarah J Nolan (author of the protocol) is now Sarah J Nevitt.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Online trials databases ‐ search strategies
Clinicaltrials.gov
Advanced Search Form
Search terms: haemophilia AND inhibitor AND prophylaxis
Study type: Interventional Studies
WHO ICTRP
Advanced Search Form
Condition: inhibitor* AND haemophilia* OR hemophilia*
Intervention: prophyla*
[Recruitment status: ALL]
Data and analyses
Comparison 1. Prophylaxis compared to on‐demand anti‐inhibitor coagulant complex (AICC) or factor eight inhibitor bypassing activity (FEIBA NF).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall bleeding rates: total number of bleeding events | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall bleeding rates: number of monthly bleeding events | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 up to 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Target joint bleeding rate: number of hemarthroses | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Target joint bleeding rate: number of joint hemorrhages | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Health‐related QoL: change from baseline in total Haem‐A‐QoL score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5.1 at 12 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Health‐related QoL: change from baseline in total EQ‐5D score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 at 12 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Health‐related QoL: change from baseline in EQ‐5D VAS score | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 at 6 months | 2 | 58 | Mean Difference (Fixed, 95% CI) | 4.11 [‐3.66, 11.88] |
| 7.2 at 12 months | 1 | 25 | Mean Difference (Fixed, 95% CI) | 9.9 [‐5.93, 25.73] |
| 8 Health‐related QoL: change from baseline in EQ‐5D Utility score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Health‐related QoL: change from baseline in SF 36 Physical Functioning Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Health‐related QoL: change from baseline in SF 36 Physical Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Health‐related QoL: change from baseline in SF 36 Bodily Pain Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 11.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Health‐related QoL: change from baseline in SF 36 General Health Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 12.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Health‐related QoL: change from baseline in SF 36 Vitality / Energy Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 13.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Health‐related QoL: change from baseline in SF 36 Social Functioning Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 14.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Health‐related QoL: change from baseline in SF 36 Emotional Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 15.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Health‐related QoL: change from baseline in SF 36 Mental Health Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 16.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Health‐related QoL: change from baseline in SF 36 Physcial Summary Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 17.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Health‐related QoL: change from baseline in SF 36 Mental Summary Score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 18.1 at 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. High‐dose compared to low‐dose recombinant factor VIIIa (rFVIIIa).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall bleeding rates: number of bleeds per month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Target joint bleeding rate: number of joint bleeds over 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Number of adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 200 μg/kg vs 100 μg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 200 μg/kg vs 25 μg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 100 μg/kg vs 25 μg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 270 μg/kg vs 90 μg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antunes 2014.
| Methods | Multicenter parallel randomized study conducted in 17 centres in Bulgaria, Russia, Croatia, Poland, Romania, USA, Japan and Brazil | |
| Participants | 36 males (prophylaxis (n = 17) or on‐demand therapy (n = 19)) Inclusion criteria: hemophilia A or B with documented history of high‐titre inhibitor (> 5 BU/mL) or low‐titre inhibitor (≤ 5 BU/mL) refractory to increased dosing of either FVIII or FIX for at least 12 months; were ≥ 4 and ≤ 65 years of age; were currently being treated on demand with bypassing agents; had ≥ 12 bleeding episodes in the previous 12 months; and a negative HIV status, or if positive, with a stable CD4 count Exclusion criteria: symptomatic liver disease; had platelet count < 100,000 per μL; were currently receiving ITI or prophylaxis; needed elective surgery; needed alpha‐interferon or protease inhibitor use; or had previous thromboembolic events |
|
| Interventions | Prophylaxis: FEIBA NF 85 +/‐ 15 units/kg intravenously bolus infusion every other day On demand: FEIBA NF, dosing depended on type of bleeding and was at discretion of the investigator Duration of treatment: 12 months (+/‐ 14 days) |
|
| Outcomes | Primary outcome: annualised bleeding rate Secondary outcomes: AJBR, overall bleeding events, target joint bleeding events, occurrence of new target joints, hemostatic efficacy, total FEIBA NF utilization, safety and quality of life |
|
| Notes | Study sponsored by Baxter Healthcare Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization in a 1:1 ratio with stratification by geography (block size not stated) |
| Allocation concealment (selection bias) | Low risk | The randomization scheme was centralized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew from on‐demand group (1 serious adverse event and 1 surgery) and 1 from the prophylaxis group (adverse event). Withdrawal rate similar across groups and all participants included in an ITT analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes defined in the methods reported in the results, no evidence of selective reporting |
| Other bias | Low risk | None identified |
Konkle 2007.
| Methods | Multicenter randomized parallel study conducted across 20 sites in Argentina, Brazil, Bulgaria, the Philippines, Poland, Romania, Russia, South Africa, Spain, Turkey and USA | |
| Participants | 22 participants (rFVIIa 270 μg/kg (n = 11) and rFVIIa 90 μg/kg (n = 11)) Inclusion criteria: males with severe congenital hemophilia A or B with a high documented history of inhibitor titre (with an inhibitor titre > 2 BU/mL in the preceding 12 months), a requirement for current treatment of bleeds with bypassing agents, and at least four bleeds requiring hemostatic drug treatment (except dental bleeds and bruises) within the previous month. Exclusion criteria: prophylaxis with any hemostatic drug within the last 3 months, ITI within the last month, known pseudotumours, platelet count < 50,000 per μL, advanced atherosclerotic disease, and congenital or acquired coagulation disorders other than hemophilia A or B |
|
| Interventions | There were 3 phases of 3‐month study period (pre‐prophylaxis, prophylaxis and post‐prophylaxis periods). Prophylaxis with high dose: activated rFVIIa 270 μg/kg once daily, slow bolus intravenously over a period of 2 minutes Prophylaxis with low dose: rFVIIa 90 μg/kg once daily |
|
| Outcomes | Primary outcome: number of bleeds per month comparing between during the prophylaxis period and pre‐prophylaxis period Secondary outcomes: number of bleeds per month comparing between post‐prophylaxis period and prophylaxis period, site specific bleeding rates, safety, HRQoL, orthopedic joint scores |
|
| Notes | On‐demand treatments of acute/breakthrough bleeds were continued as normal practice throughout the study. 38 participants were originally recruited for the study, 37 entered the pre‐treatment observation period but 15 were withdrawn Study sponsored by Novo Nordisk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized computer‐generated randomization list was used to randomly allocate participants |
| Allocation concealment (selection bias) | Low risk | Centralized computer‐generated randomization list was used to randomly allocate participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was maintained by providing an equal volume of study drug to be injected in both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals during the study period. All randomized participants analysed, ITT approach used |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcome defined in the methods mentioned in the results, some outcomes reported in more detail than others (e.g. no numerical results reported for orthopedic status) |
| Other bias | Low risk | None identified |
Leissinger 2011.
| Methods | Multicenter randomized cross‐over study conducted at 16 centres in Europe and USA | |
| Participants | 34 participants Inclusion criteria: severe hemophilia A and a history of a factor VIII inhibitor titre exceeding 5 BU, older than 2 years old, being treated with bypassing agents and had 6 or more episodes of bleeding requiring treatment in the 6 months before the study Exclusion criteria: receiving immune tolerance therapy, regular prophylaxis with any hemostatic agent, symptomatic liver disease, platelet count of less than 100,000 per μL, planned elective surgery within 12 months, used an investigational product within 1 month of study enrolment, planned to begin treatment with interferon or protease inhibitor |
|
| Interventions | Prophylaxis: AICC (FEIBA) administration at a target dose of 85 units/kg (+/‐ 15%) on 3 non‐consecutive days weekly On demand: AICC at a target dose of 85 units/kg (+/‐ 15%) for bleeding episode Duration of treatment: 6 months of prophylaxis, 3 months of washout period and 6 months of on‐demand therapy (prophylaxis first), 6 months of on‐demand therapy, 3 months of washout period and 6 months of prophylaxis (on‐demand therapy first) |
|
| Outcomes | Primary outcome: reduction of bleeding events during prophylaxis period compared with the on‐demand period Secondary outcomes: number of joint bleeding, number of target joint bleeding, HRQoL and safety |
|
| Notes | Study funded by Baxter Bioscience who provided AICC. Study investigators and a medical writer paid by Baxter Bioscience prepared the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted from a centralized call center (telephone randomization) – information from the online protocol |
| Allocation concealment (selection bias) | Low risk | Randomization conducted from a centralized call center (telephone randomization) – information from the online protocol |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For ethical and practical reasons, participants were aware of study assignments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For ethical and practical reasons, participants were aware of study assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per protocol results reported for efficacy outcomes (reduction in bleeding rates / joint bleeding rates), ITT approach for safety outcomes (monthly hemorrhage rates, adverse effects). Up to 24% of participants excluded from results: 1 patient withdrew consent before receiving study medication. The ITT group comprised 33 participants, of whom 7 did not complete the study: 1 withdrew because of an allergic reaction, 2 died, 1 was lost to follow‐up after Hurricane Katrina, and 3 withdrew consent (2 during the on‐demand period and 1 during the prophylaxis period) |
| Selective reporting (reporting bias) | Low risk | Online protocol available. All outcomes reported in protocol fully reported in the study, no evidence of selective reporting |
| Other bias | Low risk | None identified |
Ljung 2013.
| Methods | Multicenter randomized parallel study conducted over 19 sites in France, Japan, Malaysia, Serbia, Sweden, Turkey, UK, USA, South Africa | |
| Participants | 24 participants (8 to each group but 1 not dosed) Inclusion criteria: people with hemophilia A or B with high‐responding inhibitors (≥ 5 BU/mL) and frequent bleeds, age 12 ‐ 65 years, at least 2 bleeding episodes within the last month or 12 bleeding episodes within the last 6 months Exclusion criteria: low platelet count (< 50,000 per μL), active pseudotumours, advanced atherosclerotic disease, severe liver disease, renal dysfunction, coagulation disorders other than congenital hemophilia or a history of thromboembolic events |
|
| Interventions | On‐demand period: activated rFVIIa for the treatment of bleeding episodes Prophylaxis period: 40K glycoPEGylated rFVIIa bypassing agent (N7‐GP) administration at a target doses of 25, 100 and 200 μg/kg intravenously over 2 ‐ 5 minutes every second day Duration of the study: 3 months of observation period (on‐demand therapy), 3 months of prophylaxis period and 1 month of observation period |
|
| Outcomes | Primary outcome: reduction in annualised bleeding rate Secondary outcome: number of specific bleeds stratified by sites and causes |
|
| Notes | Study sponsored by Novo Nordisk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized and stratified by age, no further information given regarding generation of random sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study: placebo solution was used to provide equal injection volume irrespective of dose |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were withdrawn during the treatment period (1 from 100 μg group and 2 from 200 μg group) but all were included in analyses up to the point of withdrawal in an ITT analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available. Most outcomes defined in the methods reported in the results (target joint rate bleeds mentioned in the methods but not in the results). The study is a Phase 2 dose ranging study so the main objective of the study is safety and pharmacokinetic measures, efficacy is a secondary measure so risk of bias is judged to be low |
| Other bias | Low risk | None identified |
AICC: anti‐inhibitor coagulant complex AJBR: annualised joint bleeding rate BU: Bethesda unit FEIBA NF: nanofiltered factor eight inhibitor bypassing activity HRQoL: health‐related quality of life ITI: immune tolerance induction ITT: intention‐to‐treat rFVIIa: recombinant factor VII
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT02622321 | The study drug was not rFVIIa or APCC |
| NCT02795767 | The study drug was not rFVIIa or APCC |
| NCT02847637 | The study drug was not rFVIIa or APCC |
| NCT03020160 | The study drug was not rFVIIa or APCC |
APCC: activated prothrombin complex concentrate rFVIIa: recombinant factor VIIa
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01105546.
| Methods | Multicenter parallel study in the USA, France, Germany, Italy, Romania and Spain |
| Participants | Inclusion criteria: boys with hemophilia A who developed FVIII inhibitors, up to 8 years, ≤ 2 years from the time of inhibitor detection, high‐responding inhibitor (≥ 5 BU/mL) and known anamnestic response in case of negative inhibitor titre, candidate to start ITI with FVIII concentrates (doses ranging from 50 IU/kg/day to 200 IU/kg/day), ≤ 2 bleedings in the same joint within the last 6 months before entering the study, maximum 6 joint bleeds in the same joint within 2 years and adequate venous access Exclusion criteria: ITI already started, sensitivity to study drug, administration of study drug within 30 days prior randomization, family history of thrombosis at early age (< 40 years), known thrombophilia, any previous thrombosis, known pseudotumors, severe liver disease, platelet count < 50,000 platelet/μL and surgery within 1 month or planned for major surgery |
| Interventions | On demand: on‐demand treatment, treatment of bleeding episodes with rFVIIa 270 µg/kg (first/single dose) or 90 µg/kg IV every 2‐3 hours until bleeding resolution Prophylaxis: prophylaxis with rFVIIa 90 µg/kg/day |
| Outcomes | No information provided |
| Notes | Study sponsored by Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico |
ITI: immune tolerance induction IU: international units IV: intravenous rFVIIa: recombinant factor VIIa
Differences between protocol and review
In a post hoc change from protocol, two summary of findings tables (one for each comparison) have been added to the review. Outcomes were selected based on relevance to clinicians and consumers
Contributions of authors
Chatree Chai‐Adisaksopha assessed eligibility of studies, performed data extraction and risk of bias assessment, interpreted analyses, constructed the summary of findings tables and drafted the review text.
Sarah Nevitt performed data extraction and risk of bias assessment, performed and interpreted analyses and assisted with construction of summary of findings tables and drafting of the review text.
Mindy Simpson drafted the original protocol, assessed eligibility of studies, performed data extraction, assessed risk of bias assessment and commented on the final draft version.
Maissaa Janbain commented on the final draft version.
Barbara Konkle commented on the final draft version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Chatree Chai‐Adisaksopha (lead author): none known.
Sarah Nevitt (co‐author): none known.
Mindy Simpson (co‐author): has been compensated for participation in advisory boards by Baxter Bioscience (now Shire), Biogen Idec (now Bioverativ), CSL Behring, NovoNordisk, Grifols, and Octapharma. Rush University Medical Center receives grant support on behalf of Mindy Simpson from CSL Behring, NovoNordisk, Biogen (now Bioverativ) and Baxter Bioscience (now Shire).
Maissaa Janbain (co‐author): once received consultant fees from CSL Behring and Bayer for attending advisory board meetings.
Barbara Konkle (co‐author): receives research support from Baxter Bioscience, Biogen‐Idec, and Novo‐Nordisk and has consulted for Baxter Bioscience, Bayer, Biogen‐Idec, CSL‐Behring, Novo‐Nordisk and Pfizer.
Clarification statement added from Alan Smyth, Co‐ordinating Editor on 19 March 2020: The published protocol and review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. The review will be updated by March 2021 with the majority of review authors free of conflicts conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Antunes 2014 {published data only}
- Antunes S, Tangada S, Stasyshyn O, Mamonov V, Phillips J, Guzman‐Becerra N, et al. A prospective, open‐label, randomized, parallel study with AICC to evaluate the efficacy and safety of prophylactic vs. on‐demand treatment in hemophilia A or B subjects with inhibitors. Journal of Thrombosis and Haemostasis: JTH 2013;11 Suppl 2:982. [Abstract no: PB 4.58‐6; CENTRAL: 1014799; CRS: 5500131000000230] [Google Scholar]
- Antunes SV, Tangada S, Phillips J, Stasyshyn O, Mamonov V, Guzman‐Becerra N, et al. Comparison of historic on‐demand versus prospective on‐demand and prophylaxis bleeding episodes in hemophilia A and B patients with inhibitors treated with FEIBA NF. Haemophilia 2014;20:96. [CENTRAL: 1063882; CRS: 5500050000000504; EMBASE: 71475655] [Google Scholar]
- Antunes SV, Tangada S, Stasyshyn O, Mamonov V, Phillips J, Guzman‐Becerra N, et al. Randomized comparison of prophylaxis and on‐demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia 2013;20(1):65‐72. [CENTRAL: 961365; CRS: 5500050000000036; EMBASE: 2013812414] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasyshyn O, Antunes S, Mamanov V, Ye X, Xiong Y, Tangada S. Health‐related quality of life in hemophilia patients with inhibitors receiving prophylaxis with anti‐inhibitor coagulant complex (AICC): results from the AICC prophylaxis study. Journal of Thrombosis and Haemostasis: JTH 2013;11 Suppl 2:719. [CENTRAL: 1014800; CRS: 5500131000000232] [Google Scholar]
- Stasyshyn O, Antunes S, Mamonov V, Ye X, Epstein J, Xiong Y, et al. Prophylaxis with anti‐inhibitor coagulant complex improves health‐related quality of life in haemophilia patients with inhibitors: results from FEIBA NF Prophylaxis Study. Haemophilia 2014;20(5):644‐50. [CENTRAL: 1001041; CRS: 5500131000000129; JID:: 9442916; PUBMED: 24589084] [DOI] [PubMed] [Google Scholar]
- Varadi K, Tangada S, Loeschberger M, Montsch P, Schrenk G, Ewenstein B, et al. Pro‐ and anticoagulant factors facilitate thrombin generation and balance the haemostatic response to FEIBA in prophylactic therapy. Haemophilia 2016;22(4):615‐24. [CENTRAL: 1136893; CRS: 5500135000001495; PUBMED: 26879158] [DOI] [PubMed] [Google Scholar]
- Varadi K, Tangada S, Schrenk G, Doralt J, Ewenstein B, Turecek P. Evidence of APCC mode of action in a clinical study treating hemophilia A inhibitor patients with prophylaxis therapy. Journal of Thrombosis and Haemostasis: JTH 2015;13 Suppl 2:588. [Abstract no: PO223‐TUE; CENTRAL: 1131781; CRS: 5500050000000353; EMBASE: 71946015] [Google Scholar]
Konkle 2007 {published data only}
- Hoots WK, Ebbesen LS, Konkle BA, Auerswald GK, Roberts HR, Weatherall J, et al. Secondary prophylaxis with recombinant activated factor VII improves health‐related quality of life of haemophilia patients with inhibitors. Haemophilia 2008;14(3):466‐75. [CENTRAL: 714802; CRS: 5500100000003373] [DOI] [PubMed] [Google Scholar]
- Konkle B, Friedrich U, Abrams Z. Secondary prophylactic treatment with rFVIIa in patients with haemophilia A or B and inhibitors with high requirements for on‐demand treatment. Haemophilia 2006;12(Suppl 2):14. [Abstract no: PO363; CENTRAL: 593037; CRS: 5500100000002995] [Google Scholar]
- Konkle BA, Ebbesen LS, Auerswald GKH, Friedrich U, Ljung RCR, Roberts HR, et al. Secondary prophylaxis with rFVIIa improves quality of life of hemophilia patients with inhibitors and frequent bleeds. Blood 2006;108(11). [Abstract no: 1028; CENTRAL: 581202; CRS: 5500100000002911] [Google Scholar]
- Konkle BA, Ebbesen LS, Erhardtsen E, Bianco RP, Lissitchkov T, Rusen L, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. Journal of Thrombosis and Haemostasis: JTH 2007;5(9):1904‐13. [CENTRAL: 611680; CRS: 5500100000003138; PUBMED: 17723130] [DOI] [PubMed] [Google Scholar]
- Konkle BA, Ebbesen LS, Friedrich U, Bianco RP, Lissitchkov T, Rusen L, et al. Randomized, prospective clinical trial of rFVIIa for secondary prophylaxis in hemophilla patients with inhibitors. Blood 2006;108(11). [Abstract no: 766; CENTRAL: 593038; CRS: 5500100000002996] [Google Scholar]
Leissinger 2011 {published data only}
- Fusco F, Gringeri A, Leissinger C, Cortesi PA, Mantovani LG. Bleeding prophylaxis with an anti‐inhibitor coagulant complex (AICC) in patients with hemophilia A and inhibitors can improve quality of life: results of the PRO‐FEIBA Study. 16th Congress of the European Hematology Association; 2011 June 9‐12; London, UK. 2011. [CENTRAL: 1017613; CRS: 5500131000000290]
- Gringeri A, Leissinger C, Cortesi P, Matovani LG. Bleeding prophylaxis with an anti‐inhibitor coagulant complex (AICC) in patients with hemophilia a and inhibitors can improve quality of life: results of the pro‐FEIBA study. Journal of Thrombosis and Haemostasis: JTH 2011;9 Suppl 2:927. [Abstract no: P‐TH‐509; CENTRAL: 1015140; CRS: 5500131000000238] [Google Scholar]
- Gringeri A, Leissinger C, Cortesi PA, Jo H, Fusco F, Riva S, et al. Health‐related quality of life in patients with haemophilia and inhibitors on prophylaxis with anti‐inhibitor complex concentrate: Results from the Pro‐FEIBA study. Haemophilia 2013;19(5):736‐43. [CENTRAL: 918277; CRS: 5500050000000035; EMBASE: 2013536834] [DOI] [PubMed] [Google Scholar]
- Gringeri A, Leissinger CA, Cortesi P, Jo H, Mantovani L. Cost‐effectiveness of prophylaxis with anti‐inhibitor complex concentrate in patients with hemophilia A and inhibitors: results from the Pro‐FEIBA study. Blood 2011;118(21). [Abstract no: 4187; CENTRAL: 883157; CRS: 5500125000000183] [Google Scholar]
- Gringeri A, Lessinger C, Santagostino E, Cortesi P, Mantovani L. Pharmacoeconomic evaluation with an activated prothrombin complex concentrate (APCC) in patients with hemophilia and inhibitors (PRO‐FEIBA Study). Haemophilia 2012;18 Suppl 3:70. [Abstract no: PO‐MO‐052; CENTRAL: 868071; CRS: 5500100000011579] [Google Scholar]
- Leissinger C, Gringeri A, Antmen B, Berntorp E, Biasoli C, Carpenter S, et al. Anti‐inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. New England Journal of Medicine 2011;365(18):1684‐92. [CENTRAL: 804405; CRS: 5500100000011594; PUBMED: 22047559] [DOI] [PubMed] [Google Scholar]
- Leissinger C, Negrier C, Berntorp E, Windyga J, Serban M, Bulent A, et al. A prospective, randomized, and crossover study of an activated prothrombin complex concentrate for secondary prophylaxis in patients with hemophilia A and inhibitors (Pro‐FEIBA): subject demographics and safety data. Haemophilia 2010;16 Suppl 4:31. [Abstract no: 07P13; CENTRAL: 868062; CRS: 5500100000011569] [Google Scholar]
- Leissinger CA, Berntorp E, Biasioli C, Carpenter S, Jo H, Kavakli K, et al. Prophylactic dosing of anti‐inhibitor coagulant complex (FEIBA) reduces bleeding frequency in hemophilia A patients with inhibitors: results of the Pro‐FEIBA study. Blood 2011;118(21). [Abstract no: 720; CENTRAL: 883156; CRS: 5500125000000182] [Google Scholar]
- Leissinger CA, Gringeri A, Valentino LA, Cortesi PA. Joint disease and the potential for improved joint health in inhibitor patients who have a good response to aPCC prophylaxis: data from the profeiba study. Blood 2012;120(21). [Abstract no: 3374; CENTRAL: 883160; CRS: 5500125000000186] [Google Scholar]
- NCT00221195. Efficacy study of activated prothrombin complex for prevention of bleeds in hemophilia [A prospective, randomized, cross‐over study of an activated prothrombin complex concentrate for secondary prophylaxis in patients with hemophilia A and inhibitors]. clinicaltrials.gov/ct2/show/NCT00221195 Date first received: 16 September 2005.
- Riva S, Fusco F, Cortesi P, Fasulo M, Gringeri A. Health‐related quality of life (HRQOL) in bleeding prophylaxis with an activated prothrombin complex concentrate (APCC): results from the Pro‐FEIBA study. Haemophilia 2012;18 Suppl 3:179. [Abstract no: PO‐TU‐227; CENTRAL: 868072; CRS: 5500100000011580] [Google Scholar]
- Riva S, Fusco F, Cortesi P, Mantovani L, Lessinger C, Gringeri A. Quality of life in pro‐FEIBA study. Haemophilia 2012;18 Suppl 1:28. [Abstract no: 36; CENTRAL: 868075; CRS: 5500100000011583] [Google Scholar]
Ljung 2013 {published data only}
- Ljung R, Karim FA, Saxena K, Suzuki T, Arkhammar P, Rosholm A, et al. 40K glycoPEGylated, recombinant FVIIa: 3‐month, double‐blind, randomized trial of safety, pharmacokinetics and preliminary efficacy in hemophilia patients with inhibitors. Journal of Thrombosis and Haemostasis: JTH 2013;11(7):1260‐8. [CENTRAL: 919860; CRS: 5500050000000024; EMBASE: 2013458697] [DOI] [PubMed] [Google Scholar]
- NCT00951405. Safety and efficacy of 3 different doses of long acting factor VII in haemophilia A or B patients with inhibitors [An exploratory multi‐Centre, multi‐national, randomised, double blinded, parallel arm trial evaluating safety, pharmacokinetics and dose‐finding of prophylactic administration of long acting rFVIIa (LA‐rFVIIa) in haemophilia A or B patients with inhibitors]. clinicaltrials.gov/ct2/show/NCT00951405 Date first received: 03 August 2009.
References to studies excluded from this review
NCT02622321 {published data only}
- NCT02622321. A study to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A patients with inhibitors (HAVEN 1) [A randomized, multicenter, open‐label, phase III clinical trial to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A patients with inhibitors]. clinicaltrials.gov/ct2/show/NCT02622321 Date first received: 02 December 2015.
NCT02795767 {unpublished data only}
- NCT02795767. A study of once‐weekly emicizumab in children and adolescents with hemophilia A and Factor VIII (FVIII) inhibitors (HAVEN 2) [A single‐arm, multicenter, open‐label, phase III clinical trial to evaluate the efficacy, safety, and pharmacokinetics of once weekly subcutaneous administration of emicizumab in hemophilia A pediatric patients with inhibitors]. clinicaltrials.gov/ct2/show/NCT02795767 Date first received: 07 June 2016.
NCT02847637 {unpublished data only}
- NCT02847637. A clinical trial to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A participants without inhibitors (HAVEN 3) [A randomized, multicenter, open‐label, phase III clinical trial to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A patients without inhibitors]. clinicaltrials.gov/ct2/show/NCT02847637 Date first received: 26 July 2016.
NCT03020160 {published data only}
- NCT03020160. A study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab given every 4 weeks in participants with hemophilia A (Haven 4) [A multicenter, open‐label, phase III study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab given every 4 weeks (Q4W) in patients with hemophilia A]. clinicaltrials.gov/ct2/show/NCT03020160 Date first received: 21 December 2016.
References to studies awaiting assessment
NCT01105546 {published data only}
- NCT01105546. rFVIIa prophylaxis in children with hemophilia A and inhibitors (ENJOIH) [An investigator‐initiated study on rFVIIa prophylaxis in children with hemophilia A and inhibitors ‐ European initiative to prevent joint damage in hemophilia A children with inhibitors]. ClinicalTrials.gov/show/NCT01105546 Date first received: 15 April 2010.
Additional references
Astermark 2007
- Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood 2007;109(2):546‐51. [DOI] [PubMed] [Google Scholar]
Astermark 2010a
- Astermark J. Inhibitor development: patient‐determined risk factors. Haemophilia 2010;16(102):66‐70. [DOI] [PubMed] [Google Scholar]
Astermark 2010b
- Astermark J, Altisent C, Batorova A, Diniz MJ, Gringeri A, Holme PA, et al. Non‐genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report. Haemophilia 2010;16(5):747‐66. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Berntorp 2006
- Berntorp E, Shapiro A, Astermark J, Blanchette VS, Collins PW, Dimichele D, et al. Inhibitor treatment in haemophilias A and B: summary statement for the 2006 international consensus conference. Haemophilia 2006;12 Suppl 6:1‐7. [DOI] [PubMed] [Google Scholar]
Chuansumrit 2000
- Chuansumrit A, Isarangkura P, Angchaisuksiri P, Sriudomporn N, Tanpowpong K, Hathirat P, et al. Controlling acute bleeding episodes with recombinant factor VIIa in haemophiliacs with inhibitor: continuous infusion and bolus injection. Haemophilia 2000;6(2):61‐5. [DOI] [PubMed] [Google Scholar]
Collins 2013
- Collins PW, Chalmers E, Hart DP, Liesner R, Rangarajan S, Talks K, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors Organization. British Journal of Haematology 2013;160(2):153‐70. [DOI] [PubMed] [Google Scholar]
Darby 2004
- Darby SC, Keeling DM, Spooner RJ, Wan Kan S, Giangrande PL, Collins PW, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977‐99. Journal of Thrombosis and Haemostasis 2004;2(7):1047‐54. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration, 2011.. Available from handbook.cochrane.org.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 1998
- Hay CR. Factor VIII inhibitors in mild and moderate‐severity haemophilia A. Haemophilia 1998;4(4):558‐63. [DOI] [PubMed] [Google Scholar]
Hay 2012
- Hay CR, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012;119(6):1335‐44. [DOI] [PubMed] [Google Scholar]
Hedner 2000
- Hedner U. Recombinant coagulation factor VIIa: from the concept to clinical application in hemophilia treatment in 2000. Seminars in Thrombosis and Hemostasis 2000;26(4):363‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iorio 2010a
- Iorio A, Halimeh S, Holzhauer S, Goldenberg N, Marchesini E, Marcucci M, et al. Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma‐derived or recombinant factor VIII concentrates: a systematic review. Journal of Thrombosis and Haemostasis 2010;8(6):1256‐65. [DOI] [PubMed] [Google Scholar]
Iorio 2010b
- Iorio A, Matino D, D'Amico R, Makris M. Recombinant factor VIIa concentrate versus plasma derived concentrates for the treatment of acute bleeding episodes in people with haemophilia and inhibitors. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004449.pub3] [DOI] [PubMed] [Google Scholar]
Iorio 2011
- Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding‐related complications in people with hemophilia A or B. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD003429.pub4] [DOI] [PubMed] [Google Scholar]
Kavakli 2006
- Kavakli K, Makris M, Zulfikar B, Erhardtsen E, Abrams ZS, Kenet G, et al. Home treatment of haemarthroses using a single dose regimen of recombinant activated factor VII in patients with haemophilia and inhibitors. A multi‐centre, randomised, double‐blind, cross‐over trial. Thrombosis and Haemostasis 2006;95(4):600‐5. [PubMed] [Google Scholar]
Lusher 1998
- Lusher JM, Roberts HR, Davignon G, Joist JH, Smith H, Shapiro A, et al. A randomized, double‐blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. rFVIIa Study Group. Haemophilia 1998;4(6):790‐8. [DOI] [PubMed] [Google Scholar]
MASAC 2013
- National Hemophilia Foundation. MASAC recommendation regarding prophylaxis with bypassing agents in patients with hemophilia and high titer inhibitors. www.hemophilia.org/Researchers‐Healthcare‐Providers/Medical‐and‐Scientific‐Advisory‐Council‐MASAC/MASAC‐Recommendations/MASAC‐Recommendation‐Regarding‐Prophylaxis‐with‐Bypassing‐Agents‐in‐Patients‐with‐Hemophilia‐and‐High‐Titer‐Inhibitors (accessed prior to 20 June 2017).
Matzinger 1994
- Matzinger P. Tolerance, danger, and the extended family. Annual Review of Immunology 1994;12:991‐1045. [DOI] [PubMed] [Google Scholar]
Matzinger 2012
- Matzinger P. The evolution of the danger theory. Interview by Lauren Constable, Commissioning Editor. Expert Review of Clinical Immunology 2012;8(4):311‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruthi 2007
- Pruthi RK, Mathew P, Valentino LA, Sumner MJ, Seremetis S, Hoots WK, et al. Haemostatic efficacy and safety of bolus and continuous infusion of recombinant factor VIIa are comparable in haemophilia patients with inhibitors undergoing major surgery. Results from an open‐label, randomized, multicenter trial. Thrombosis and Haemostasis 2007;98(4):726‐32. [PubMed] [Google Scholar]
Santagostino 2006
- Santagostino E, Mancuso ME, Rocino A, Mancuso G, Scaraggi F, Mannucci PM. A prospective randomized trial of high and standard dosages of recombinant factor VIIa for treatment of hemarthroses in hemophiliacs with inhibitors. Journal of Thrombosis and Haemostasis 2006;4(2):367‐71. [DOI] [PubMed] [Google Scholar]
Scalone 2006
- Scalone L, Mantovani LG, Mannucci PM, Gringeri A, COCIS Study Investigators. Quality of life is associated to the orthopaedic status in haemophilic patients with inhibitors. Haemophilia 2006;12(2):154‐62. [DOI] [PubMed] [Google Scholar]
Seremetis 1994
- Seremetis SV. The clinical use of factor VIIa in the treatment of factor VIII inhibitor patients. Seminars in Hematology 1994;31(2 Suppl 4):53‐5. [PubMed] [Google Scholar]
Shapiro 1998
- Shapiro AD, Gilchrist GS, Hoots WK, Cooper HA, Gastineau DA. Prospective, randomised trial of two doses of rFVIIa (NovoSeven) in haemophilia patients with inhibitors undergoing surgery. Thrombosis and Haemostasis 1998;80(5):773‐8. [PubMed] [Google Scholar]
Shapiro 2003
- Shapiro, A. Inhibitor treatment: state of the art. Disease‐a‐Month 2003;49(1):22‐38. [DOI] [PubMed] [Google Scholar]
Valentino 2010
- Valentino LA. Assessing the benefits of FEIBA prophylaxis in haemophilia patients with inhibitors. Haemophilia 2010;16(2):263‐71. [DOI] [PubMed] [Google Scholar]
Young 2008
- Young G, Shafer FE, Rojas P, Seremetis S. Single 270 microg kg(‐1)‐dose rFVIIa vs. standard 90 microg kg(‐1)‐dose rFVIIa and APCC for home treatment of joint bleeds in haemophilia patients with inhibitors: a randomized comparison. Haemophilia 2008;14(2):287‐94. [DOI] [PubMed] [Google Scholar]


