6.
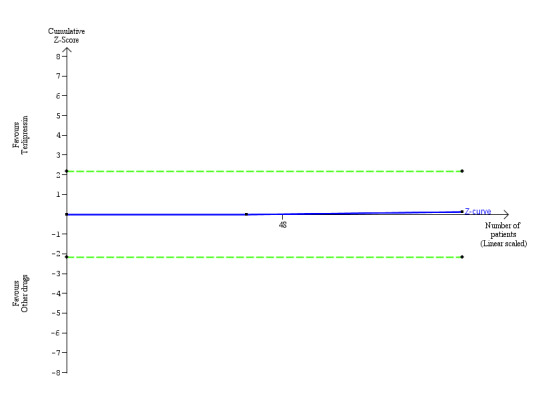
Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.
